Non functioning pituitary adenomas nonfunctioning pituitary neuroendocrine tumores











- Slides: 71

Non functioning pituitary adenomas, non-functioning pituitary neuroendocrine tumores (NF-Pit. NETS) Clinical cases Akbar Soltani. MD. MSc. Endocrinologist Evidence Based Medicine Research Center Critical Thinking Group Tehran University of Medical Sciences www. ebm. ir

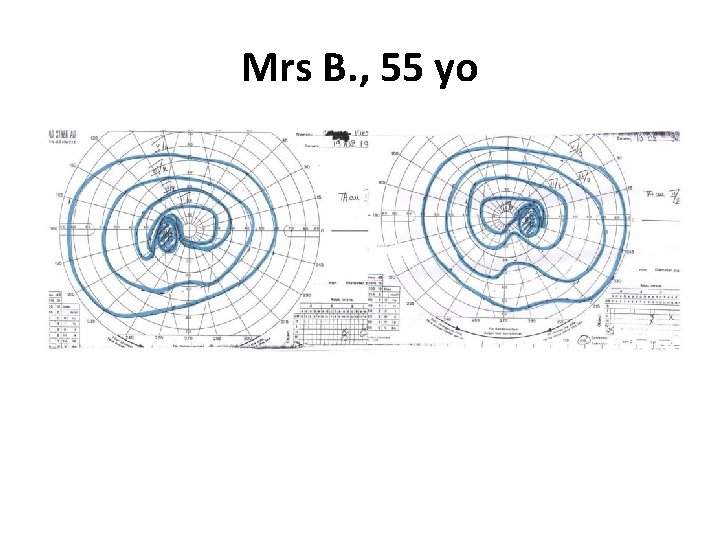
1 st case: Mrs B. , 55 yo • Previous history –Mammary benign cyst –Bilateral ovariectomy when 48 yo, HRT for menopause –Hysterectomy when 49 yo (endometriosis) • Recent history: –Consults an endocrinologist for weight gain, –He measures PRL level which is increased to 120 ng/ml (N<20) • Normal clinical examination

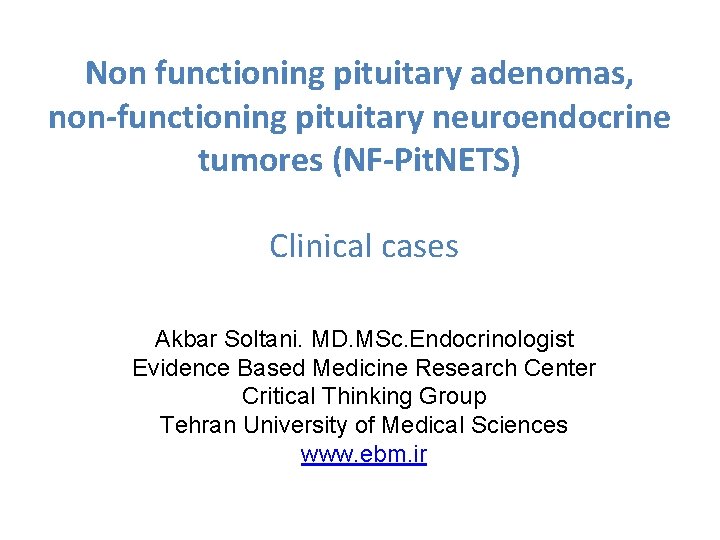
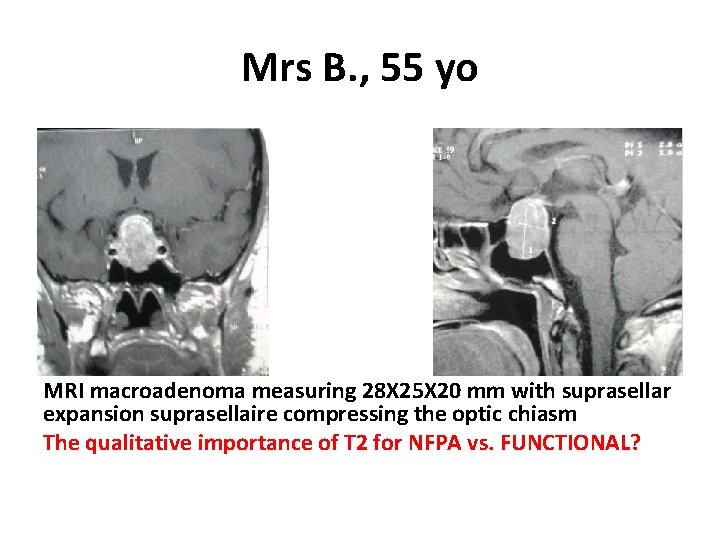
Mrs B. , 55 yo MRI macroadenoma measuring 28 X 25 X 20 mm with suprasellar expansion suprasellaire compressing the optic chiasm The qualitative importance of T 2 for NFPA vs. FUNCTIONAL?

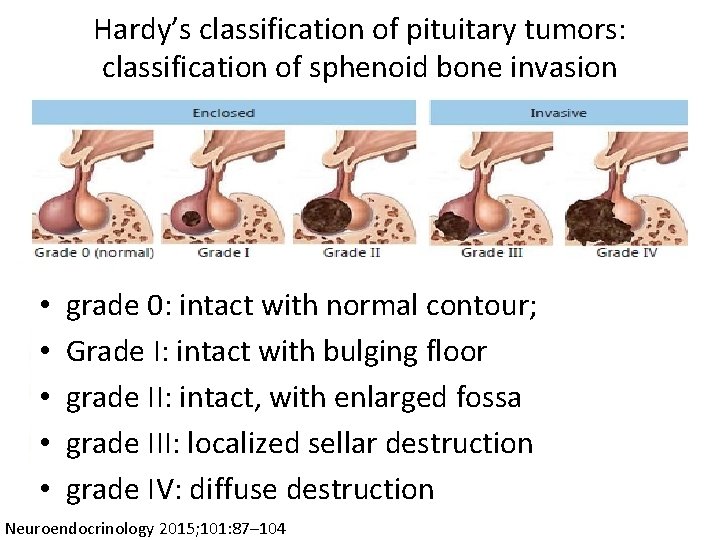
Hardy’s classification of pituitary tumors: classification of sphenoid bone invasion • • • grade 0: intact with normal contour; Grade I: intact with bulging floor grade II: intact, with enlarged fossa grade III: localized sellar destruction grade IV: diffuse destruction Neuroendocrinology 2015; 101: 87– 104

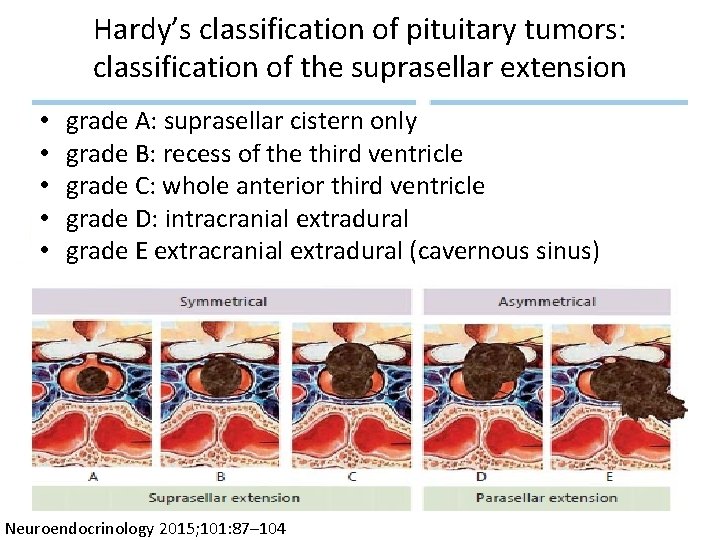
Hardy’s classification of pituitary tumors: classification of the suprasellar extension • • • grade A: suprasellar cistern only grade B: recess of the third ventricle grade C: whole anterior third ventricle grade D: intracranial extradural grade E extracranial extradural (cavernous sinus) Neuroendocrinology 2015; 101: 87– 104

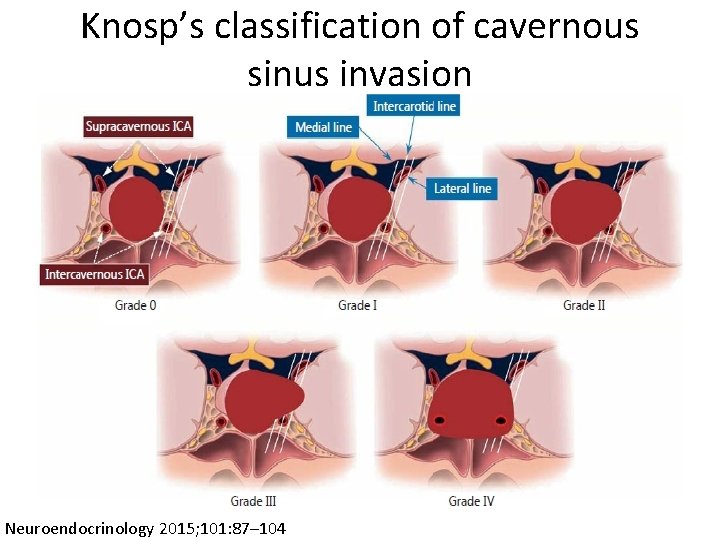
Knosp’s classification of cavernous sinus invasion Neuroendocrinology 2015; 101: 87– 104

Mrs B. , 55 yo

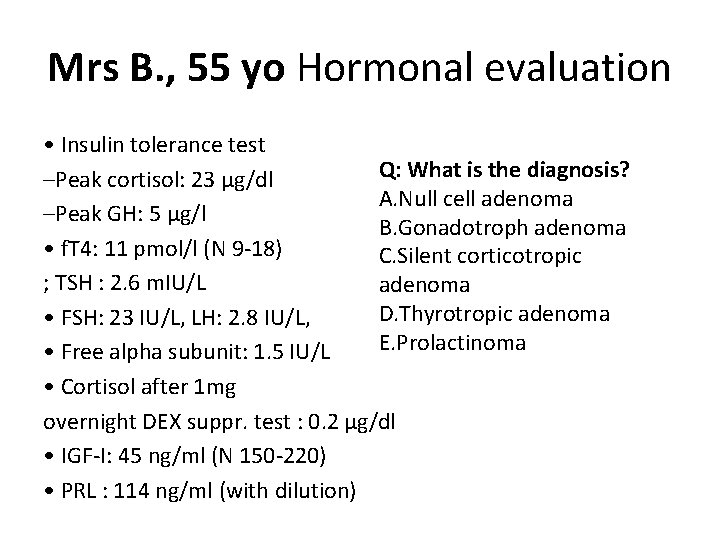
Q 1: Which hormonal work up do you plan for this patient? • • • 1. Basal FSH, LH, alpha-subunit 2. TRH-test 3. ACTH-stimulation test 4. Gn. RH-test 5. Basal f. T 4, f. T 3, TSH

Mrs B. , 55 yo Hormonal evaluation • Insulin tolerance test Q: What is the diagnosis? –Peak cortisol: 23 μg/dl A. Null cell adenoma –Peak GH: 5 μg/l B. Gonadotroph adenoma • f. T 4: 11 pmol/l (N 9 -18) C. Silent corticotropic ; TSH : 2. 6 m. IU/L adenoma D. Thyrotropic adenoma • FSH: 23 IU/L, LH: 2. 8 IU/L, E. Prolactinoma • Free alpha subunit: 1. 5 IU/L • Cortisol after 1 mg overnight DEX suppr. test : 0. 2 μg/dl • IGF-I: 45 ng/ml (N 150 -220) • PRL : 114 ng/ml (with dilution)

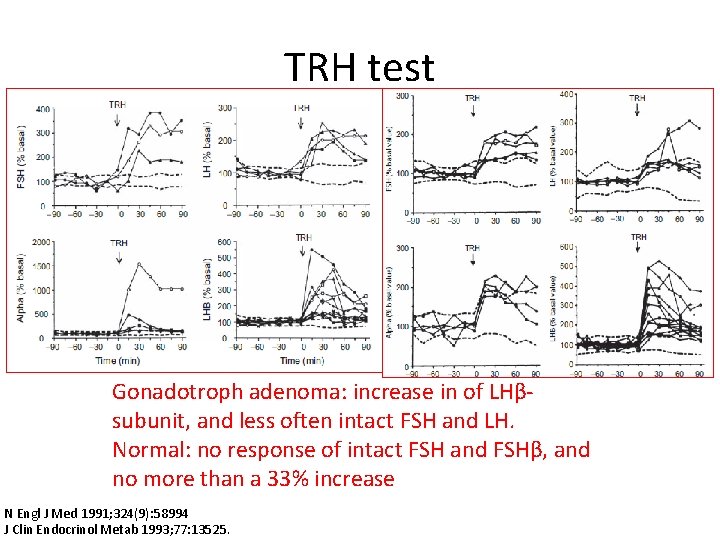
TRH test Gonadotroph adenoma: increase in of LHβsubunit, and less often intact FSH and LH. Normal: no response of intact FSH and FSHβ, and no more than a 33% increase N Engl J Med 1991; 324(9): 58994 J Clin Endocrinol Metab 1993; 77: 13525.

Mrs B. , 55 yo Diagnosis • Pituitary macroadenoma • Moderate hyperprolactinemia suggestive of stalk interruption • No thyrotropic deficiency, no corticotropic deficiency • GH deficiency • Increased FSH levels…but low LH levels in a postmenopausal woman • (No evidence for a « silent » corticotropic adenoma) Likely gonadotropic adenoma (secreting FSH and free alpha-subunit)

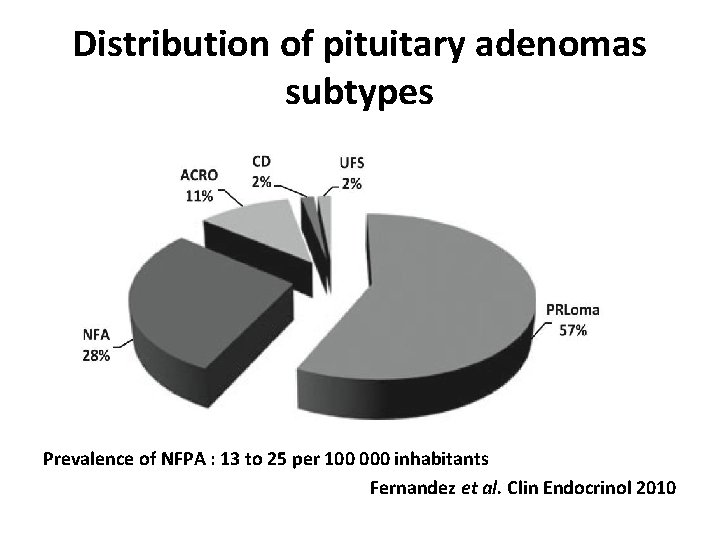
Distribution of pituitary adenomas subtypes Prevalence of NFPA : 13 to 25 per 100 000 inhabitants Fernandez et al. Clin Endocrinol 2010

Mrs B. , 55 yo Treatment • Transsphenoidal removal of the macroadenoma (considered as complete by the neurosurgeon) • Histology : adenoma with few cytological signs of secretion • Immunocytochemistry : –Positivity for anti alpha. SU: <5% of cells –Positivity for anti ß-LH: <5% of cells –Positivity for anti ß-FSH: 50 à 75% of cells Gonadotropic adenoma

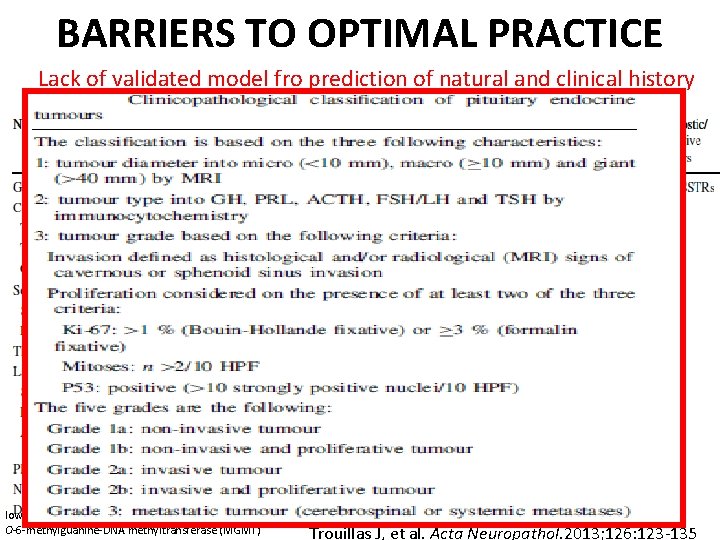
BARRIERS TO OPTIMAL PRACTICE Lack of validated model fro prediction of natural and clinical history low molecular weight cytokeratin (LMWCK) [ O-6 -methylguanine-DNA methyltransferase (MGMT) Trouillas J, et al. Acta Neuropathol. 2013; 126: 123 -135

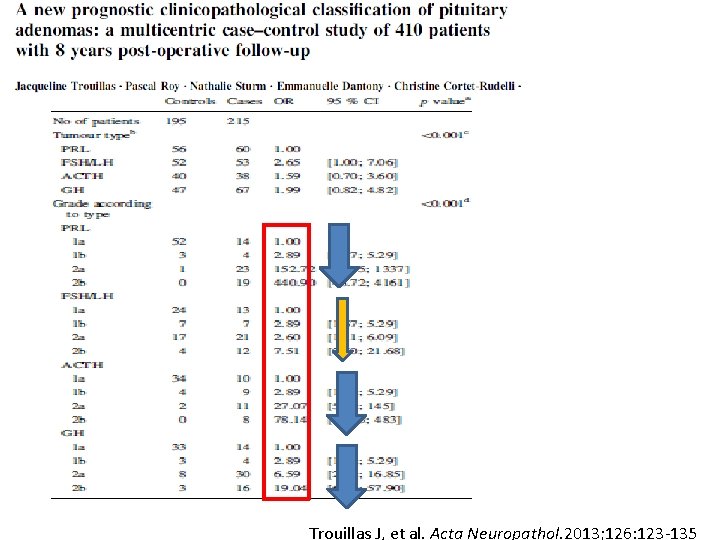
Trouillas J, et al. Acta Neuropathol. 2013; 126: 123 -135

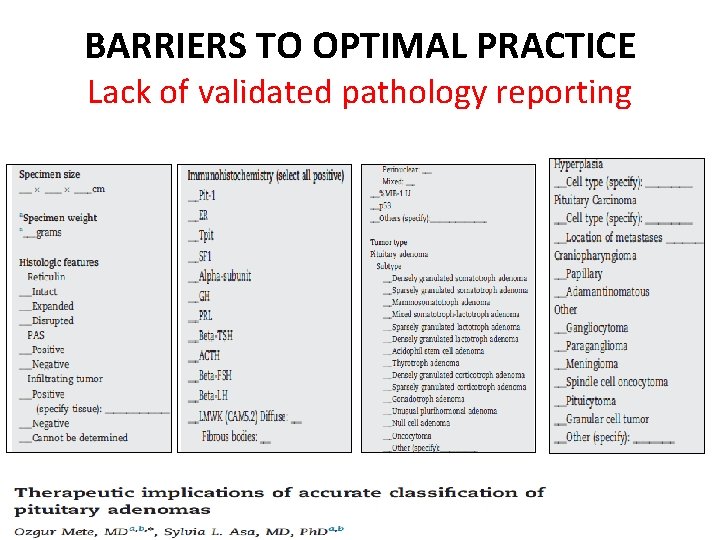
BARRIERS TO OPTIMAL PRACTICE Lack of validated pathology reporting

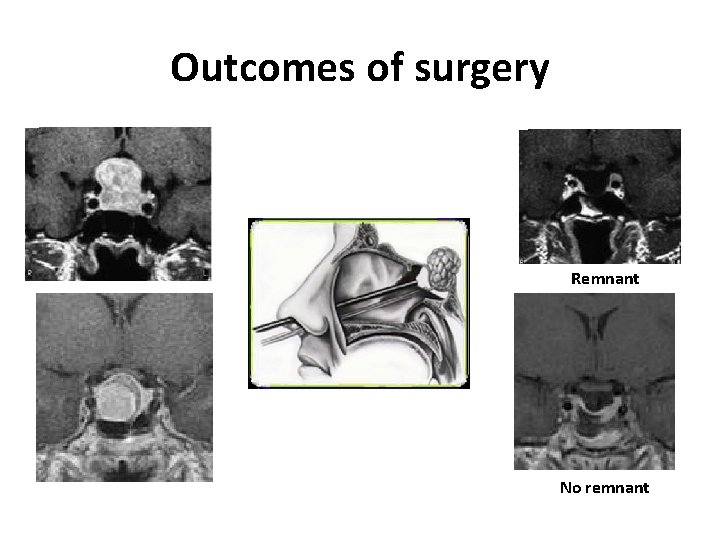
Outcomes of surgery Remnant No remnant


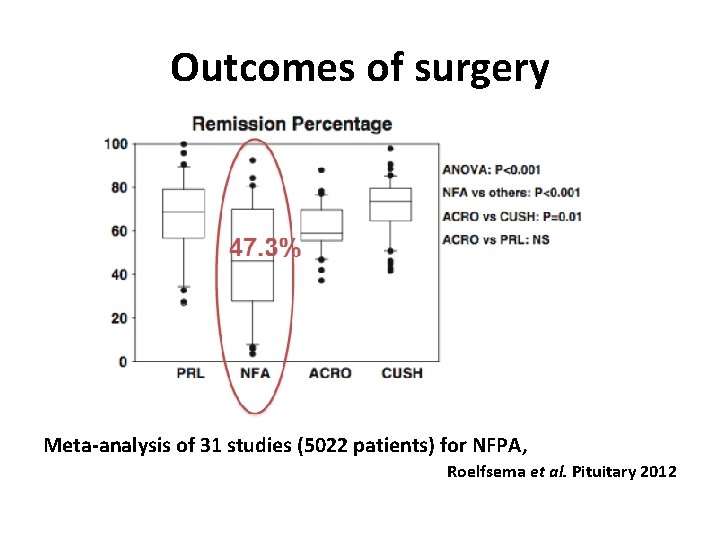
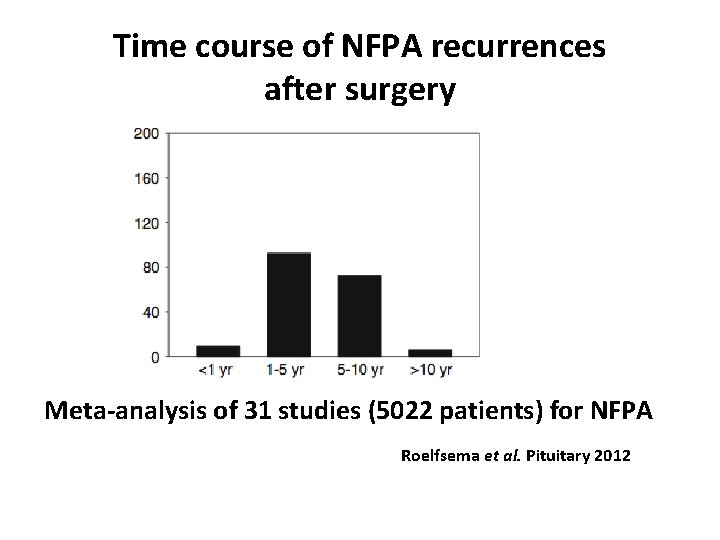
Outcomes of surgery Meta-analysis of 31 studies (5022 patients) for NFPA, Roelfsema et al. Pituitary 2012

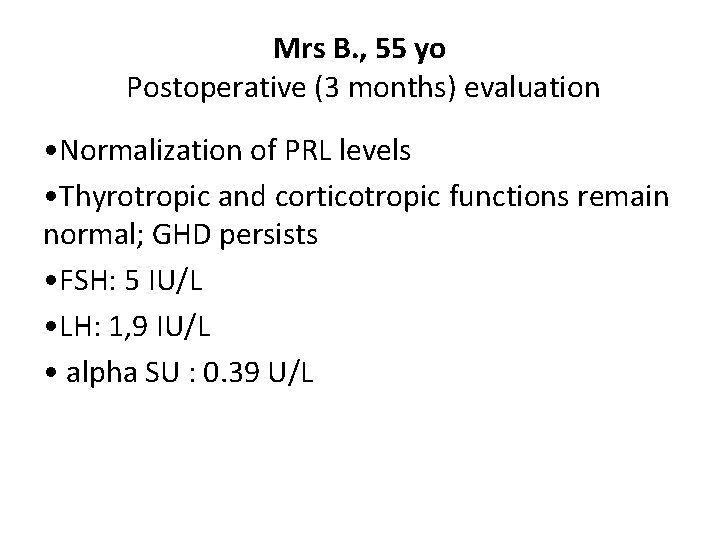
Mrs B. , 55 yo Postoperative (3 months) evaluation • Normalization of PRL levels • Thyrotropic and corticotropic functions remain normal; GHD persists • FSH: 5 IU/L • LH: 1, 9 IU/L • alpha SU : 0. 39 U/L

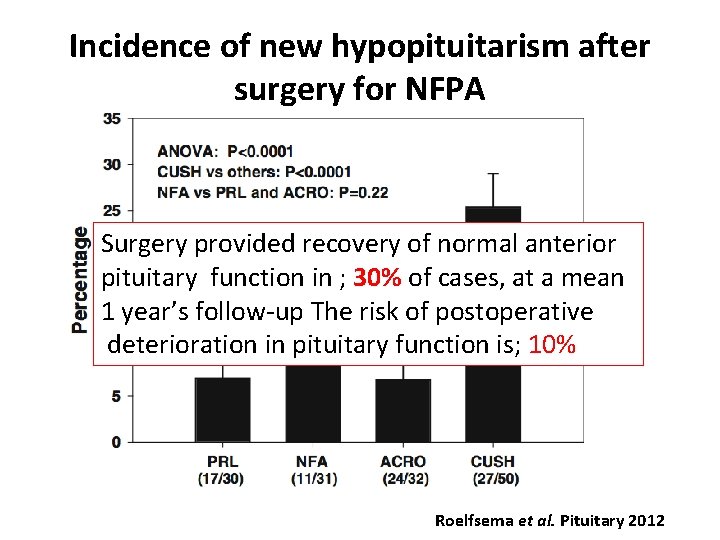
Incidence of new hypopituitarism after surgery for NFPA Surgery provided recovery of normal anterior pituitary function in ; 30% of cases, at a mean 1 year’s follow-up The risk of postoperative deterioration in pituitary function is; 10% Roelfsema et al. Pituitary 2012

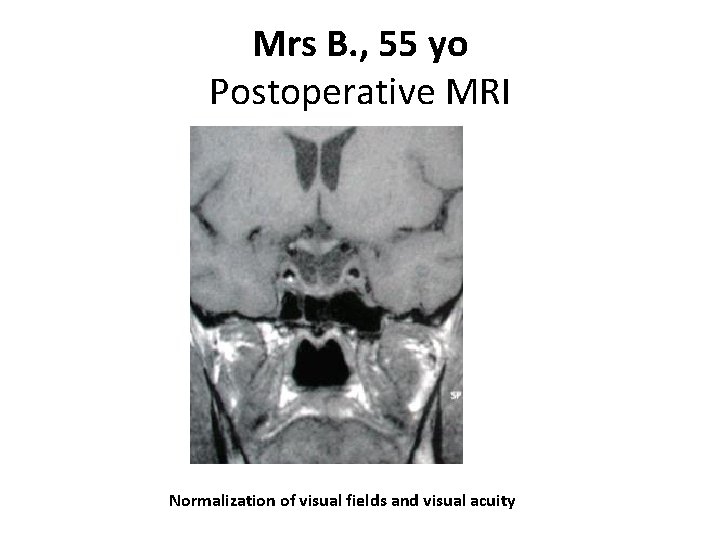
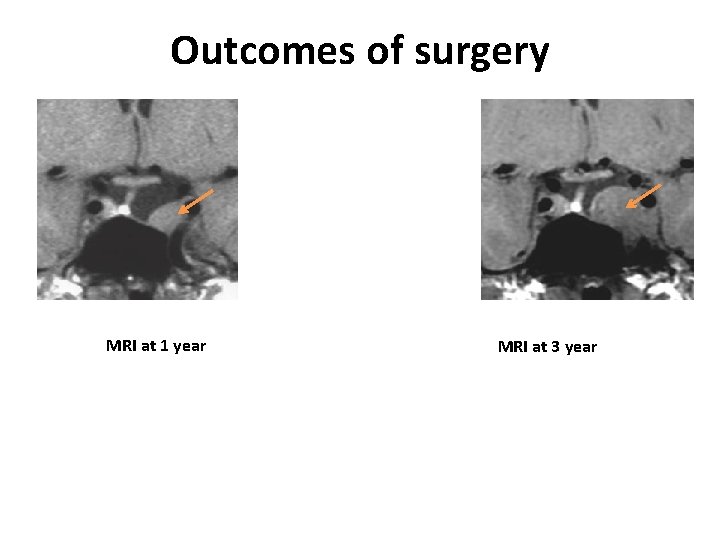
Mrs B. , 55 yo Postoperative MRI Normalization of visual fields and visual acuity

Q: At this point, what is your attitude in this patient without remnant? 1. Surveillance (watch and see) 2. Systematic adjuvant fractionated radiotherapy 3. Systematic adjuvant gamma-knife

Mrs B. , 55 yo Decision of simple surveillance Long term follow up : no recurrence

2 nd case: Mr T. , 30 yo • Recent history –Headaches, sight • Ophtalmologic eval. : –AV LE 4/10; RE 10/10 –VF: BT hemianopsia • MRI

Mr T. , 30 yo • Transsphenoidal surgery : incomplete removal of the pituitary adenoma • Immunocytochemistry: FSHß +, Ki 67+ (2%), p 53 • Oph. evaluation after 1 mth: –AV 10/10 (both eyes) –Improvement in VF • MRI at 6 months postop. Decision of follow up…

BARRIERS TO OPTIMAL PRACTICE Nonavailability of genetic testing • Up to 5% of PA are familial adenomas • Familial Isolated Pituitary Adenomas (FIPA): – Germline mutations in aryl hydrocarbon receptorinteracting protein (AIP) gene in 20% of FIPA (nonfunctioning tumor is rare) • MEN -1 and MEN-4 • Screening for both AIP and MEN-1 mutations needs to be considered in patients diagnosed with pituitary adenoma under the age of 21 years • Of clinical importance is that adenomas in patients with MEN 1 or AIP mutations are big, invasive, and may not respond well to standard medical therapy. Vierimaa O, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science (New York, NY). 2006; 312(5777): 1228 -1230.

Outcomes of surgery MRI at 1 year MRI at 3 year

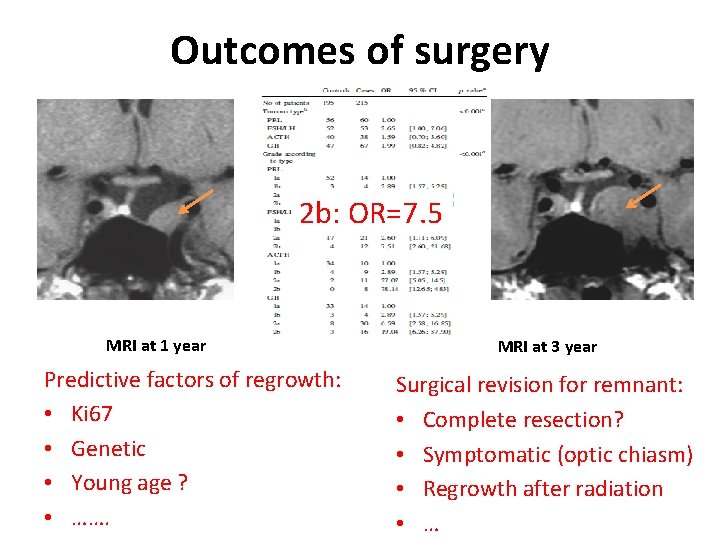
Q: At this point, what is your attitude in this patient with a remnant which increases ? 1. Pursue visual and MRI surveillancce 2. Fractionated radiotherapy 3. Repeat surgery and then surveillance (avoid radiotherapy) 4. Repeat surgery for decreasing the volume and propose gamma-knife 5. Treatment with cabergoline

Outcomes of surgery 2 b: OR=7. 5 MRI at 1 year Predictive factors of regrowth: • Ki 67 • Genetic • Young age ? • ……. MRI at 3 year Surgical revision for remnant: • Complete resection? • Symptomatic (optic chiasm) • Regrowth after radiation • …

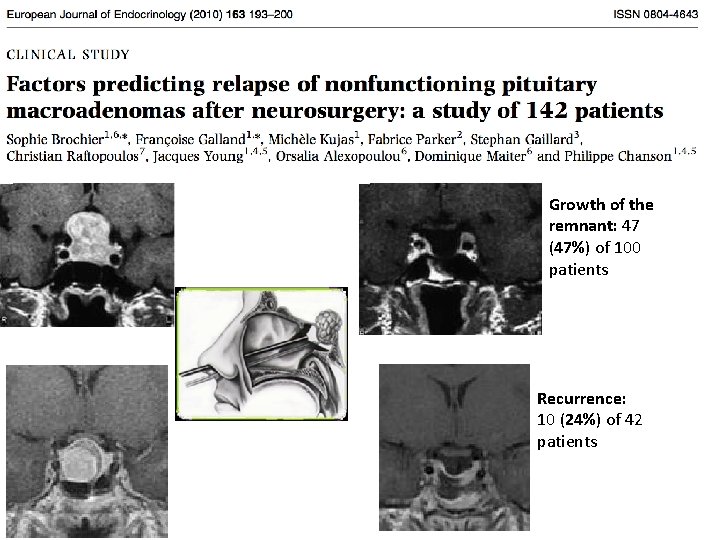
Growth of the remnant: 47 (47%) of 100 patients Recurrence: 10 (24%) of 42 patients

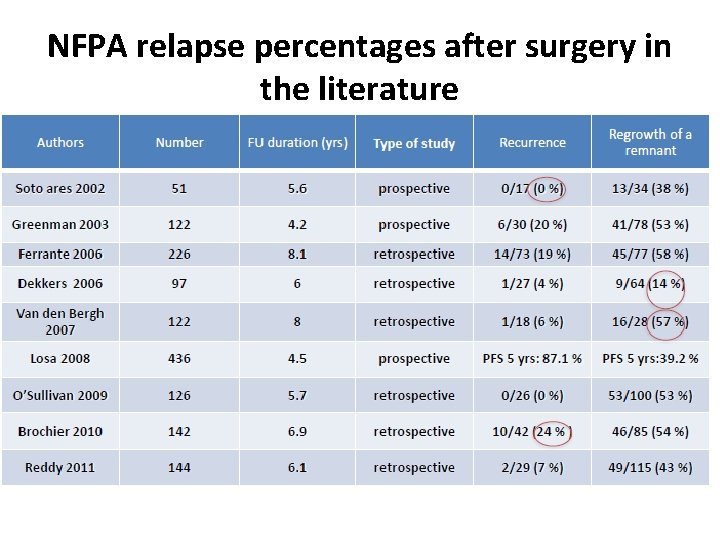
NFPA relapse percentages after surgery in the literature

Time course of NFPA recurrences after surgery Meta-analysis of 31 studies (5022 patients) for NFPA Roelfsema et al. Pituitary 2012

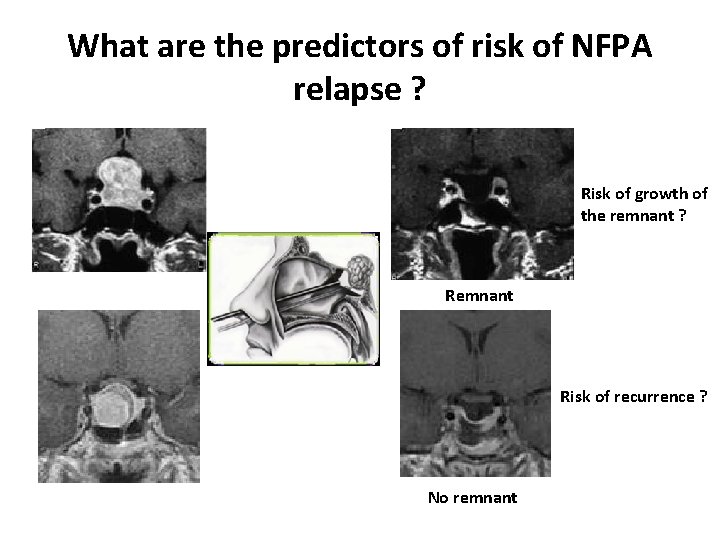
What are the predictors of risk of NFPA relapse ? Risk of growth of the remnant ? Remnant Risk of recurrence ? No remnant


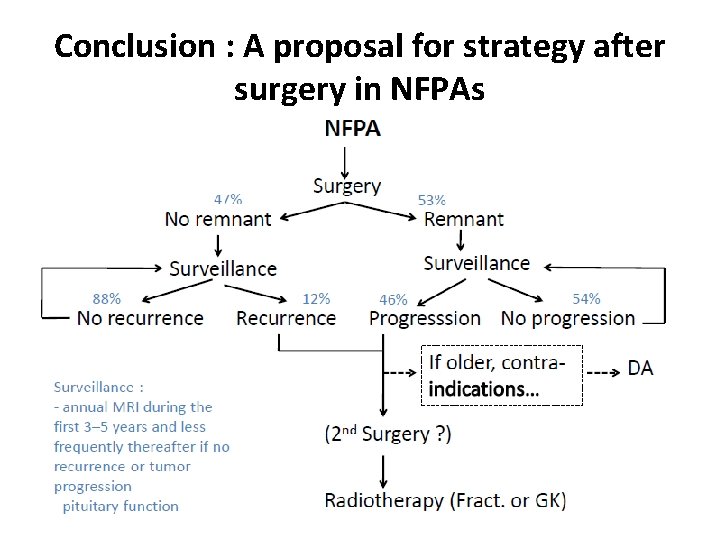
Management of relapsing NFPA 1 Conservative management • In the absence of remnant, the risk of recurrence after surgery is 12%, mainly between 5 and 15 years… MRI surveillance +++ • In the presence of a remnant (e. g. in the cavernous sinus), the risk of regrowth is 44% at 5 years and 60% at 10 years… MRI surveillance possible. But at which frequency? Roelfsema et al. Pituitary 2012

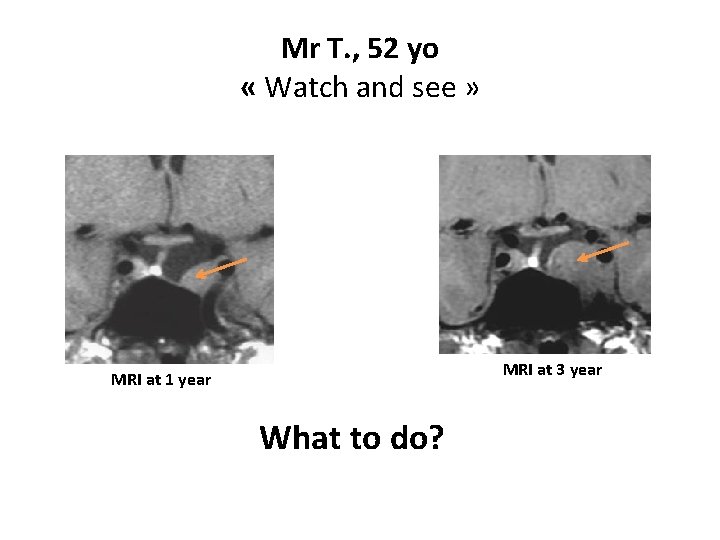
Mr T. , 52 yo « Watch and see » MRI at 3 year MRI at 1 year What to do?

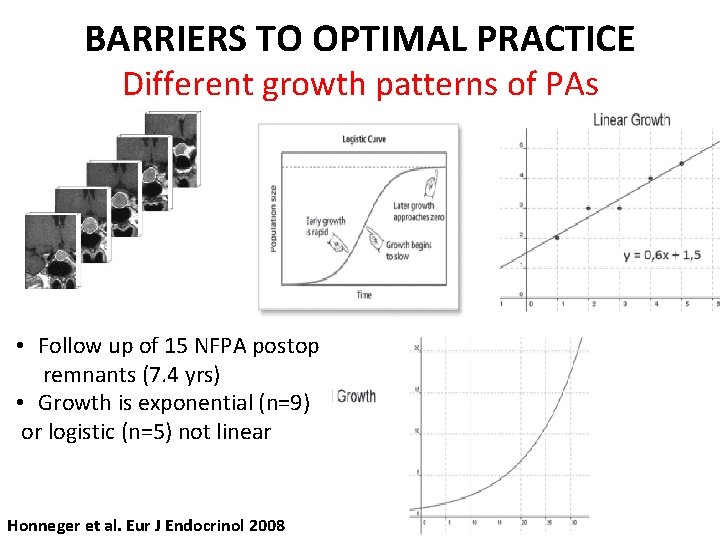
BARRIERS TO OPTIMAL PRACTICE Different growth patterns of PAs • Follow up of 15 NFPA postop remnants (7. 4 yrs) • Growth is exponential (n=9) or logistic (n=5) not linear Honneger et al. Eur J Endocrinol 2008

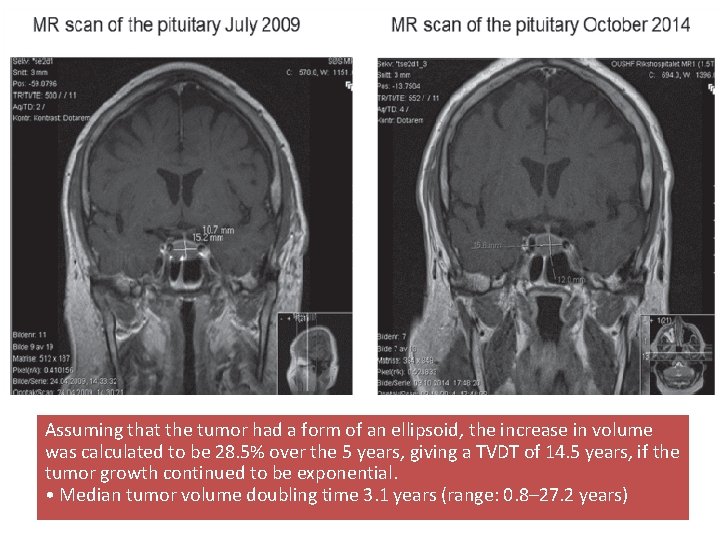
Assuming that the tumor had a form of an ellipsoid, the increase in volume was calculated to be 28. 5% over the 5 years, giving a TVDT of 14. 5 years, if the tumor growth continued to be exponential. • Median tumor volume doubling time 3. 1 years (range: 0. 8– 27. 2 years)

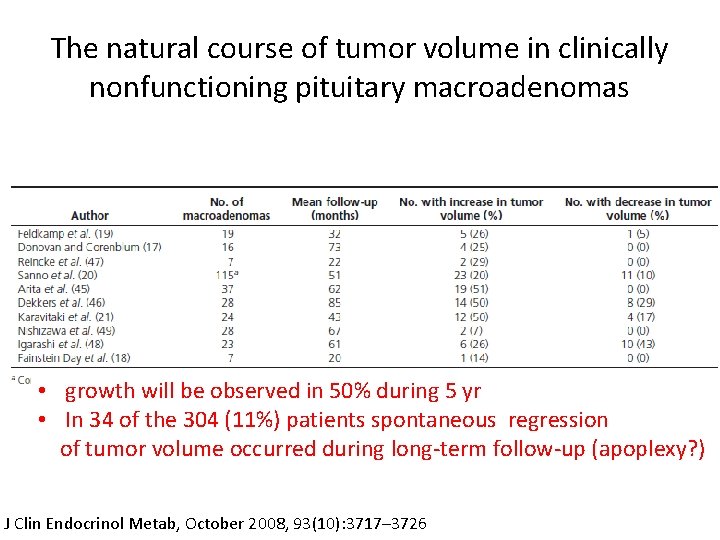
The natural course of tumor volume in clinically nonfunctioning pituitary macroadenomas • growth will be observed in 50% during 5 yr • In 34 of the 304 (11%) patients spontaneous regression of tumor volume occurred during long-term follow-up (apoplexy? ) J Clin Endocrinol Metab, October 2008, 93(10): 3717– 3726

BARRIERS TO OPTIMAL PRACTICE Follow-Up Strategy • MRI – In a series of nonoperated patients – mean increase in diameter : 0. 6 mm/yr – below the detection limit of currently used MRIs • Moreover, it is important to compare sequential MRIs with the first postoperative MRI • Visual assessment – low negative predictive value for recurrence – especially : with large distance between pituitary tumor and optic chiasm.

Management of relapsing NFPA 2 Postoperative radiotherapy It works!

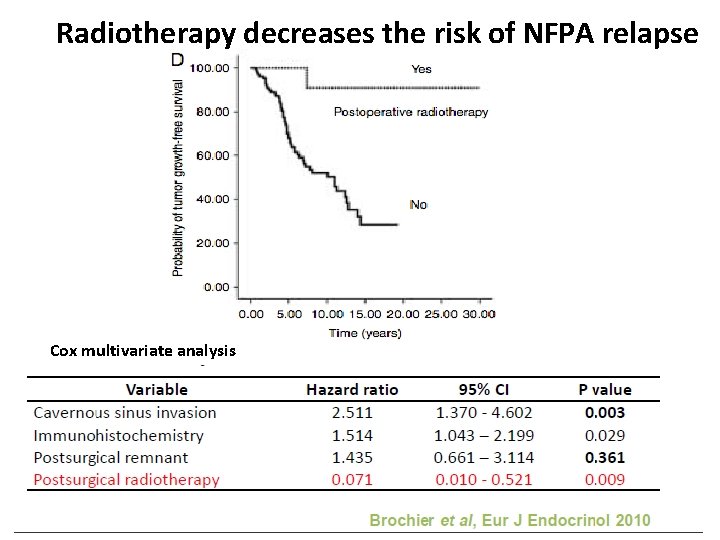
Radiotherapy decreases the risk of NFPA relapse Cox multivariate analysis

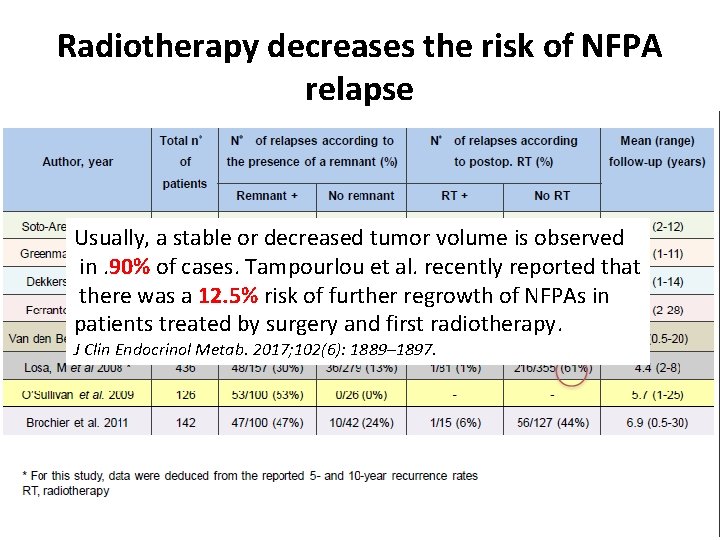
Radiotherapy decreases the risk of NFPA relapse Usually, a stable or decreased tumor volume is observed in. 90% of cases. Tampourlou et al. recently reported that there was a 12. 5% risk of further regrowth of NFPAs in patients treated by surgery and first radiotherapy. J Clin Endocrinol Metab. 2017; 102(6): 1889– 1897.

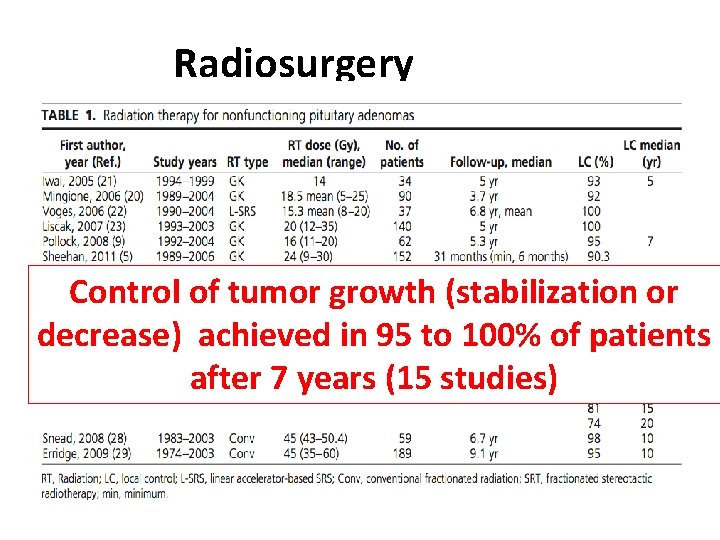
Radiosurgery Control of tumor growth (stabilization or decrease) achieved in 95 to 100% of patients after 7 years (15 studies)

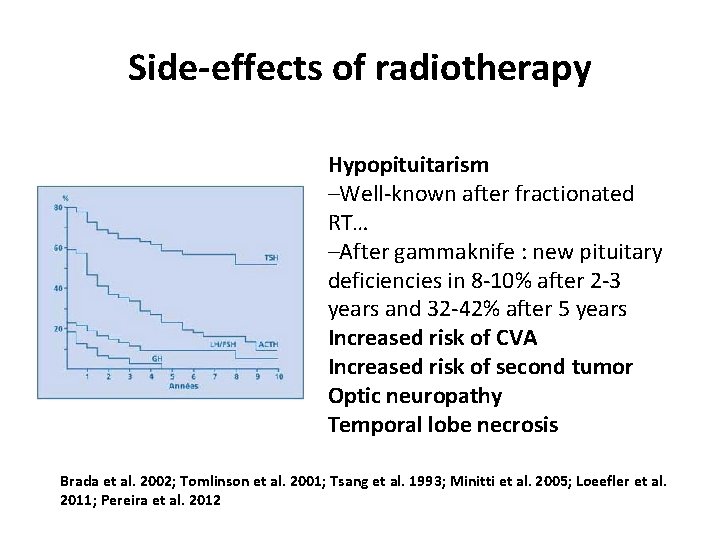
Side-effects of radiotherapy Hypopituitarism –Well-known after fractionated RT… –After gammaknife : new pituitary deficiencies in 8 -10% after 2 -3 years and 32 -42% after 5 years Increased risk of CVA Increased risk of second tumor Optic neuropathy Temporal lobe necrosis Brada et al. 2002; Tomlinson et al. 2001; Tsang et al. 1993; Minitti et al. 2005; Loeefler et al. 2011; Pereira et al. 2012

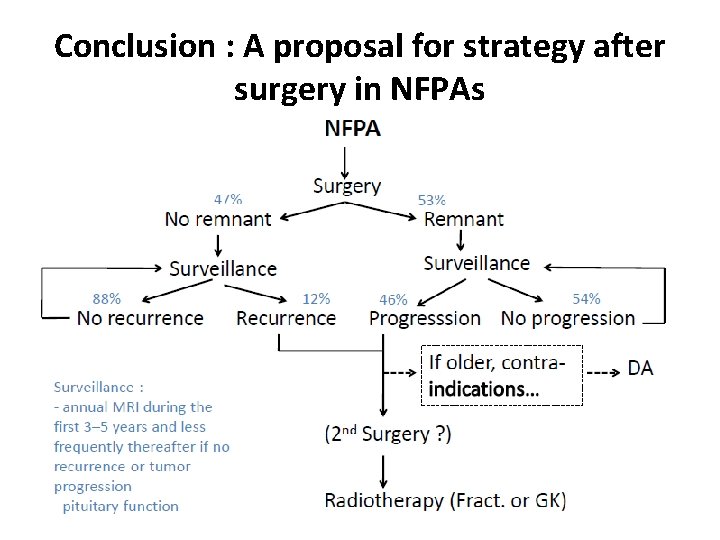
Conclusion : A proposal for strategy after surgery in NFPAs

Management of relapsing NFPA 2 Medical treatment ? • Gn. RH analogues : no antitumoral effects and risk of pituitary apoplexy ! Liuzzi et al. 1991; Boute et al. 1991; Colombo et al. 1994; Ando et al. 1995; Chanson et al. 1995; Morsi et al. 1996; Schuval et al. 1996; Chanson et al. 1997

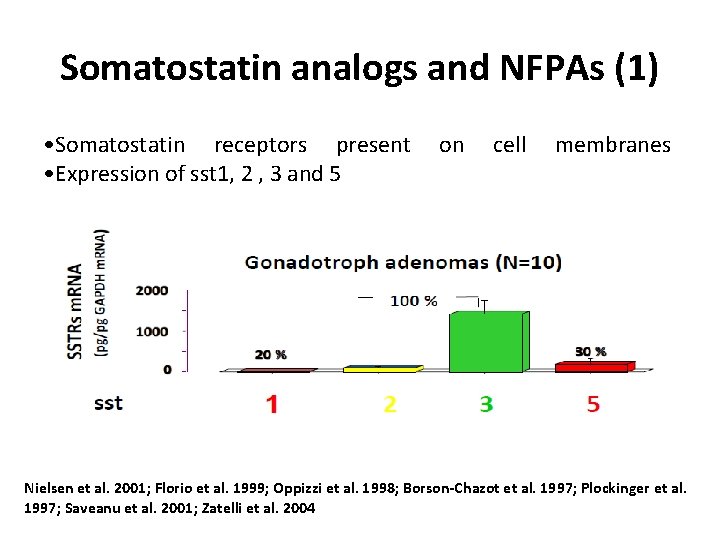
Somatostatin analogs and NFPAs (1) • Somatostatin receptors present • Expression of sst 1, 2 , 3 and 5 on cell membranes Nielsen et al. 2001; Florio et al. 1999; Oppizzi et al. 1998; Borson-Chazot et al. 1997; Plockinger et al. 1997; Saveanu et al. 2001; Zatelli et al. 2004

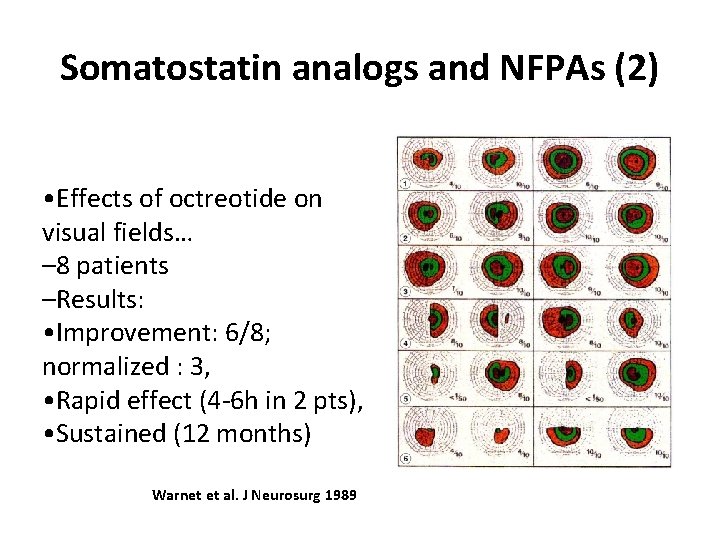
Somatostatin analogs and NFPAs (2) • Effects of octreotide on visual fields… – 8 patients –Results: • Improvement: 6/8; normalized : 3, • Rapid effect (4 -6 h in 2 pts), • Sustained (12 months) Warnet et al. J Neurosurg 1989

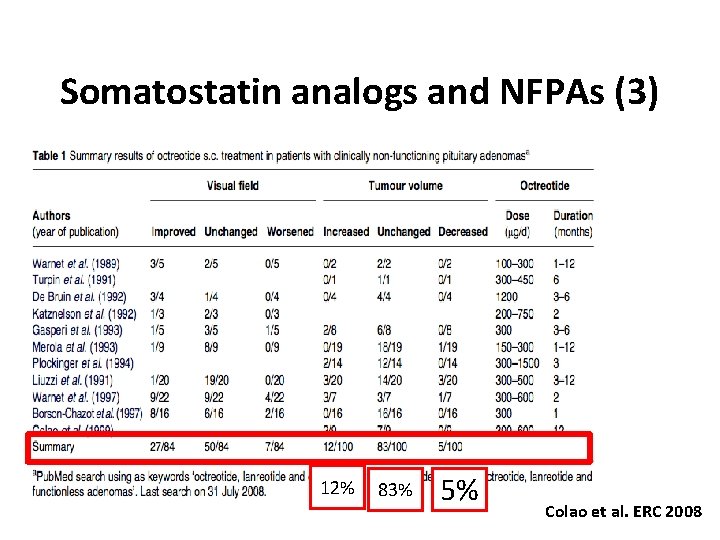
Somatostatin analogs and NFPAs (3) 12% 83% 5% Colao et al. ERC 2008

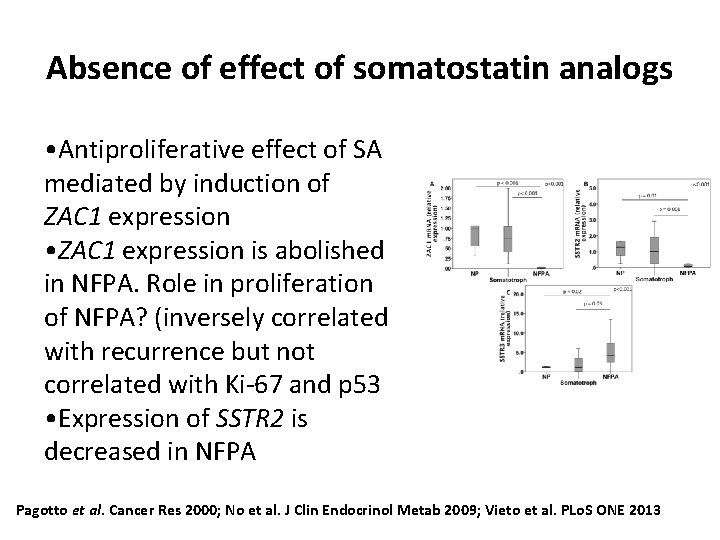
Absence of effect of somatostatin analogs • Antiproliferative effect of SA mediated by induction of ZAC 1 expression • ZAC 1 expression is abolished in NFPA. Role in proliferation of NFPA? (inversely correlated with recurrence but not correlated with Ki-67 and p 53 • Expression of SSTR 2 is decreased in NFPA Pagotto et al. Cancer Res 2000; No et al. J Clin Endocrinol Metab 2009; Vieto et al. PLo. S ONE 2013

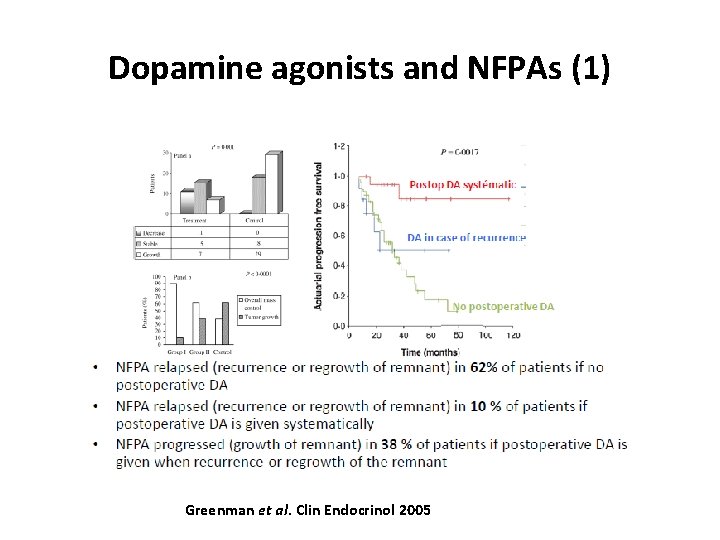
Dopamine agonists and NFPAs (1) Greenman et al. Clin Endocrinol 2005

Dopamine agonists and NFPAs (1) Greenman et al. Clin Endocrinol 2005

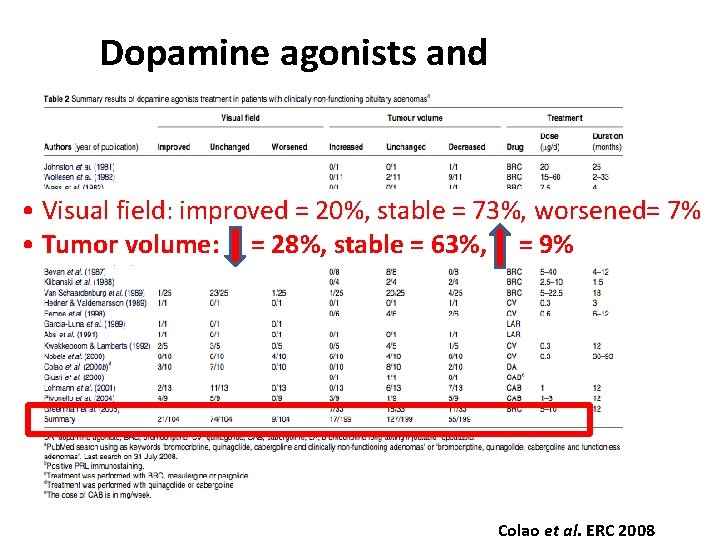
Dopamine agonists and NFPAs (3) • Visual field: improved = 20%, stable = 73%, worsened= 7% • Tumor volume: = 28%, stable = 63%, = 9% Colao et al. ERC 2008

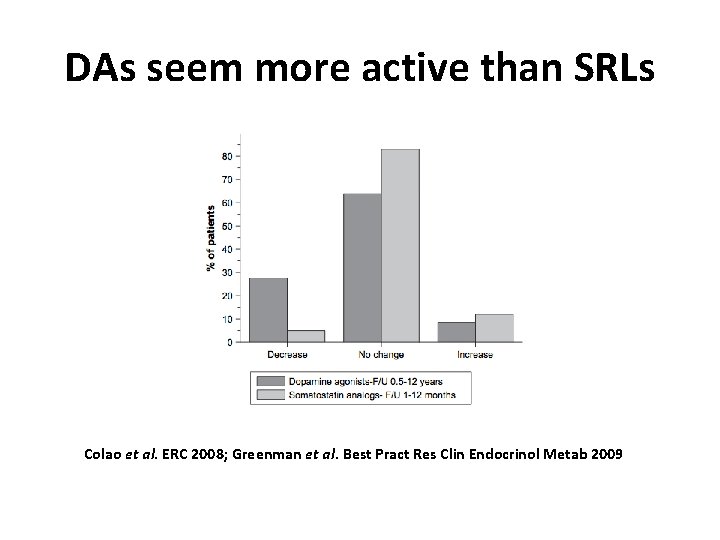
DAs seem more active than SRLs Colao et al. ERC 2008; Greenman et al. Best Pract Res Clin Endocrinol Metab 2009

Conclusion : A proposal for strategy after surgery in NFPAs

J Clin Endocrinol Metab, October 2008, 93(10): 3717– 3726



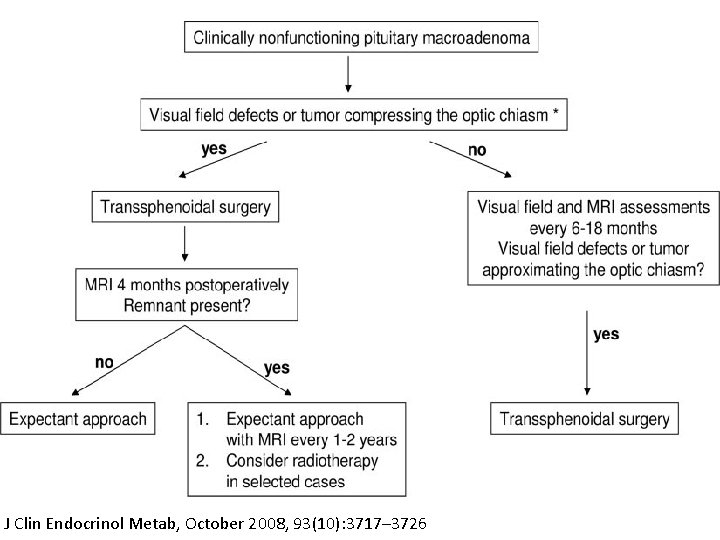
STRATEGIES FOR MANAGEMENT At the Time of Diagnosis Surgery and the Management of Symptomatic NFPAs With Visual Disorders. With surgery, improved vision is reported in; 80% to 90% of cases (including both partial and total recovery) (2– 4). Recoverymay be progressive, over a period of up to 1 year after surgery. Some studies highlighted a correlation between percentage recovery and duration and severity of visual field defect (acuity , 1/10 or optic atrophy being of poor prognosis) (5, 6). In the case of demonstrable visual disorder, surgery should therefore not be delayed; the emergency depends on the severity of visual impact. There are no clear data for a threshold time beyond which visual recovery after chiasma decompression is no longer possible. Visual disorders are an indication for surgery, even though complete recovery cannot be guaranteed.

NFPAs With Pituitary Deficiency The risk of onset of further pituitary deficiency associated with macroadenomais estimated at 12% per year. Arafah et al. (7, 8) correlated preoperative deficiency, headaches, and hyperprolactinemia to the potential for postoperative recovery; when all the criteria were met, postoperative recovery of deficiency and improvement in headaches were more frequent, correlating with intrasellar pressure. Surgery provided recovery of normal anterior pituitary function in ; 30% of cases, at a mean 1 year’s follow-up (9); the rate was higher for earlier management. Some teams indeed recommend surgery at the asymptomatic stage of macroadenoma (9). The risk of postoperative deterioration in pituitary function is; 10% (5). Pituitary deficiency is thus probably not the main factor indicating surgery, postoperative recovery being uncertain.

NFPAs With Headaches. The involvement of pituitary adenoma in headaches can only be established after ruling out all other possible causes (possibly after referral to a neurologist) and determining time of onset in relation to the natural history of the adenoma. Disabling headache implicating adenoma could be an indication for nonemergency surgery, warning the patient that no direct causal relation can be proven and thus results cannot be guaranteed.

Wait-and-See Attitude or Surgery in the Management of Asymptomatic NFPAs Surgical indications should be based on several factors. Size of the Tumor. Progression is slow in microadenoma and surgery is not indicated. The natural progression of macroadenoma may indicate nonemergency surgery, depending on evolutive status, proximity to the optic pathways, and the patient’s age. The spontaneous evolution of a nonsecreting macroadenoma has been evaluated at 17% increase and 12% decrease with a follow-up. 4 years, and 30% to 40% increase with a longer follow-up (10).

Patient Age Nonemergency surgery may be considered in young patients without awaiting progression, given the low risk of postoperative visual impairment, the almost inevitable progression of adenoma over the long-term, and the risk of definitive postoperative visual impairment if surgery is delayed awaiting onset of a campimetric visual-field effect. For young female patients, surgery should respond quickly to any impairment of visual field. Gonadotroph deficiency is also an indication for surgery, although postoperative recovery is unsure. Pregnancy is not theoretically a risk factor for increasing adenoma volume, but there is a risk of visual field defect if the adenoma is close to the optic pathways.

Patient Age In that case, surgery should be considered; if it is not implemented, close clinical surveillance and visual field testing should accompany the pregnancy. If no pregnancy is planned and the adenoma shows no impact, MRI surveillance should be proposed, or else surgical intervention without awaiting progression. Indications in 65 - to 75 -year-olds are the same as in young subjects, if comorbidity, anesthesia risk, and patient impact are well assessed. In the case of surgery, the approach should preferably be transsphenoidal, so as not to increase the risk of complications. In the absence of visual impact, simple annual MRI surveillance is sufficient. The lack of data for. 75 -year-olds is to be borne in mind.

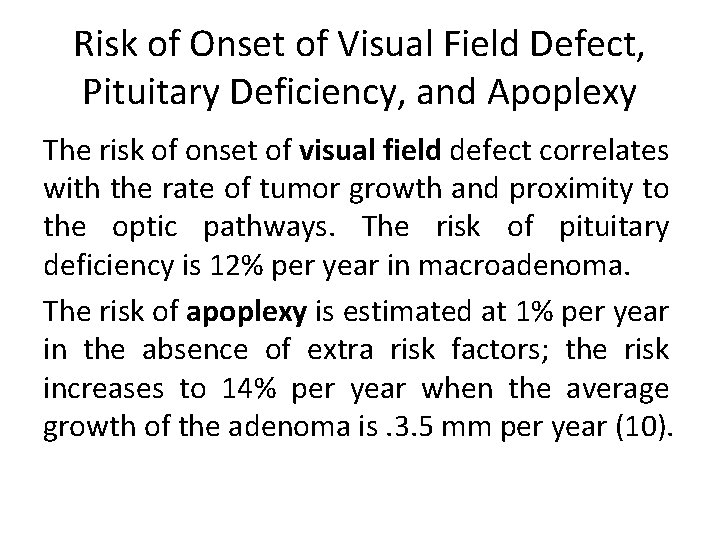
Risk of Onset of Visual Field Defect, Pituitary Deficiency, and Apoplexy The risk of onset of visual field defect correlates with the rate of tumor growth and proximity to the optic pathways. The risk of pituitary deficiency is 12% per year in macroadenoma. The risk of apoplexy is estimated at 1% per year in the absence of extra risk factors; the risk increases to 14% per year when the average growth of the adenoma is. 3. 5 mm per year (10).

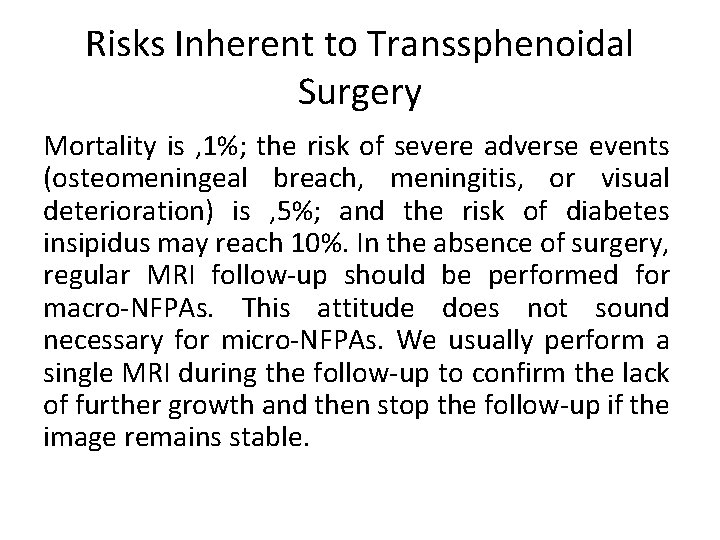
Risks Inherent to Transsphenoidal Surgery Mortality is , 1%; the risk of severe adverse events (osteomeningeal breach, meningitis, or visual deterioration) is , 5%; and the risk of diabetes insipidus may reach 10%. In the absence of surgery, regular MRI follow-up should be performed for macro-NFPAs. This attitude does not sound necessary for micro-NFPAs. We usually perform a single MRI during the follow-up to confirm the lack of further growth and then stop the follow-up if the image remains stable.

After Surgery The follow-up is based on regular pituitary MRI. The first MRI should be done 3 to 4 months after the surgery; the results of this MRI will determine the following therapeutic procedures (11, 12).

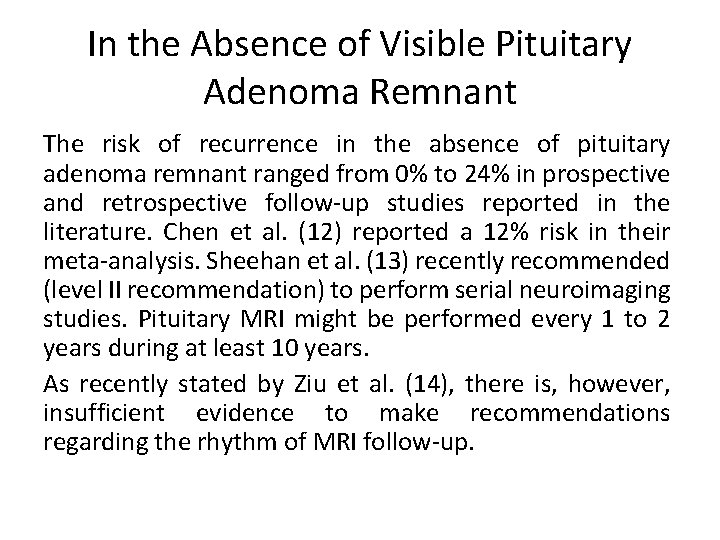
In the Absence of Visible Pituitary Adenoma Remnant The risk of recurrence in the absence of pituitary adenoma remnant ranged from 0% to 24% in prospective and retrospective follow-up studies reported in the literature. Chen et al. (12) reported a 12% risk in their meta-analysis. Sheehan et al. (13) recently recommended (level II recommendation) to perform serial neuroimaging studies. Pituitary MRI might be performed every 1 to 2 years during at least 10 years. As recently stated by Ziu et al. (14), there is, however, insufficient evidence to make recommendations regarding the rhythm of MRI follow-up.

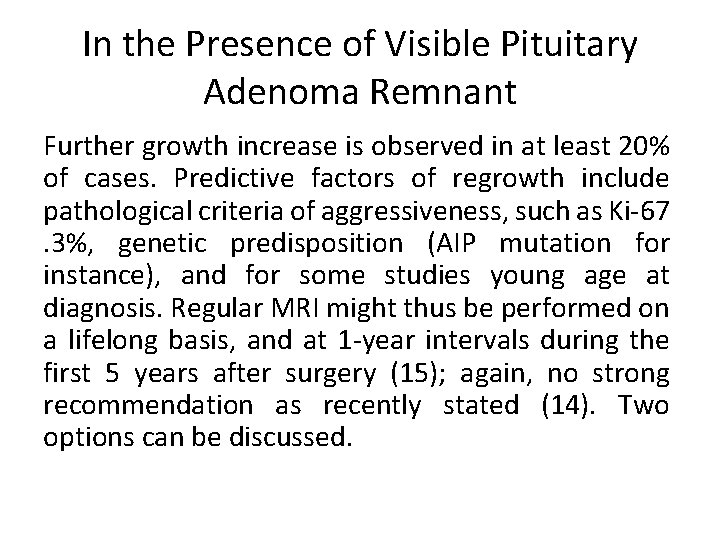
In the Presence of Visible Pituitary Adenoma Remnant Further growth increase is observed in at least 20% of cases. Predictive factors of regrowth include pathological criteria of aggressiveness, such as Ki-67. 3%, genetic predisposition (AIP mutation for instance), and for some studies young age at diagnosis. Regular MRI might thus be performed on a lifelong basis, and at 1 -year intervals during the first 5 years after surgery (15); again, no strong recommendation as recently stated (14). Two options can be discussed.