Novel Oral Anticoagulants NOAC Dan Moellentin Pharm D














































- Slides: 82

Novel Oral Anticoagulants (NOAC) Dan Moellentin, Pharm. D, BCPS, Associate Professor Husson University

Strokes in Atrial Fibrillation • 1/5 of strokes are caused by a. fib • 1/3 cardiac arrhythmias hospitalizations • 2 million Americans affected by a. fib ~50% are anti-coagulated?

What would be the best anticoagulation Fast onset Antidote

Brief History of Anticoagulation in Atrial Fibrillation (a. fib) Warfarin was approved in 1954 • INR wasn’t created by the World Health Organization until the 1980’s Until 2009, warfarin was considered the gold standard for anticoagulation in a. fib. Now there are three new oral anticoagulants FDA approved for anticoagulation in a. fib

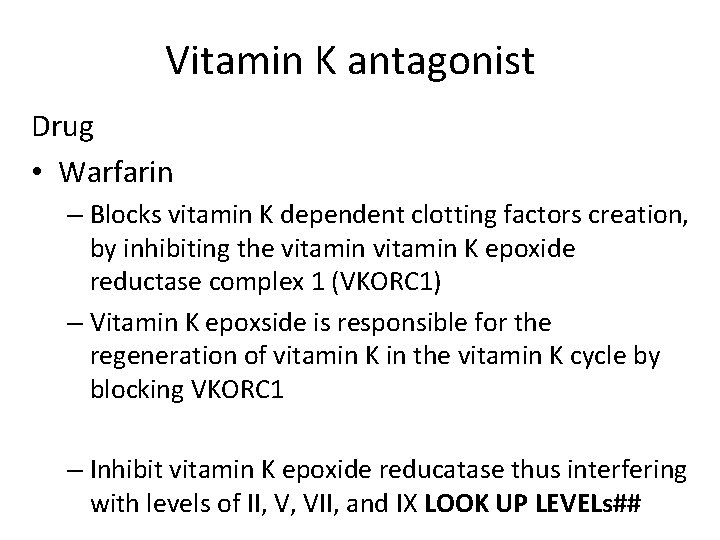
Vitamin K antagonist Drug • Warfarin – Blocks vitamin K dependent clotting factors creation, by inhibiting the vitamin K epoxide reductase complex 1 (VKORC 1) – Vitamin K epoxside is responsible for the regeneration of vitamin K in the vitamin K cycle by blocking VKORC 1 – Inhibit vitamin K epoxide reducatase thus interfering with levels of II, V, VII, and IX LOOK UP LEVELs##

Patient Case #1 • The majority of warfarin is elimination via metabolism – Clinical example---pt post bowel resection--

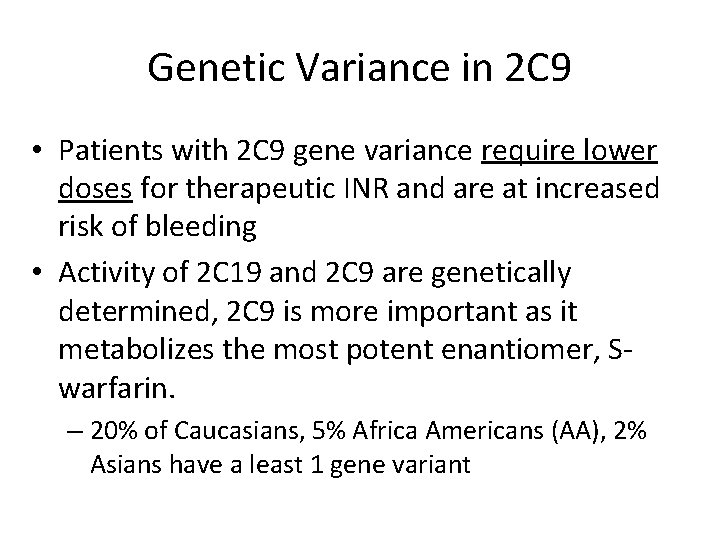
Genetic Variance in 2 C 9 • Patients with 2 C 9 gene variance require lower doses for therapeutic INR and are at increased risk of bleeding • Activity of 2 C 19 and 2 C 9 are genetically determined, 2 C 9 is more important as it metabolizes the most potent enantiomer, Swarfarin. – 20% of Caucasians, 5% Africa Americans (AA), 2% Asians have a least 1 gene variant

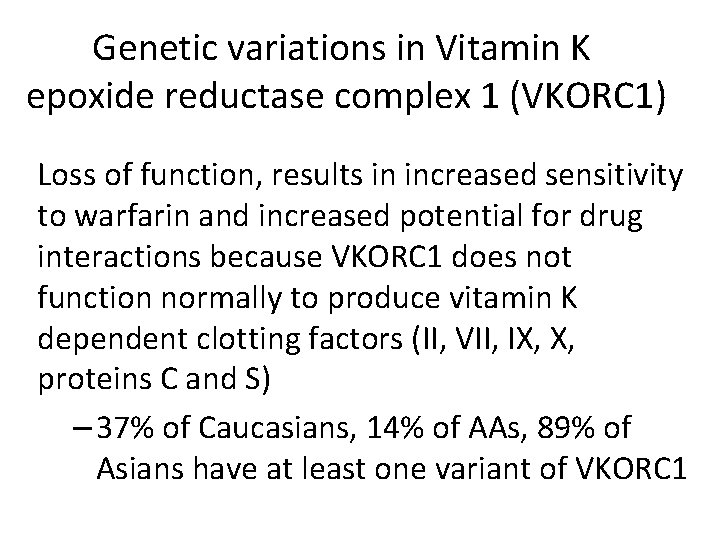
Genetic variations in Vitamin K epoxide reductase complex 1 (VKORC 1) Loss of function, results in increased sensitivity to warfarin and increased potential for drug interactions because VKORC 1 does not function normally to produce vitamin K dependent clotting factors (II, VII, IX, X, proteins C and S) – 37% of Caucasians, 14% of AAs, 89% of Asians have at least one variant of VKORC 1

Clinical Significance of Genetic Testing before Warfarin initiation • Not all centers have on-site testing • Genetic testing costs about $200 • Is it paid for by Medicare part D or private insurance? • Adding a few extra days to hospitalization is expensive • Is it only useful in guiding early dosing, ie. does it just save one dose? • “Drug interactions with warfarin may negate the influence of genetic variations and must be considered”.

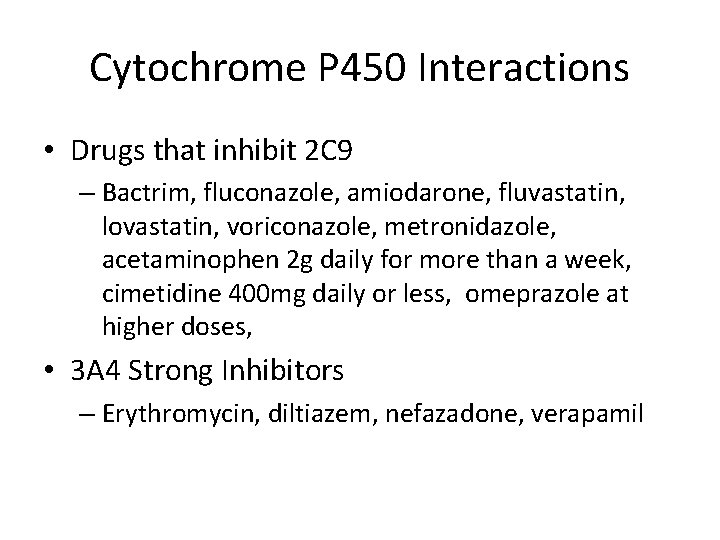
Cytochrome P 450 Interactions • Drugs that inhibit 2 C 9 – Bactrim, fluconazole, amiodarone, fluvastatin, lovastatin, voriconazole, metronidazole, acetaminophen 2 g daily for more than a week, cimetidine 400 mg daily or less, omeprazole at higher doses, • 3 A 4 Strong Inhibitors – Erythromycin, diltiazem, nefazadone, verapamil

Drugs Affecting Absorption Case #1 Cholestyramine or Colestipol • Get Dan to write case

Warfarin MOA: Vitamin K Antagonist (VKA) Initial dose: 10 mg PO x 2 days, then dose based on INR Half-life: 40 hours Monitoring: • If INR is ≤ 0. 5 from the target range, keep the same dose and check INR in 1 -2 weeks. • Once INR is stabilized, one INR reading up to every 12 weeks is adequate.

Only One is Also Approved for DVT/PE Treatment/Prophylaxis Rivaroxaban • “Treatment of deep vein thrombosis (DVT) pulmonary embolism” • “Reduction in the risk of recurrence of DVT” • “PE for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery”

Newly Approved PO anticoagulants • All 3 are FDA indicated for – “reducing the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation” • Direct Thrombin Inhibitor – Pradaxa® (dabigatran) • Approved in 2010 • Xa Inhibitors – Xarelto® (rivaroxaban) • Approved in 2011 – Eliquis® (Apixaban) • Approved in 2013

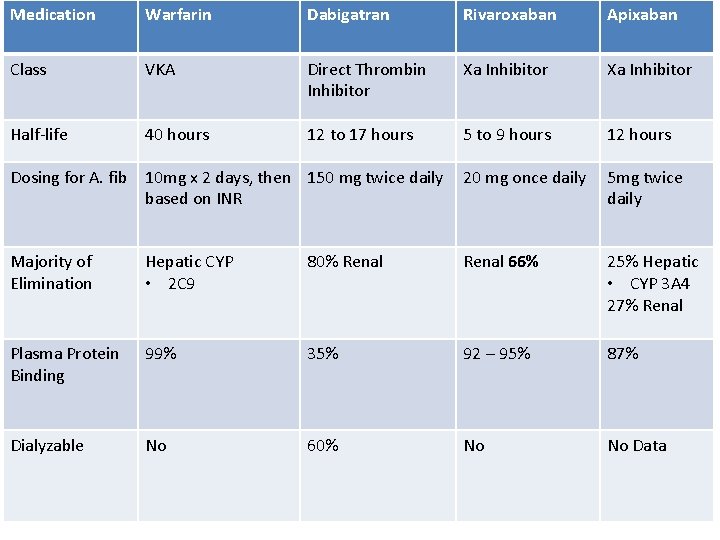
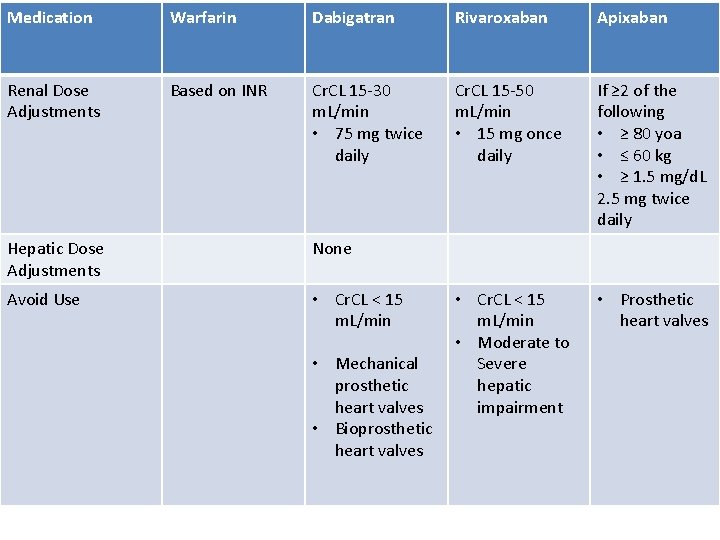
Medication Warfarin Dabigatran Rivaroxaban Apixaban Class VKA Direct Thrombin Inhibitor Xa Inhibitor Half-life 40 hours 12 to 17 hours 5 to 9 hours 12 hours Dosing for A. fib 10 mg x 2 days, then 150 mg twice daily based on INR 20 mg once daily 5 mg twice daily Majority of Elimination Hepatic CYP • 2 C 9 80% Renal 66% 25% Hepatic • CYP 3 A 4 27% Renal Plasma Protein Binding 99% 35% 92 – 95% 87% Dialyzable No 60% No No Data

Medication Warfarin Dabigatran Rivaroxaban Apixaban Renal Dose Adjustments Based on INR Cr. CL 15 -30 m. L/min • 75 mg twice daily Cr. CL 15 -50 m. L/min • 15 mg once daily If ≥ 2 of the following • ≥ 80 yoa • ≤ 60 kg • ≥ 1. 5 mg/d. L 2. 5 mg twice daily • Cr. CL < 15 m. L/min • Moderate to Severe hepatic impairment • Prosthetic heart valves Hepatic Dose Adjustments None Avoid Use • Cr. CL < 15 m. L/min • Mechanical prosthetic heart valves • Bioprosthetic heart valves

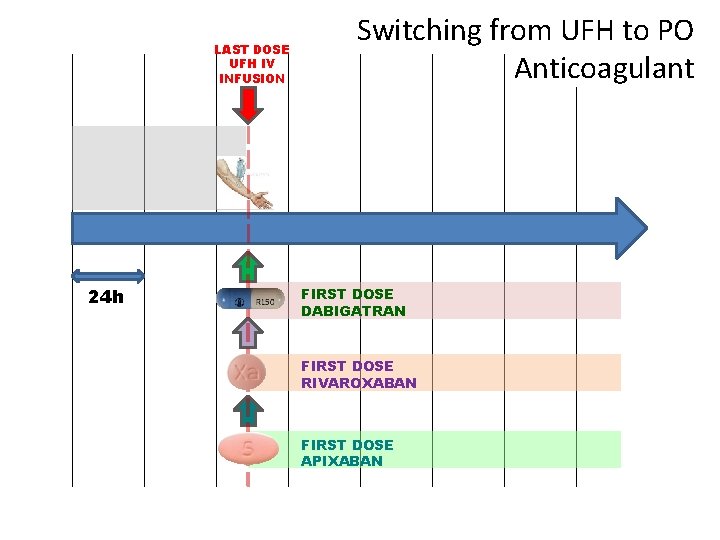
LAST DOSE UFH IV INFUSION 24 h Switching from UFH to PO Anticoagulant FIRST DOSE DABIGATRAN FIRST DOSE RIVAROXABAN FIRST DOSE APIXABAN

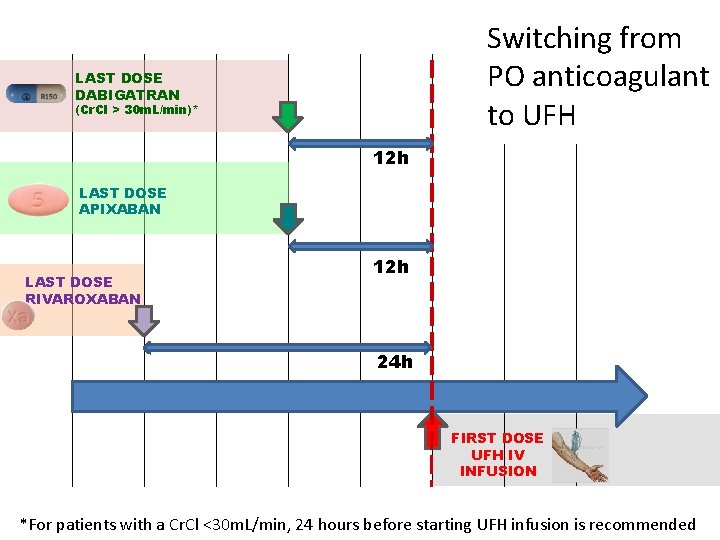
Switching from PO anticoagulant to UFH LAST DOSE DABIGATRAN (Cr. Cl > 30 m. L/min)* 12 h LAST DOSE APIXABAN LAST DOSE RIVAROXABAN 12 h 24 h FIRST DOSE UFH IV INFUSION *For patients with a Cr. Cl <30 m. L/min, 24 hours before starting UFH infusion is recommended

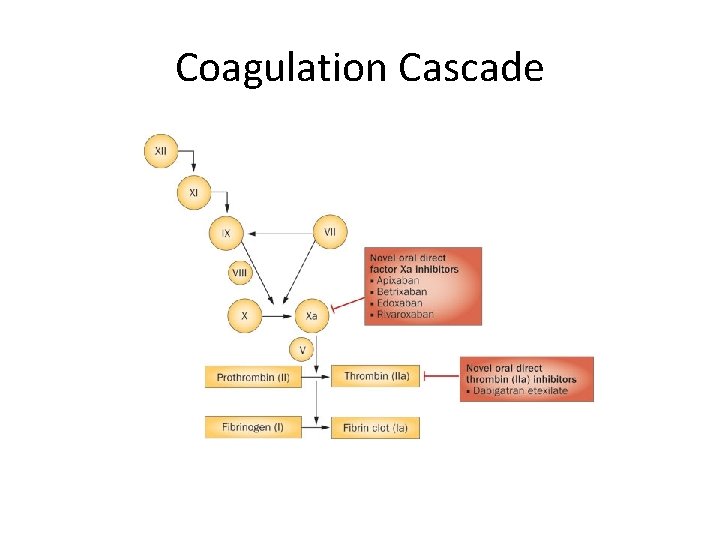
Coagulation Cascade

® Pradaxa (dabigatran) MOA: Direct Thrombin Inhibitor Half-life: 12 to 17 hours Dosing: • 150 mg PO twice daily • Cr. CL 15 -30 m. L/min – 75 mg twice daily Adverse Effects: • Dyspepsia • GI bleeding

Prescribing Information Drug Interactions for Dabigatran • USA – Amiodarone, dronedarone, quinidine, verapamil, clarithromycin, and ketoconazole, rifampin. • Canada – Amiodarone, dronedarone, verapamil, quinidine, rifampicin – Contraindications: Ketoconazole • EMA – Strong P-gp inhibitors: Amiodarone, , verapamil, quinidine, clarithromycin – P-gp inducers: rifampicin – Contraindications: Ketoconazole, itraconazole, tacrolimus, dronedarone, cyclosporine

Xarelto® (rivaroxaban) MOA: Xa Inhibitor Half-life: 5 to 9 hours Dosing: • A. Fib: – Cr. CL >50 m. L/min • 20 mg once daily – Cr. CL 15 -50 m. L/min • 15 mg once daily Adverse Effects

Eliquis® (apixaban) MOA: Xa Inhibitor Half-life: 12 hours Dosing: 5 mg PO twice daily • 2. 5 mg twice daily – If ≥ 2 of the following • ≥ 80 yoa • ≤ 60 kg • ≥ 1. 5 mg/d. L Adverse Effects:

Pt casen #1 dabigitran plus amiodarone • Pgp- problem is fda lit

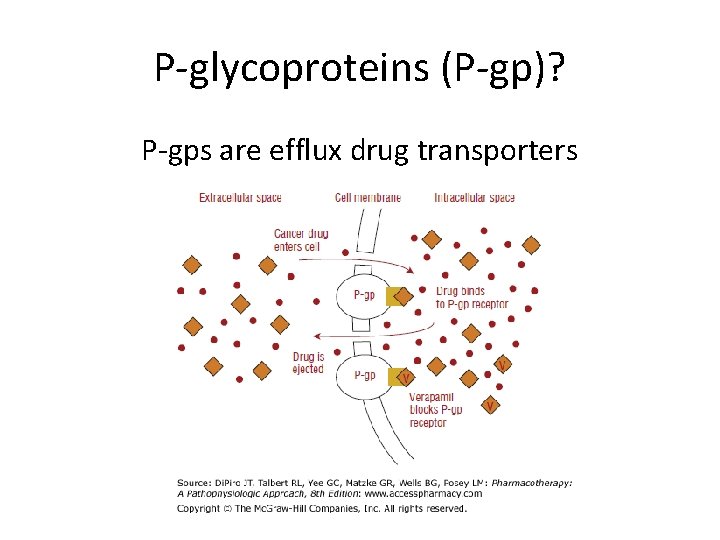
P-glycoproteins (P-gp)? P-gps are efflux drug transporters

P-gp function is to excrete or protect the tissues from xenobiotic absorption. • Also referred as PGY 1, multidrug resistance protein-1 (MDR 1). • Member of the adenosine triphosphate (ATP) binding cassette (ABC) gene • ABCB 1 is the gene that encodes for P-gp

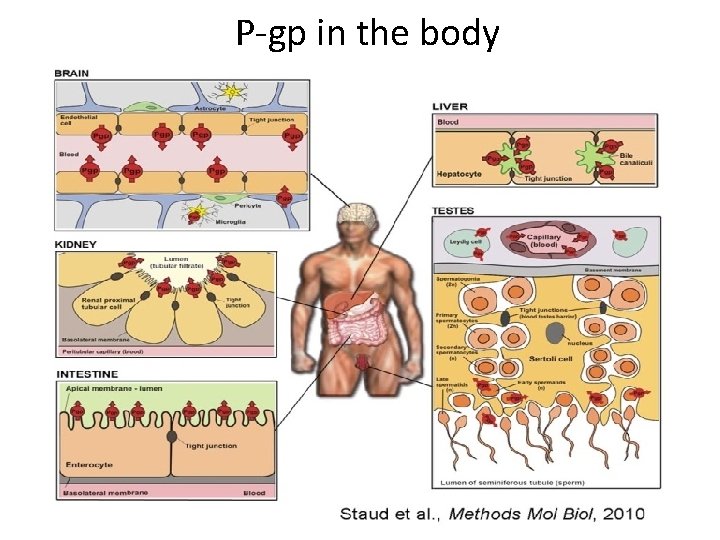
P-gp in the body

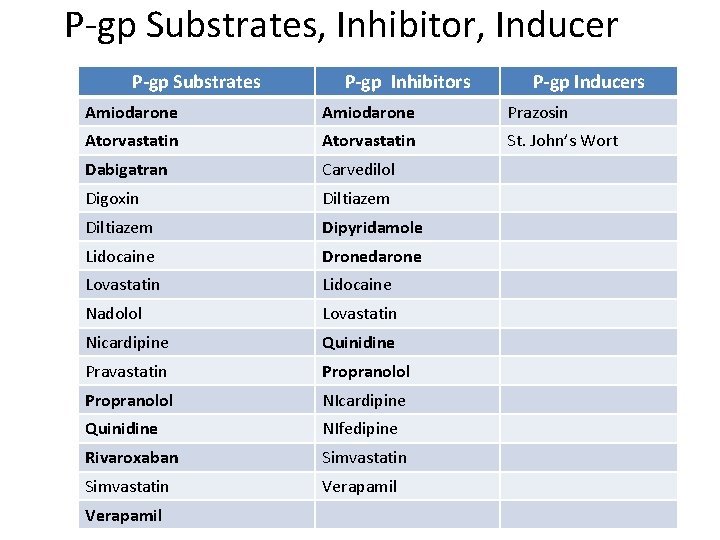
P-gp Substrates, Inhibitor, Inducer P-gp Substrates P-gp Inhibitors P-gp Inducers Amiodarone Prazosin Atorvastatin St. John’s Wort Dabigatran Carvedilol Digoxin Diltiazem Dipyridamole Lidocaine Dronedarone Lovastatin Lidocaine Nadolol Lovastatin Nicardipine Quinidine Pravastatin Propranolol NIcardipine Quinidine NIfedipine Rivaroxaban Simvastatin Verapamil

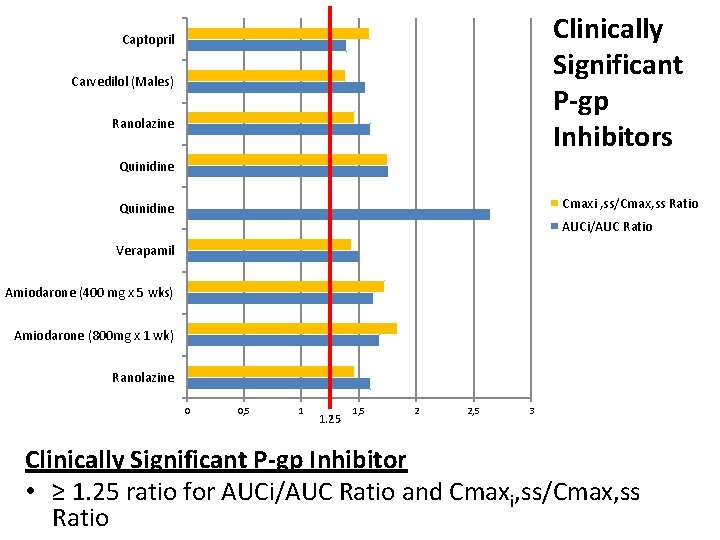
Clinically Significant P-gp Inhibitors Captopril Carvedilol (Males) Ranolazine Quinidine Cmaxi , ss/Cmax, ss Ratio Quinidine AUCi/AUC Ratio Verapamil Amiodarone (400 mg x 5 wks) Amiodarone (800 mg x 1 wk) Ranolazine 0 0, 5 1 1. 25 1, 5 2 2, 5 3 Clinically Significant P-gp Inhibitor • ≥ 1. 25 ratio for AUCi/AUC Ratio and Cmaxi, ss/Cmax, ss Ratio

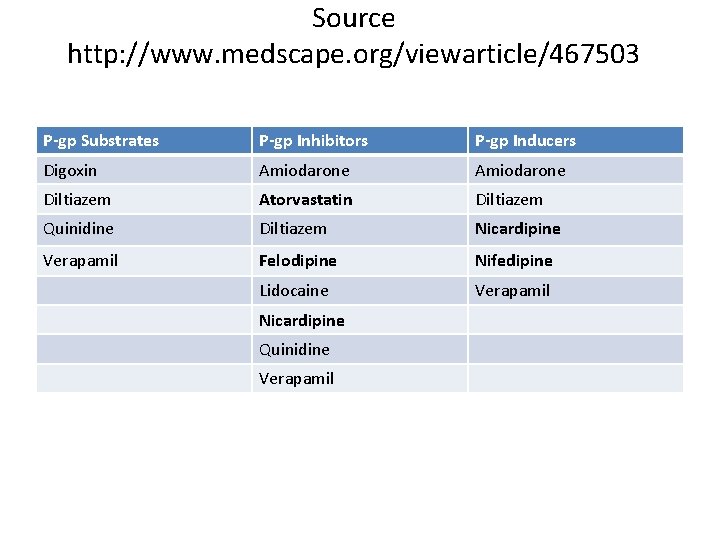
Source http: //www. medscape. org/viewarticle/467503 P-gp Substrates P-gp Inhibitors P-gp Inducers Digoxin Amiodarone Diltiazem Atorvastatin Diltiazem Quinidine Diltiazem Nicardipine Verapamil Felodipine Nifedipine Lidocaine Verapamil Nicardipine Quinidine Verapamil

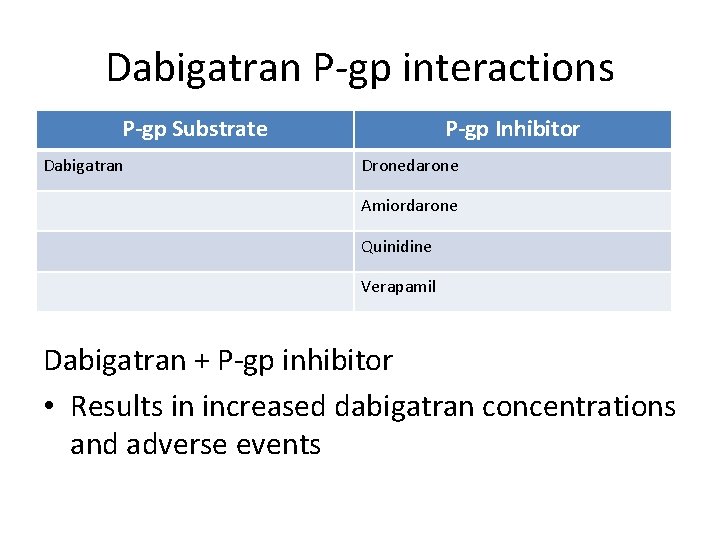
Dabigatran P-gp interactions P-gp Substrate Dabigatran P-gp Inhibitor Dronedarone Amiordarone Quinidine Verapamil Dabigatran + P-gp inhibitor • Results in increased dabigatran concentrations and adverse events

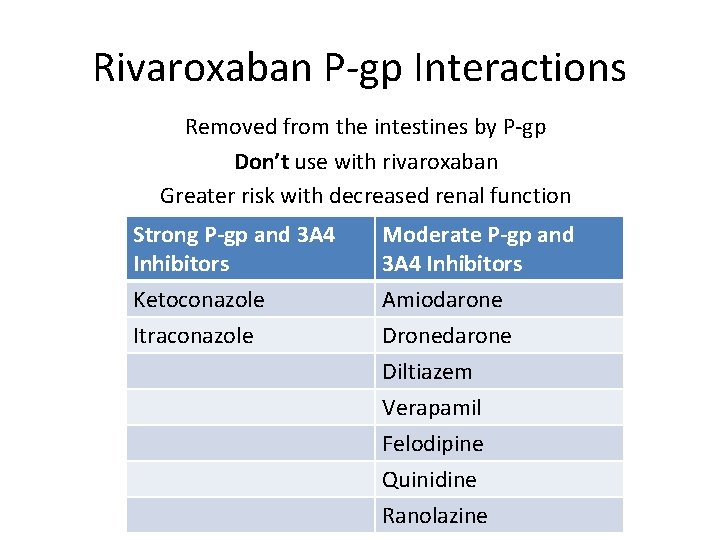
Rivaroxaban P-gp Interactions Removed from the intestines by P-gp Don’t use with rivaroxaban Greater risk with decreased renal function Strong P-gp and 3 A 4 Inhibitors Ketoconazole Itraconazole Moderate P-gp and 3 A 4 Inhibitors Amiodarone Dronedarone Diltiazem Verapamil Felodipine Quinidine Ranolazine

Apixaban P-gp Interactions Apixaban • ↑Apixaban conc. – Itraconazole, ketoconazole, ritonavir, and clarithromycin

Case #3 rivaroxaban

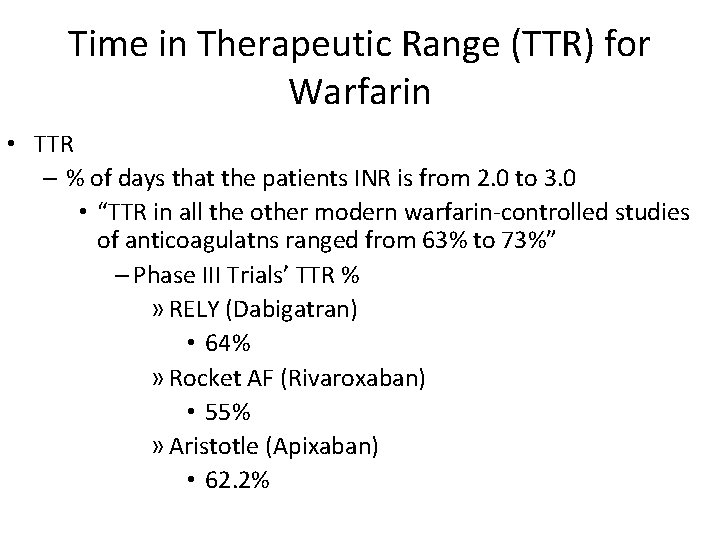
Time in Therapeutic Range (TTR) for Warfarin • TTR – % of days that the patients INR is from 2. 0 to 3. 0 • “TTR in all the other modern warfarin-controlled studies of anticoagulatns ranged from 63% to 73%” – Phase III Trials’ TTR % » RELY (Dabigatran) • 64% » Rocket AF (Rivaroxaban) • 55% » Aristotle (Apixaban) • 62. 2%

Is Rivaroxaban Dosing Appropriate? Half-life: 5 to 9 hours Non-valvular A. Fib • 20 mg PO once daily Phase II trials suggested that 10 mg twice daily could be safer than 20 mg once daily

FDA WORDING • INADEQUATE ON DABIGITRAN • DOSING LIKELY WRONG FOR DVT PROP ON RIVAROX APIXIBAN DOSING CORRECTNESS IS UNKNOWN • WHAT IS DIFFERENT ON LAST 2 DRUGS IS THAT DECISION FOR DOSE ADJUSTMENT IS NOW A PROVIDER JUDGEMENT BASED ON 3 A 4 AND PGP

No NOAC Are Approved for ACS

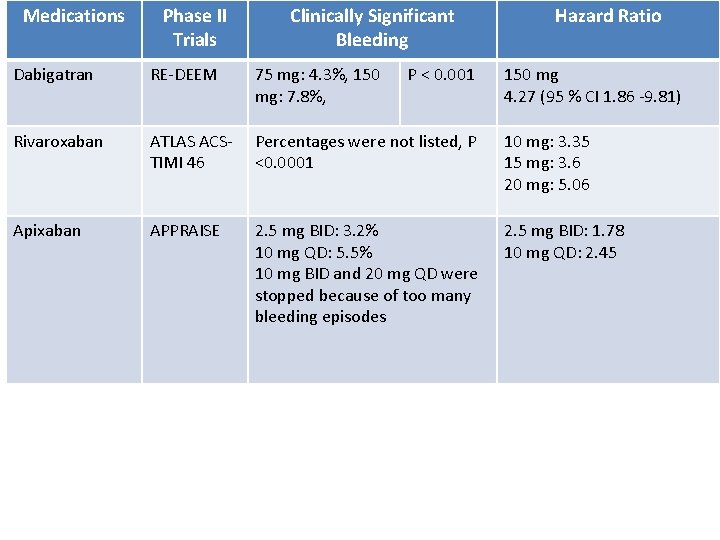
Medications Phase II Trials Clinically Significant Bleeding P < 0. 001 Hazard Ratio Dabigatran RE-DEEM 75 mg: 4. 3%, 150 mg: 7. 8%, 150 mg 4. 27 (95 % CI 1. 86 -9. 81) Rivaroxaban ATLAS ACSTIMI 46 Percentages were not listed, P <0. 0001 10 mg: 3. 35 15 mg: 3. 6 20 mg: 5. 06 Apixaban APPRAISE 2. 5 mg BID: 3. 2% 10 mg QD: 5. 5% 10 mg BID and 20 mg QD were stopped because of too many bleeding episodes 2. 5 mg BID: 1. 78 10 mg QD: 2. 45

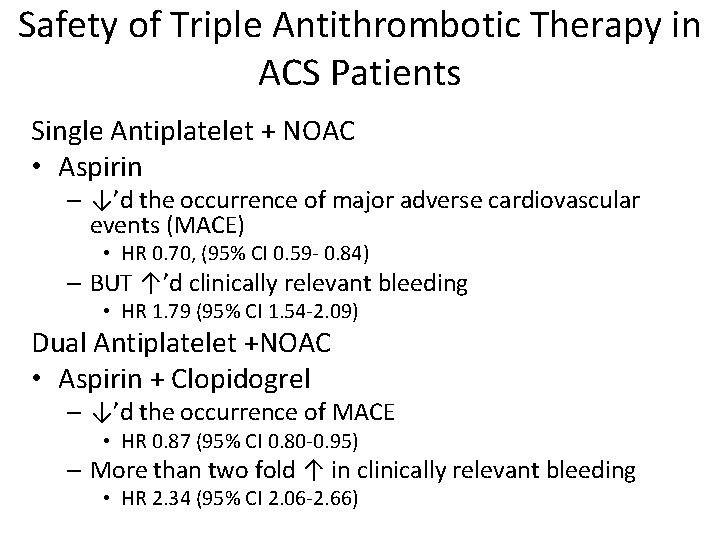
Safety of Triple Antithrombotic Therapy in ACS Patients Single Antiplatelet + NOAC • Aspirin – ↓’d the occurrence of major adverse cardiovascular events (MACE) • HR 0. 70, (95% CI 0. 59 - 0. 84) – BUT ↑’d clinically relevant bleeding • HR 1. 79 (95% CI 1. 54 -2. 09) Dual Antiplatelet +NOAC • Aspirin + Clopidogrel – ↓’d the occurrence of MACE • HR 0. 87 (95% CI 0. 80 -0. 95) – More than two fold ↑ in clinically relevant bleeding • HR 2. 34 (95% CI 2. 06 -2. 66)

Risk Assessment Physicians will need assess the patient’s risk for • Thromboembolism – CHADS 2 Score ≥ 2 = probably require triple antithrombotic therapy • Hemorrhaging “ Clinicians should strive to limit the use of dual antiplatelet agents with concurrent antithrombotics in patients who are at the highest risk for thromoembolic events and ensure that these patients are instructed to report any signs and sympotms of bleeding or recurrent thrombosis” Dager W Pharm. D PCPS (AQ Cardiology)

Reversal and Monitoring of n. OAC



Reversal of Dabigatran

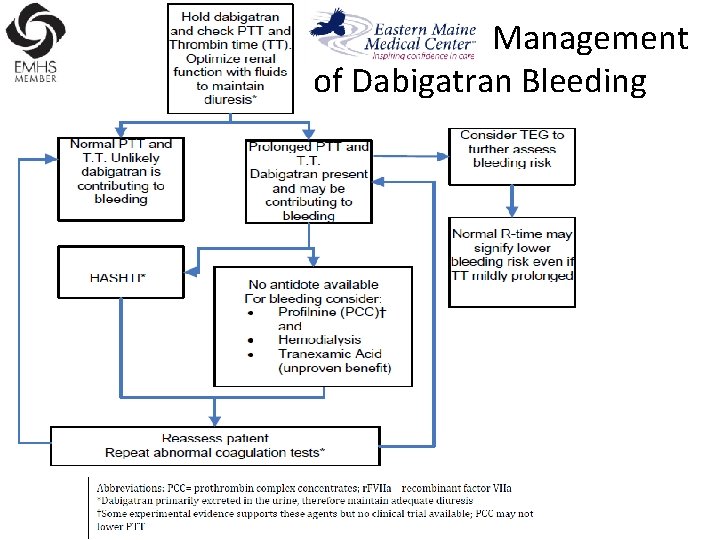
Management of Dabigatran Bleeding

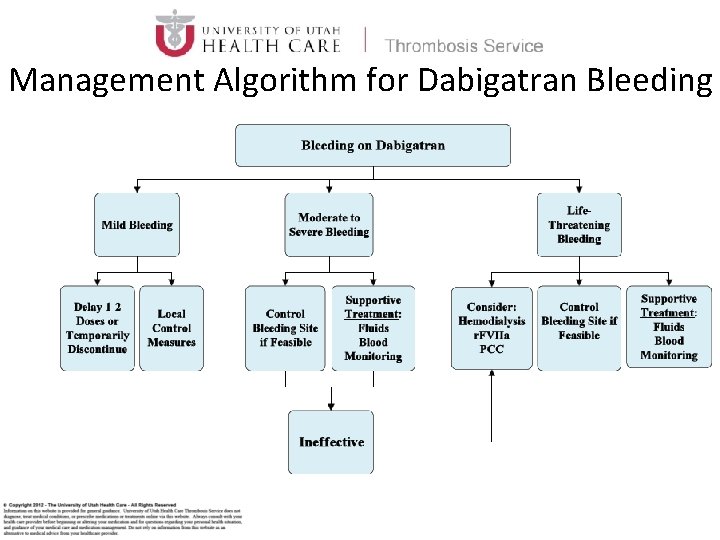
Management Algorithm for Dabigatran Bleeding


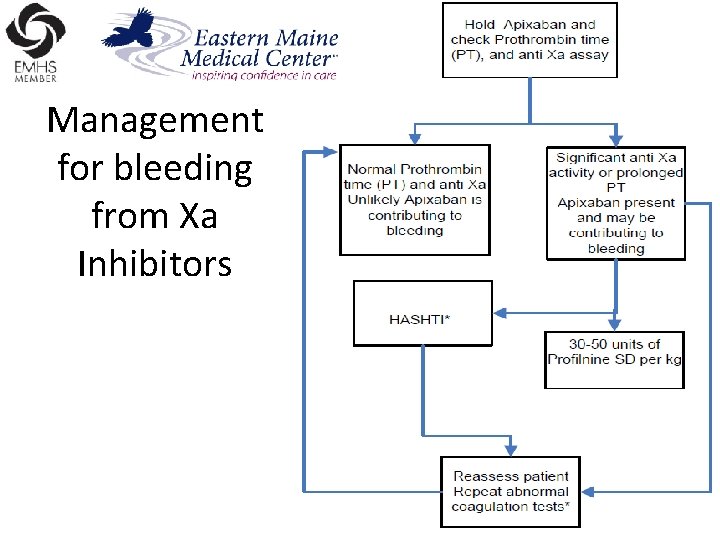
Management for bleeding from Xa Inhibitors

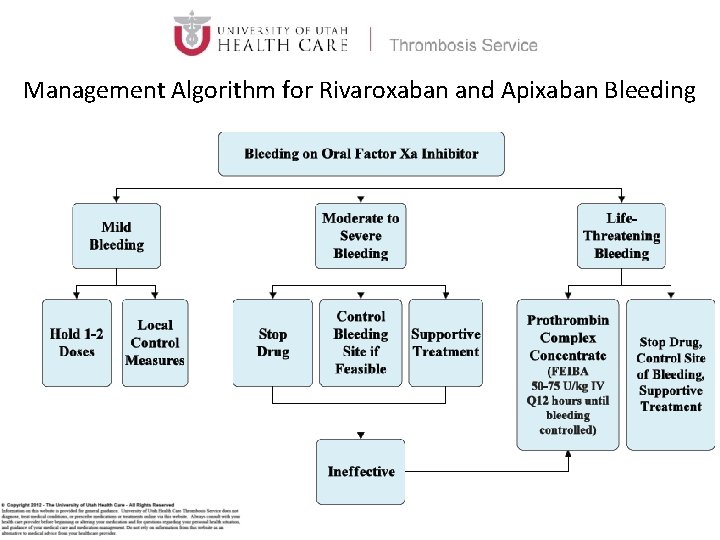
Management Algorithm for Rivaroxaban and Apixaban Bleeding

Reversal of Dabigatran • Since dabigatran directly blocks thrombin and does not decrease the coagulation factors, using coagulation factors like, PCC, FFP is NOT expected to be completely effective as a reversal agent. • The best method of reversing dabigatran is to be excreted out by the kidneys.

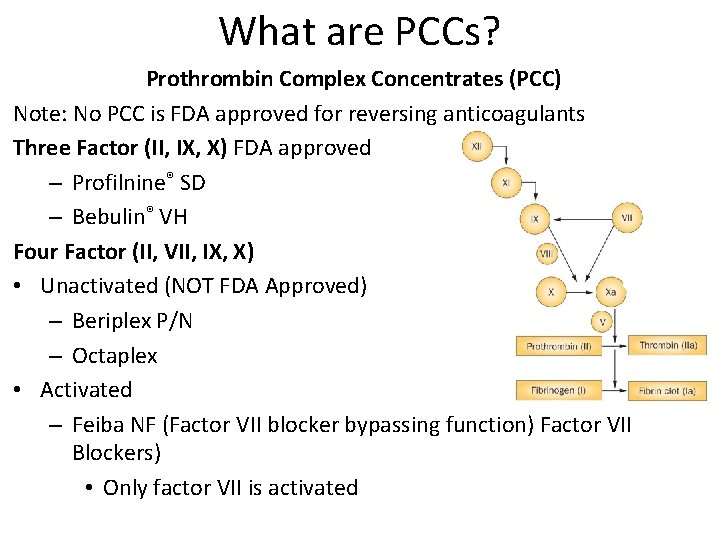
What are PCCs? Prothrombin Complex Concentrates (PCC) Note: No PCC is FDA approved for reversing anticoagulants Three Factor (II, IX, X) FDA approved – Profilnine® SD – Bebulin® VH Four Factor (II, VII, IX, X) • Unactivated (NOT FDA Approved) – Beriplex P/N – Octaplex • Activated – Feiba NF (Factor VII blocker bypassing function) Factor VII Blockers) • Only factor VII is activated

Antibody Antidote for Dabigatran? Boehringer Ingelheim: manufacture dabigatran (Pradaxa®) • Currently developing and studying pre-clinically a humanized antibody fragment (Fab) that could be used as a reversal agent for dabigatran. – Rats were given dabigatran and “there was a rapid, dose-dependent decrease in bleeding time after IV injection of Fab” – Additionally, the Fab reversed clotting ex vivo as well.

Reversal of Rivaroxaban? Phase I Trials • Four Factor PCC (Factor II, VII, IX and X) – After 3 days of rivaroxaban and one dose of PCC PT and ETP was statistically significantly decreased • PRT 4445 – New recombinant protein that blocks Xa inhibitors by serving as a decoy. – The manufacters of PRT 4445 has report its safe and tolerable and claims it reverses Xa inhibitors in 5 minutes and last 3 hours, however, the study isn’t available online yet.

So What is the “Right” Answer? Pre-clinical studies Studies showing PCC only improves labs not bleeding in apixaban and rivaroxaban

Warfarin Reversal • Is warfarin really “reversed? ” • Example intracranial hemorrhage (ICH)….

Gastrointestinal Bleeding • Higher risk for DVT/PE treatment vs. DVT/PE prophylaxis after hip/knee replacement surgery – Rivaroxaban 10 mg once daily • Hip: 35 days • Knee: 12 days – Maybe a dose-dependent effect • Dabigatran and rivaroxaban – Higher risk than apixaban

Numbers of post-marketing cases of ICH and retroperitoneal hemorrhages Apixaban Rivaroxaban Dabigatran

Post Marketing • Incidence of stroke, major bleeding, ICH

Post Marketing • Bleeding Risk with Dabigatran in the Frail Elderly NEJM http: //www. nejm. org/doi/full/10. 1056/NEJM c 1112874

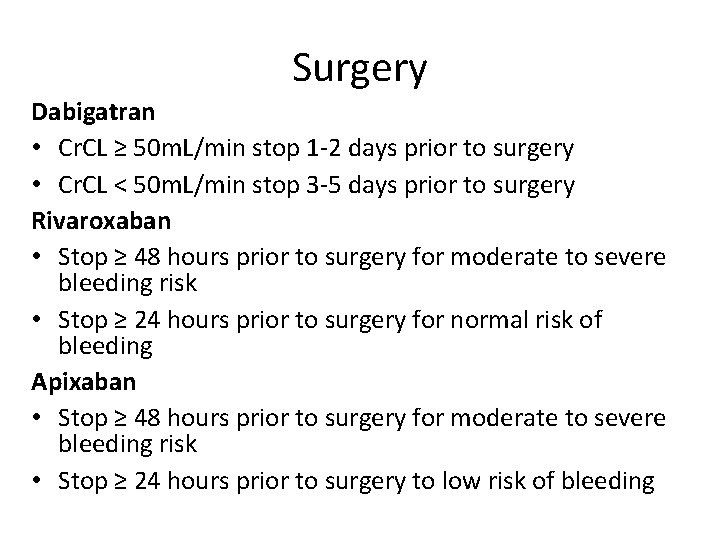
Surgery Dabigatran • Cr. CL ≥ 50 m. L/min stop 1 -2 days prior to surgery • Cr. CL < 50 m. L/min stop 3 -5 days prior to surgery Rivaroxaban • Stop ≥ 48 hours prior to surgery for moderate to severe bleeding risk • Stop ≥ 24 hours prior to surgery for normal risk of bleeding Apixaban • Stop ≥ 48 hours prior to surgery for moderate to severe bleeding risk • Stop ≥ 24 hours prior to surgery to low risk of bleeding

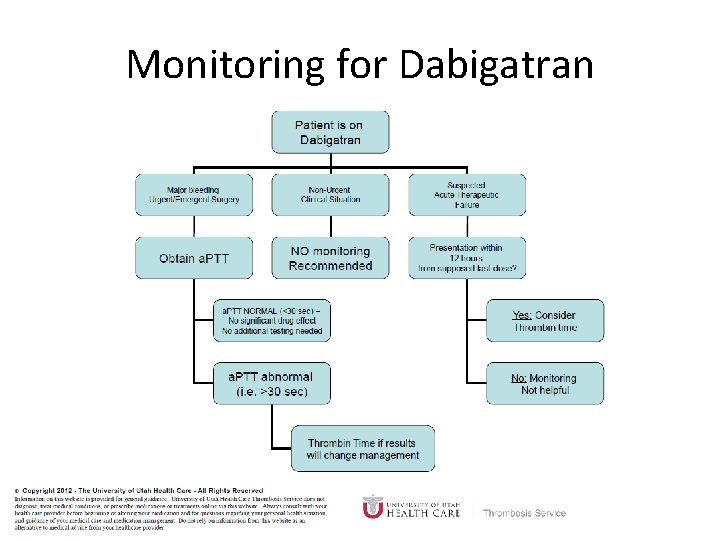
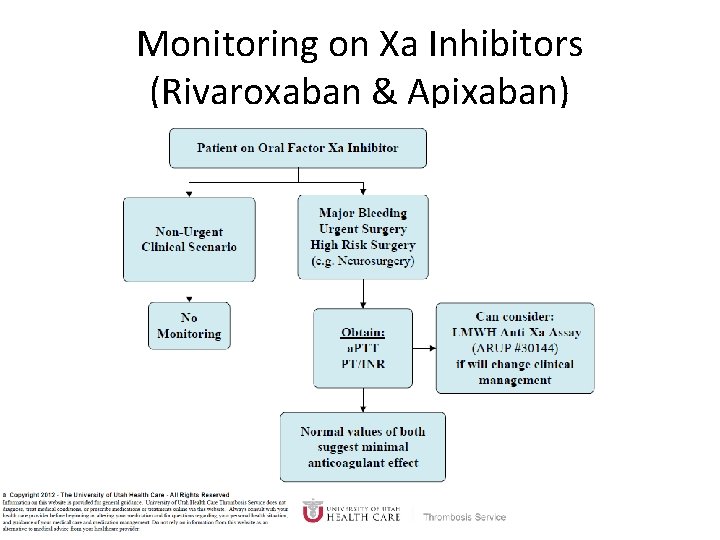
Monitoring Dabigatran • Useful in establishing if drug in present or not – Normal a. PTT: barely any dabigatran present – TT (Thrombin Time): linear dose-response curve for dabigatran, NOT after steady-state. Rivaroxaban and Apixaban? ? • a. PTT • PT/INR • Anti-Xa Level

Monitoring for Dabigatran

Monitoring on Xa Inhibitors (Rivaroxaban & Apixaban)

Why Not Just Use Anti-Xa Assays to Monitor Xa Inhibitors



Dosing Adjustments

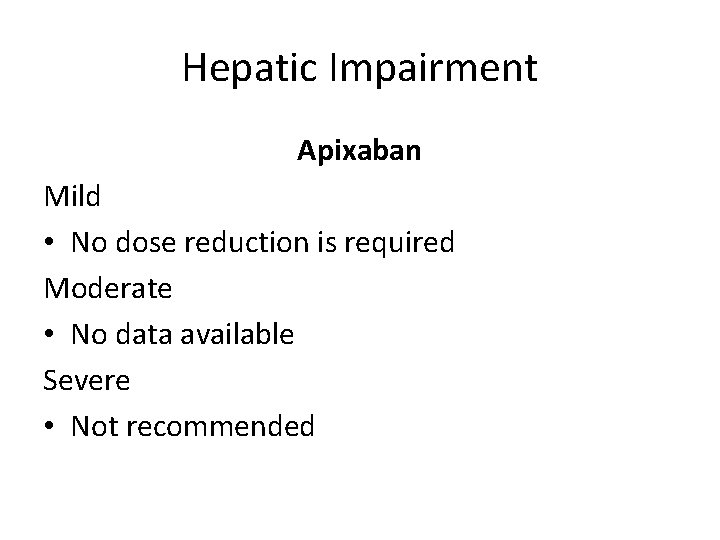
Hepatic Impairment Apixaban Mild • No dose reduction is required Moderate • No data available Severe • Not recommended

Hepatic Impairment Rivaroxban • Don’t use – Moderate and severe impairment – Liver disease that involves bleeding disorders

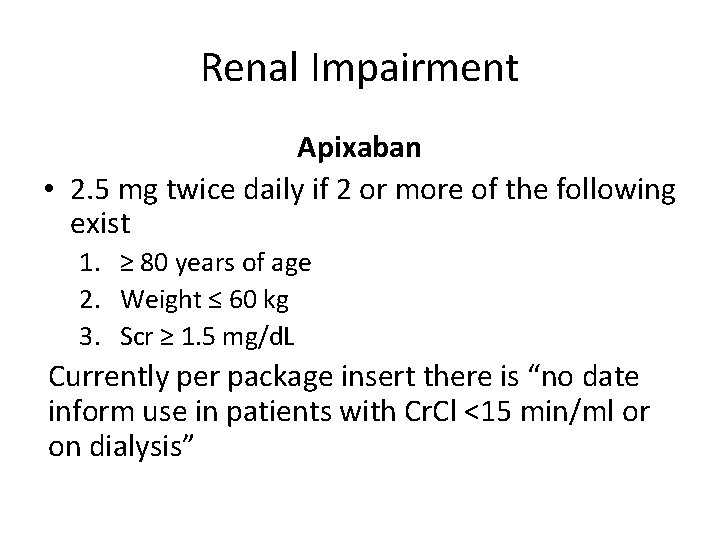
Renal Impairment Apixaban • 2. 5 mg twice daily if 2 or more of the following exist 1. ≥ 80 years of age 2. Weight ≤ 60 kg 3. Scr ≥ 1. 5 mg/d. L Currently per package insert there is “no date inform use in patients with Cr. Cl <15 min/ml or on dialysis”

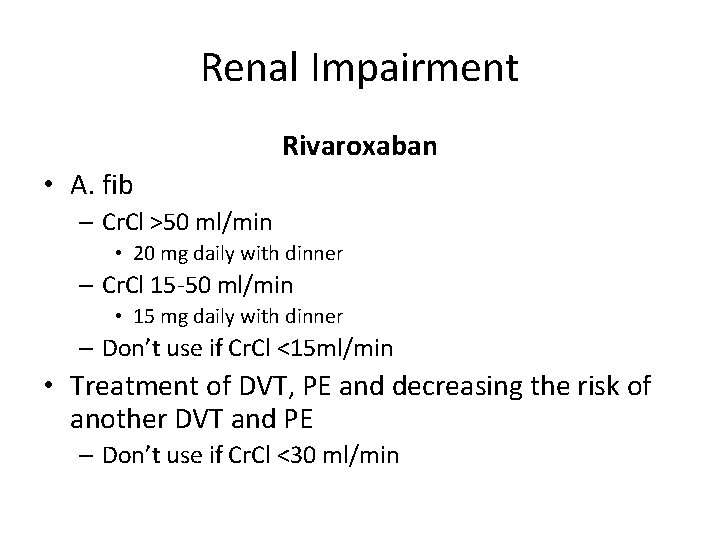
Renal Impairment Rivaroxaban • A. fib – Cr. Cl >50 ml/min • 20 mg daily with dinner – Cr. Cl 15 -50 ml/min • 15 mg daily with dinner – Don’t use if Cr. Cl <15 ml/min • Treatment of DVT, PE and decreasing the risk of another DVT and PE – Don’t use if Cr. Cl <30 ml/min

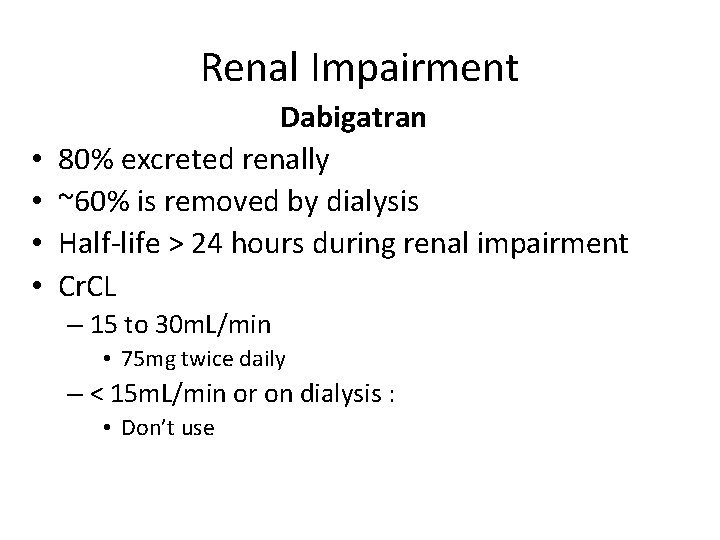
Renal Impairment • • Dabigatran 80% excreted renally ~60% is removed by dialysis Half-life > 24 hours during renal impairment Cr. CL – 15 to 30 m. L/min • 75 mg twice daily – < 15 m. L/min or on dialysis : • Don’t use

Institute for Safe Medication Practices (ISMP) ISMP • Federally certified, nonprofit organization dedicated to patient safety by – Education on safe medication use – Compile and examine medication errors, side effects, and near misses. – Distribute up to date medication safety news, provide ways of preventing errors, and tools to decrease risks

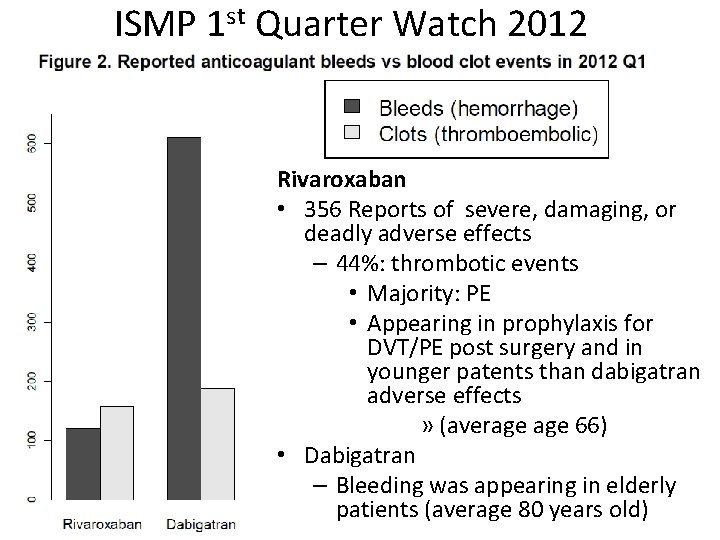
ISMP 1 st Quarter Watch 2012 Rivaroxaban • 356 Reports of severe, damaging, or deadly adverse effects – 44%: thrombotic events • Majority: PE • Appearing in prophylaxis for DVT/PE post surgery and in younger patents than dabigatran adverse effects » (average 66) • Dabigatran – Bleeding was appearing in elderly patients (average 80 years old)

ISMP 2012 2 nd Quarter Watch Dabigatran – The odds of recorded death from dabigatran were about five fold greater than warfarin. Rivaroxaban – The odds of recorded death from rivaroxaban were about less than two times greater than warfarin. – 10 mg once daily vs. 20 mg once daily • 10 mg once daily – 7 fold greater odds of a recoded emoblicthrombotic occurrence.

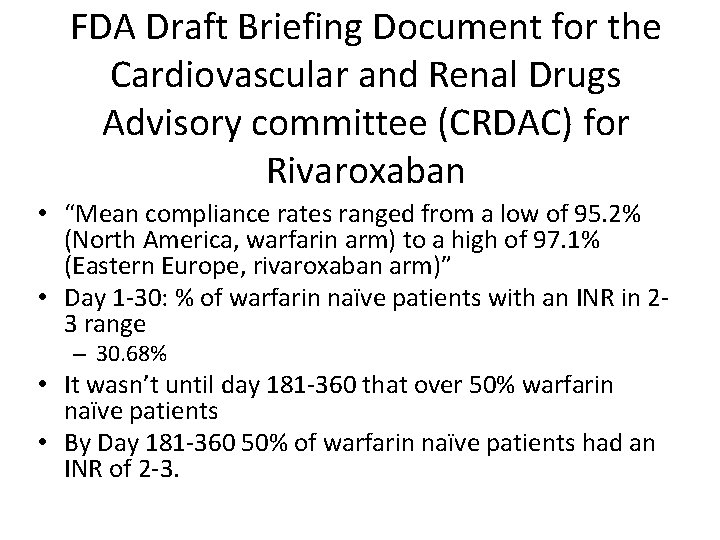
FDA Draft Briefing Document for the Cardiovascular and Renal Drugs Advisory committee (CRDAC) for Rivaroxaban • “Mean compliance rates ranged from a low of 95. 2% (North America, warfarin arm) to a high of 97. 1% (Eastern Europe, rivaroxaban arm)” • Day 1 -30: % of warfarin naïve patients with an INR in 23 range – 30. 68% • It wasn’t until day 181 -360 that over 50% warfarin naïve patients • By Day 181 -360 50% of warfarin naïve patients had an INR of 2 -3.

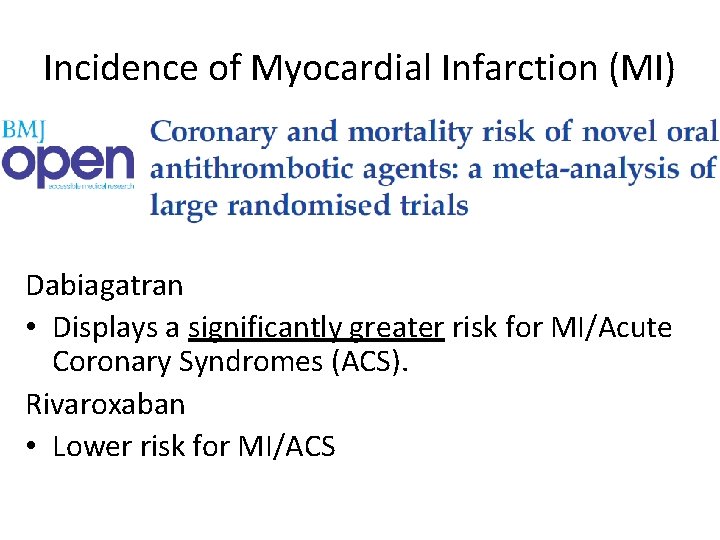
Incidence of Myocardial Infarction (MI) Dabiagatran • Displays a significantly greater risk for MI/Acute Coronary Syndromes (ACS). Rivaroxaban • Lower risk for MI/ACS

When to discontinue Apixaban Rivaroxaban Dabigatran

Cardioversion • Dabigatran has been shown to be an acceptable alternative to warfarin • No studies have evaluated rivaroxaban or apixaban

Maybe a slide • wha. T PERCENTAGE OF NEW RX FOR A FIB ARE NON WARFARIN DRUGS

Summary
 Noac yokosuka
Noac yokosuka Kcentra
Kcentra Jean marie connors
Jean marie connors Pauta harmonització anticoagulants
Pauta harmonització anticoagulants Types of anticoagulants
Types of anticoagulants Les anticoagulants ifsi
Les anticoagulants ifsi Embolie pulmonaire ifsi
Embolie pulmonaire ifsi Intrinsic pathway
Intrinsic pathway What is the transport system of the body
What is the transport system of the body Library.med.utah.edu/kw/pharm/hyper heart.html
Library.med.utah.edu/kw/pharm/hyper heart.html Outfield pharm
Outfield pharm Letazol
Letazol Pharm406
Pharm406 Bc bio-pharm
Bc bio-pharm Pharm link
Pharm link Pharm gkb
Pharm gkb Secur pharm
Secur pharm Noel pharm
Noel pharm Pharmaceutical science atar
Pharmaceutical science atar Pharm
Pharm Klucel exf
Klucel exf Friciton
Friciton Pharm id
Pharm id Ambio pharm
Ambio pharm Define epistolary novel
Define epistolary novel Features of the victorian novel
Features of the victorian novel Heart of darkness as a modern novel
Heart of darkness as a modern novel Internal conflict in to kill a mockingbird
Internal conflict in to kill a mockingbird The victorian novel
The victorian novel Techniques in victorian poetry
Techniques in victorian poetry Sentimental novel definition
Sentimental novel definition Summary the reading public
Summary the reading public The great gatsby party chart answers
The great gatsby party chart answers Gothic literature frankenstein
Gothic literature frankenstein Bildungsroman novel
Bildungsroman novel Proposal penelitian sastra novel
Proposal penelitian sastra novel Objectives of the study
Objectives of the study Terry eagleton what is a novel
Terry eagleton what is a novel Persepolis graphic novel
Persepolis graphic novel Graphic novel terms
Graphic novel terms Novel sunkris
Novel sunkris Nilai agama laut bercerita
Nilai agama laut bercerita Father of indian english novel
Father of indian english novel Cinderella plot diagram
Cinderella plot diagram Graphic weight graphic novel definition
Graphic weight graphic novel definition Hatchet cover book
Hatchet cover book What is a splash page in a graphic novel
What is a splash page in a graphic novel Graphic novel elements
Graphic novel elements Bleed comic definition
Bleed comic definition Definition gothic novel
Definition gothic novel What is the definition of gothic literature
What is the definition of gothic literature Mary shelley literary influences
Mary shelley literary influences Intrinsic and extrinsic elements of novel
Intrinsic and extrinsic elements of novel Elements of gothic literature
Elements of gothic literature Short story definition
Short story definition Element of novel
Element of novel Dystopian novels meaning
Dystopian novels meaning Newer drug delivery system
Newer drug delivery system In the novel a tale of two cities dgp
In the novel a tale of two cities dgp Fiction and drama venn diagram
Fiction and drama venn diagram How did the northerners react to the novel?
How did the northerners react to the novel? Zbirka novel boccaccia
Zbirka novel boccaccia Wb novel
Wb novel Warm up comic
Warm up comic Unsur ekstrinsik prosa
Unsur ekstrinsik prosa Their eyes were watching god anticipation guide
Their eyes were watching god anticipation guide Hatchet title
Hatchet title Augustan novel
Augustan novel Graphic novel definition
Graphic novel definition Apa itu sinopsis buku
Apa itu sinopsis buku Novel clinical drug trial design
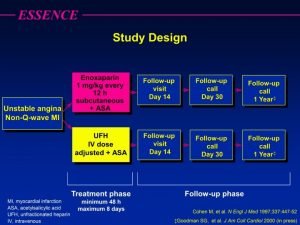
Novel clinical drug trial design Elements of a mystery
Elements of a mystery Kitchen novel banana yoshimoto
Kitchen novel banana yoshimoto Unsur intrinsik novel komet tere liye
Unsur intrinsik novel komet tere liye Kari graphic novel
Kari graphic novel Jane eyre victorian novel
Jane eyre victorian novel Flowers for algernon short story vs novel
Flowers for algernon short story vs novel Dystopia literature definition
Dystopia literature definition Daniel defoe the father of english novel
Daniel defoe the father of english novel Developing and acquiring information systems
Developing and acquiring information systems Define bildungsroman novel
Define bildungsroman novel Gothic literature definition
Gothic literature definition Apa yang dimaksud kalimat imbauan
Apa yang dimaksud kalimat imbauan