National Institute for Pharmaceutical Technology and Education NIPTE




- Slides: 15

National Institute for Pharmaceutical Technology and Education (NIPTE) Interim Risk Assessment Report

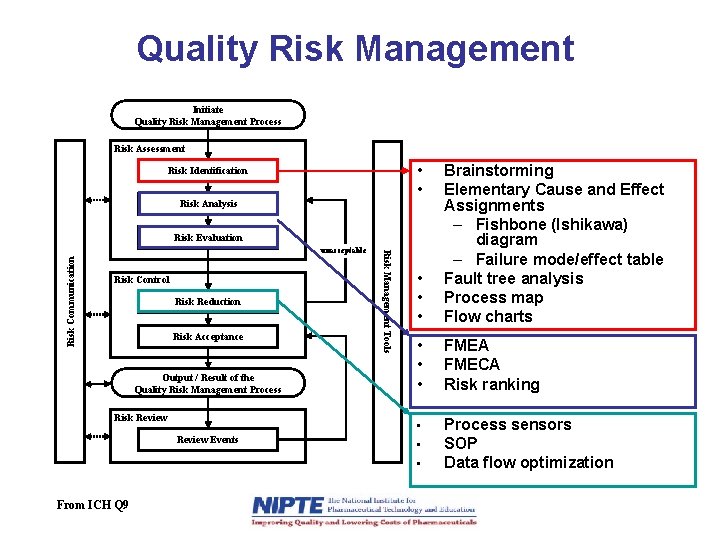
Quality Risk Management Initiate Quality Risk Management Process Risk Assessment • • Risk Identification Risk Analysis • • • Brainstorming Elementary Cause and Effect Assignments – Fishbone (Ishikawa) diagram – Failure mode/effect table Fault tree analysis Process map Flow charts • • • FMEA FMECA Risk ranking • Process sensors SOP Data flow optimization Risk Evaluation Risk Communication Risk Control Risk Reduction Risk Acceptance Output / Result of the Quality Risk Management Process Risk Review Events Risk Management Tools unacceptable • • From ICH Q 9

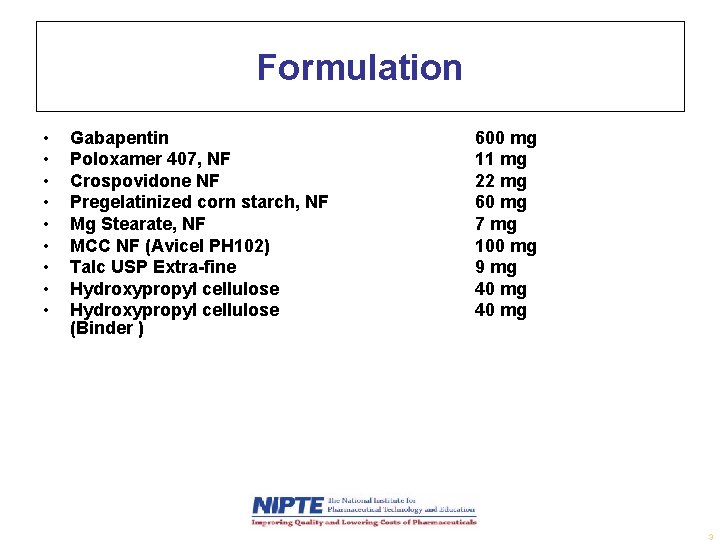
Formulation • • • Gabapentin Poloxamer 407, NF Crospovidone NF Pregelatinized corn starch, NF Mg Stearate, NF MCC NF (Avicel PH 102) Talc USP Extra-fine Hydroxypropyl cellulose (Binder ) 600 mg 11 mg 22 mg 60 mg 7 mg 100 mg 9 mg 40 mg 3

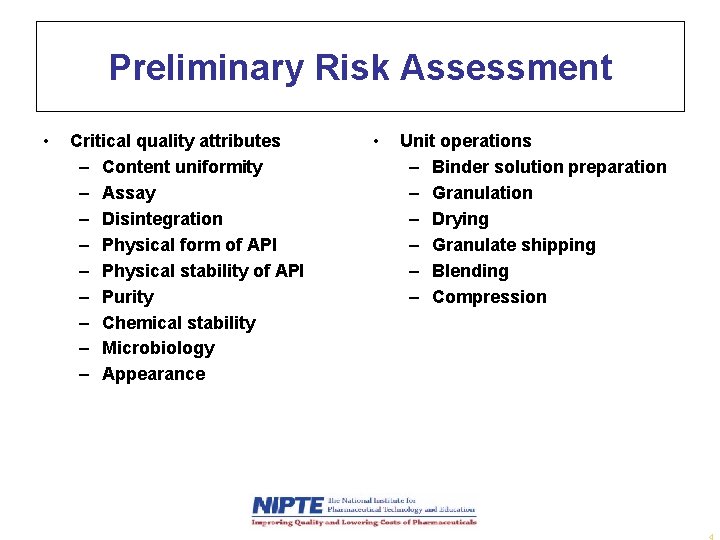
Preliminary Risk Assessment • Critical quality attributes – Content uniformity – Assay – Disintegration – Physical form of API – Physical stability of API – Purity – Chemical stability – Microbiology – Appearance • Unit operations – Binder solution preparation – Granulation – Drying – Granulate shipping – Blending – Compression 4

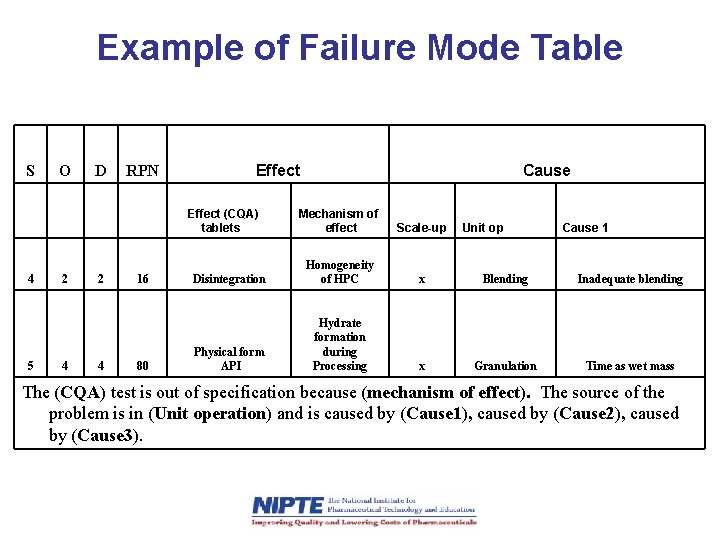
Example of Failure Mode Table S O D RPN Effect (CQA) tablets 4 5 2 4 16 80 Cause Mechanism of effect Scale-up Disintegration Homogeneity of HPC x Blending Inadequate blending Physical form API Hydrate formation during Processing x Granulation Time as wet mass Unit op Cause 1 The (CQA) test is out of specification because (mechanism of effect). The source of the problem is in (Unit operation) and is caused by (Cause 1), caused by (Cause 2), caused by (Cause 3).

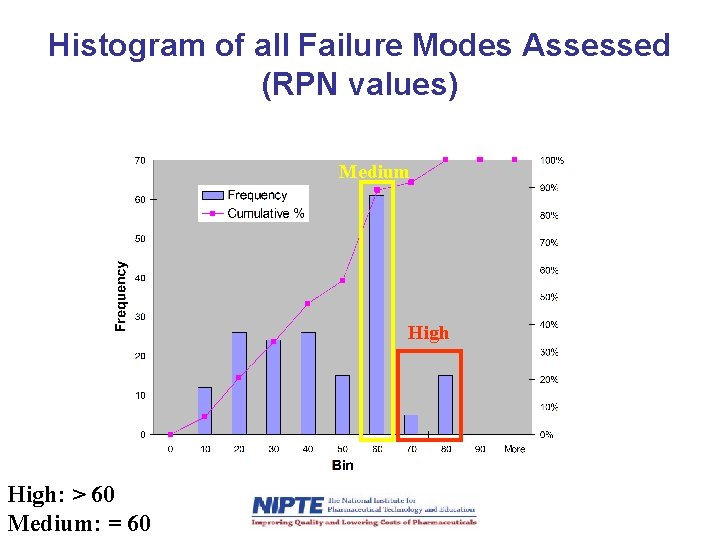
Histogram of all Failure Modes Assessed (RPN values) Medium High: > 60 Medium: = 60

Preliminary RPN Results: Risks sorted by Unit Operation • Binder solution preparation – Number of risks assessed: 11 – No high or medium risk failure modes • Granulation – Number of risks assessed: 75 – High risk failure modes: 13 /75 – Medium risk failure modes: 20 /75 • Fluid bed drying – Number of risks assessed: 30 – High risk failure modes: 3 /30 – Medium risk failure modes: 16 /30

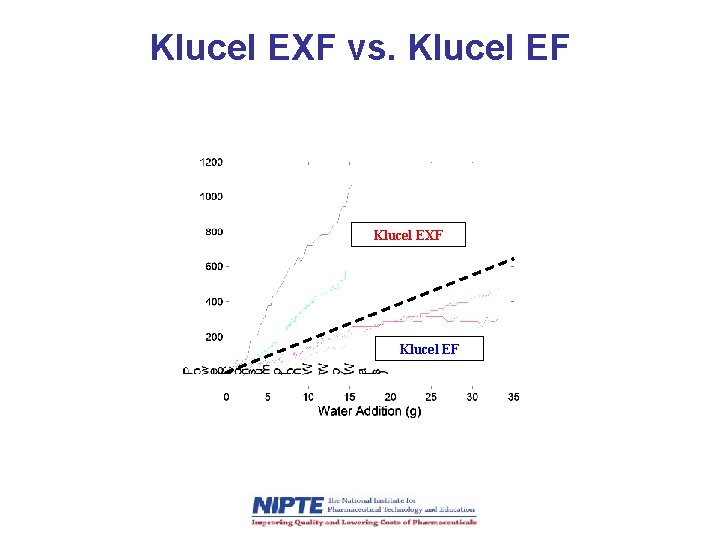
Qb. D Approach to Formulation Optimization • The original HPC grade (Klucel EXF, fine PSD) was sensitive to small changes in water content (lacking robustness) • A second HPC grade (Klucel EF, larger PSD) was more robust. • Similar PSDs were achieved using Klucel EF at 5. 5% water as with Klucel EXF at 2. 5%.

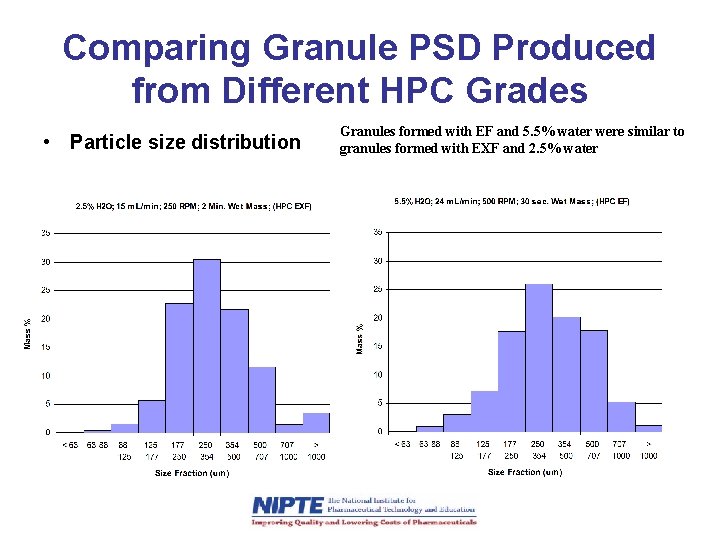
Comparing Granule PSD Produced from Different HPC Grades • Particle size distribution Granules formed with EF and 5. 5% water were similar to granules formed with EXF and 2. 5% water

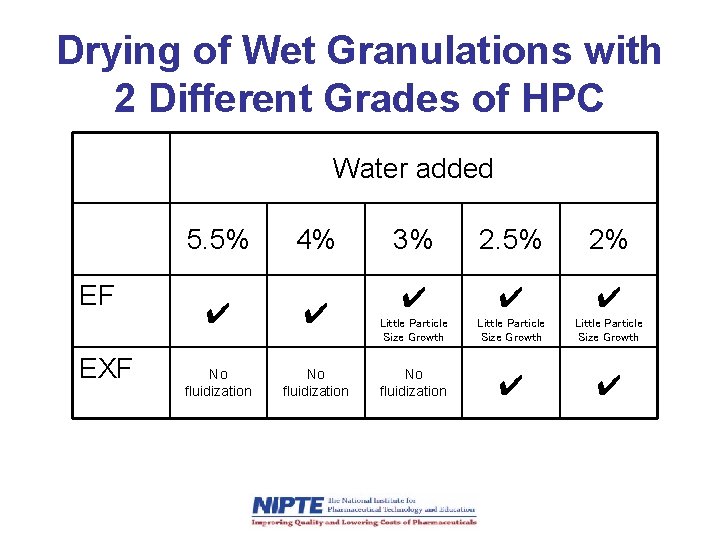
Drying of Wet Granulations with 2 Different Grades of HPC Water added 5. 5% EF EXF 4% ✔ ✔ No fluidization 3% 2. 5% 2% ✔ ✔ ✔ Little Particle Size Growth No fluidization ✔ ✔

Klucel EXF vs. Klucel EF Klucel EXF Klucel EF

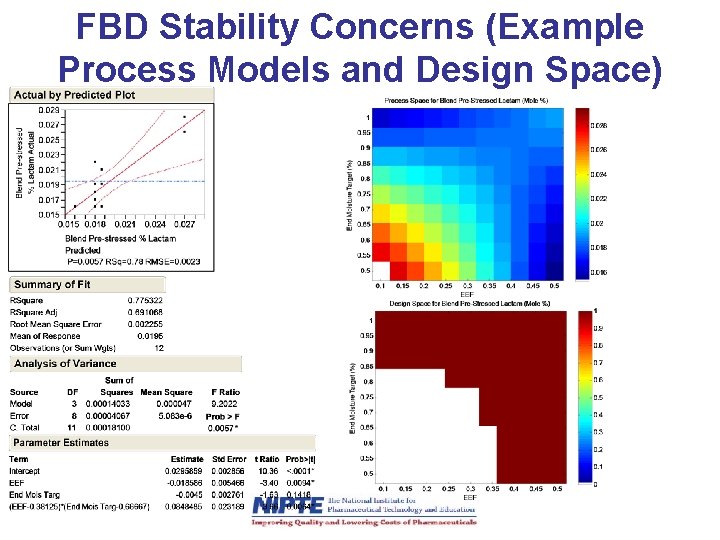
FBD Stability Concerns (Example Process Models and Design Space)

Summary of Drying Stability Specs • Residual moisture stabilizes granules and blends from lactam formation – Some residual moisture is good to decrease lactam formation, but too much residual moisture has flow and hardness consequences, which could result in content uniformity and disintegration problems. • High temperature drying results in higher lactam formation. – There was no improvement in efficiency by drying at higher temperatures due to increased cooling times, so low temperature drying is optimum.

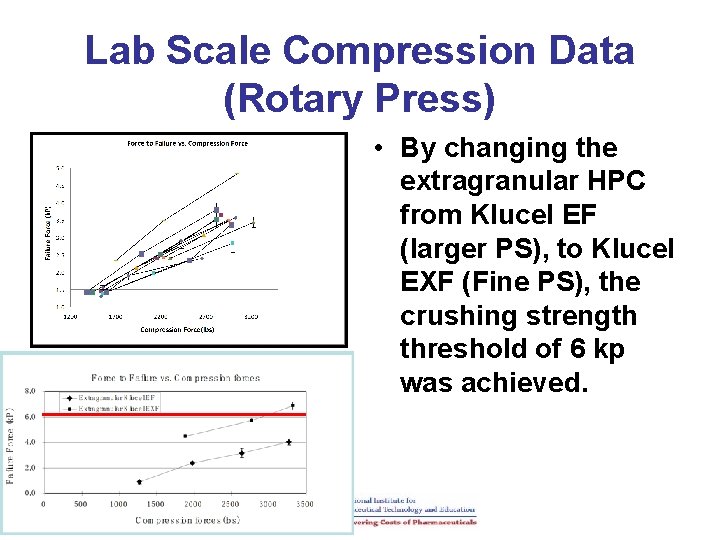
Lab Scale Compression Data (Rotary Press) • By changing the extragranular HPC from Klucel EF (larger PS), to Klucel EXF (Fine PS), the crushing strength threshold of 6 kp was achieved.

Additional Assessment Efforts • Evaluate all stability data • Update design space models at each scale – Develop design space model across scales • Identify and document – Risks to product quality – Advantages of current methods for reducing risk • Modeling • Process monitoring/control
 Ulsan national institute of science and technology (unist)
Ulsan national institute of science and technology (unist) National institute of standards and technology
National institute of standards and technology Assistive technology implementation plan
Assistive technology implementation plan National institute of agricultural technology
National institute of agricultural technology Biopharmaceutical technology transfer
Biopharmaceutical technology transfer American council on pharmaceutical education
American council on pharmaceutical education Vidhyadeep institute of engineering and technology
Vidhyadeep institute of engineering and technology Masdar institute of science and technology
Masdar institute of science and technology Madhav institute of technology and science
Madhav institute of technology and science Vocational education & training in uae
Vocational education & training in uae Sagar institute of research and technology
Sagar institute of research and technology Schorarly
Schorarly Download r for windows
Download r for windows Indian institute of space science and technology
Indian institute of space science and technology Shri dadaji institute of technology and science
Shri dadaji institute of technology and science Prof ram meghe institute of technology and research
Prof ram meghe institute of technology and research





























