Nomenclature A System of Naming Compounds Compounds are







![Epsom Salts [Mg. SO 4 * 7 H 20] Epsom Salts [Mg. SO 4 * 7 H 20]](https://slidetodoc.com/presentation_image_h2/dc22698488702cb43bc1bb7b057aa21f/image-19.jpg)





- Slides: 33

Nomenclature • A System of Naming Compounds • Compounds are two or more atoms of different elements bonded together.


Polyatomic ions • Poly = Many • Atomic = having to do with atoms • ions = having a charge + or –

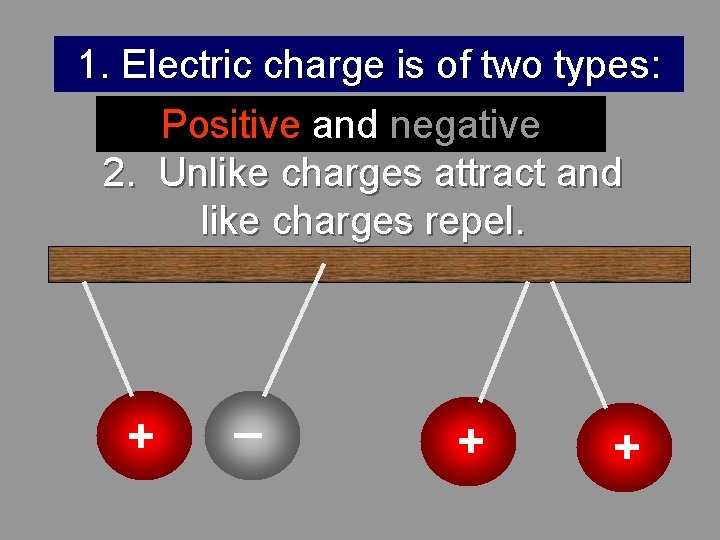
1. Electric charge is of two types: Positive and negative 2. Unlike charges attract and like charges repel. + _ + +

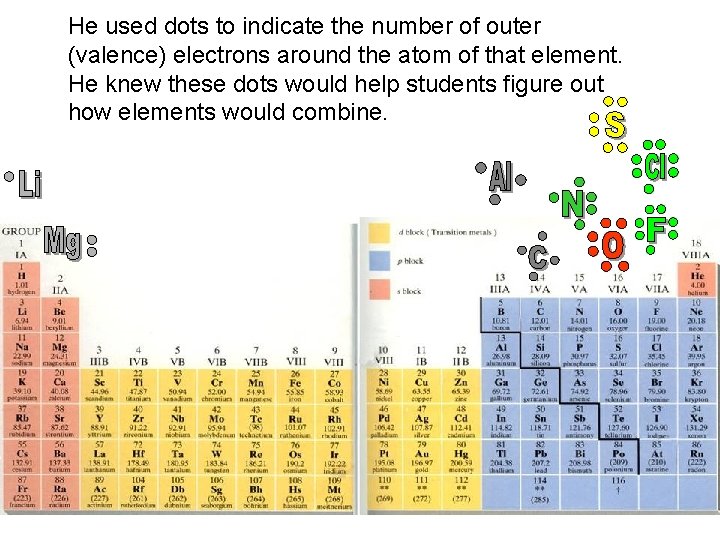
Gilbert Lewis invents a chemistry learning technique.

He used dots to indicate the number of outer (valence) electrons around the atom of that element. He knew these dots would help students figure out how elements would combine.

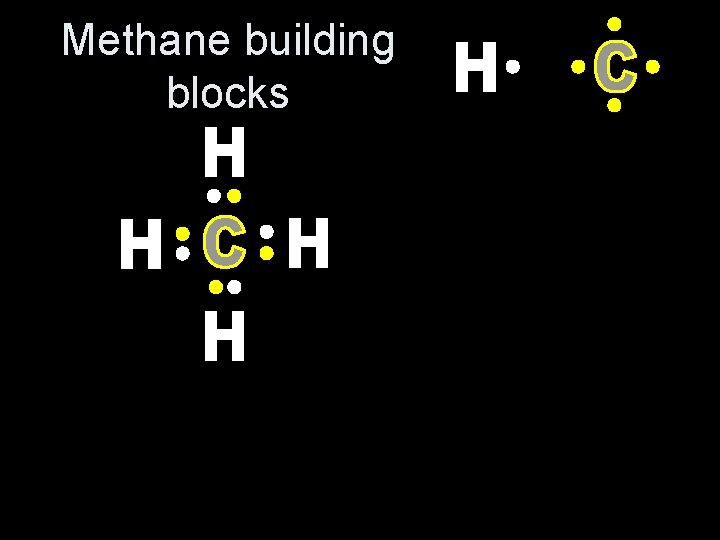
Methane building blocks

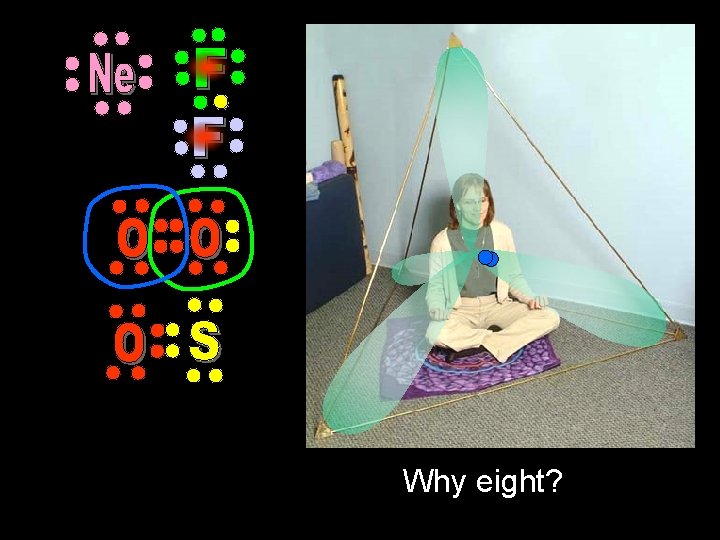
Why eight?


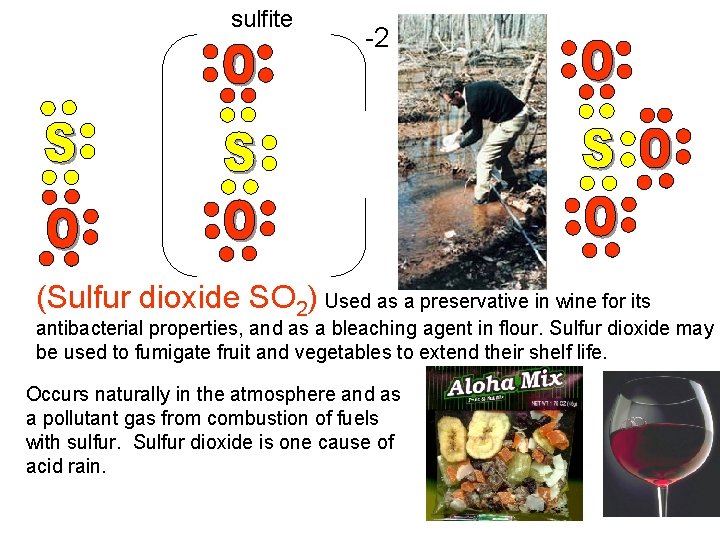
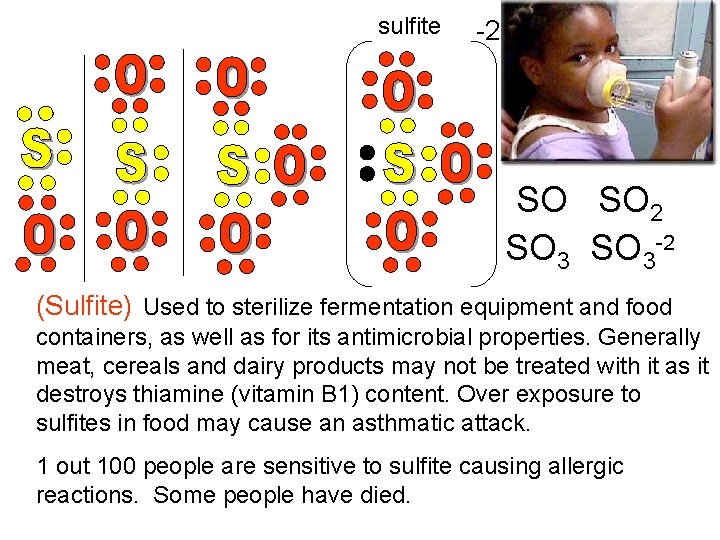
sulfite -2 - - (Sulfur dioxide SO 2) Used as a preservative in wine for its antibacterial properties, and as a bleaching agent in flour. Sulfur dioxide may be used to fumigate fruit and vegetables to extend their shelf life. Occurs naturally in the atmosphere and as a pollutant gas from combustion of fuels with sulfur. Sulfur dioxide is one cause of acid rain.

sulfite -2 SO SO 2 SO 3 -2 (Sulfite) Used to sterilize fermentation equipment and food containers, as well as for its antimicrobial properties. Generally meat, cereals and dairy products may not be treated with it as it destroys thiamine (vitamin B 1) content. Over exposure to sulfites in food may cause an asthmatic attack. 1 out 100 people are sensitive to sulfite causing allergic reactions. Some people have died.

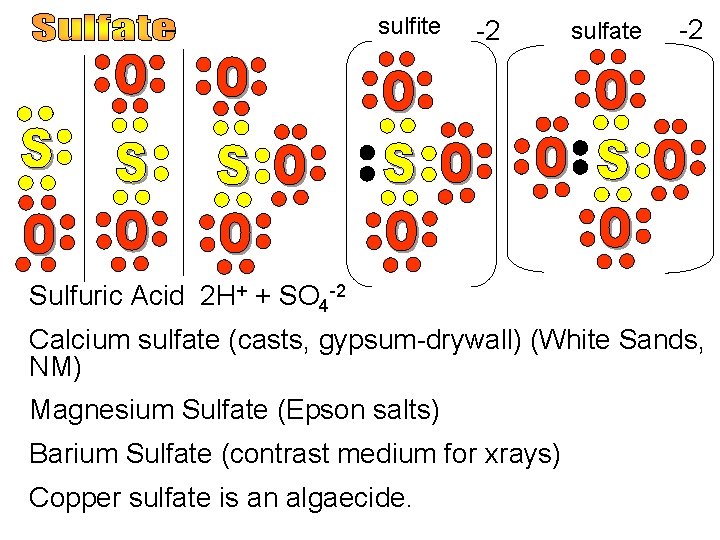
sulfite -2 sulfate -2 Sulfuric Acid 2 H+ + SO 4 -2 Calcium sulfate (casts, gypsum-drywall) (White Sands, NM) Magnesium Sulfate (Epson salts) Barium Sulfate (contrast medium for xrays) Copper sulfate is an algaecide.

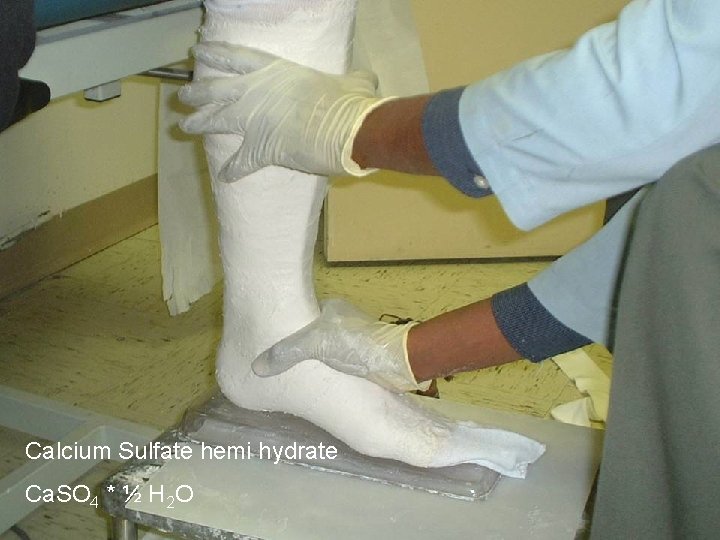
Calcium Sulfate hemi hydrate Ca. SO 4 * ½ H 2 O

Calcium Sulfate hemi hydrate Ca. SO 4 * ½ H 2 O

Ca. SO 4 * 2 H 2 O

White Sands, NM Ca. SO 4 * 2 H 2 O

White Sands, NM Ca. SO 4 * 2 H 2 O

Desert Rose
![Epsom Salts Mg SO 4 7 H 20 Epsom Salts [Mg. SO 4 * 7 H 20]](https://slidetodoc.com/presentation_image_h2/dc22698488702cb43bc1bb7b057aa21f/image-19.jpg)
Epsom Salts [Mg. SO 4 * 7 H 20]

H 2 SO 4 Sulfuric acid (battery acid)

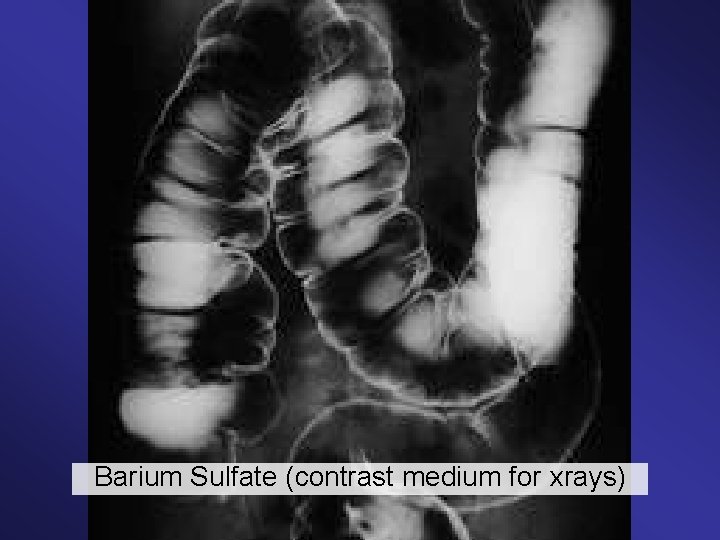
Barium Sulfate (contrast medium for xrays)


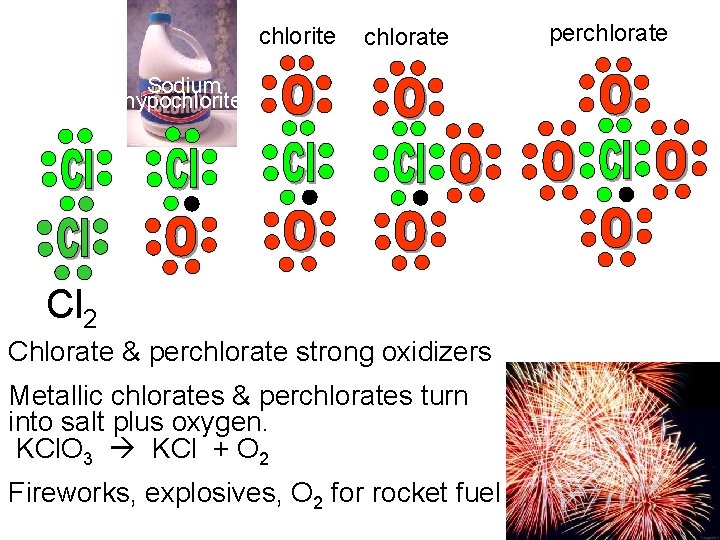
chlorite chlorate perchlorate Sodium hypochlorite Cl 2 Cl. O 3 Chlorate & perchlorate strong oxidizers Metallic chlorates & perchlorates turn into salt plus oxygen. KCl. O 3 KCl + O 2 Fireworks, explosives, O 2 for rocket fuel Cl. O 4


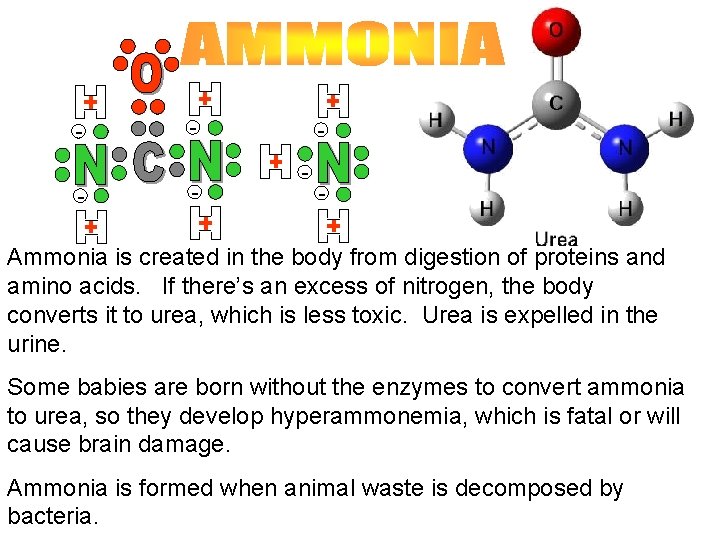
- - Ammonia is created in the body from digestion of proteins and amino acids. If there’s an excess of nitrogen, the body converts it to urea, which is less toxic. Urea is expelled in the urine. Some babies are born without the enzymes to convert ammonia to urea, so they develop hyperammonemia, which is fatal or will cause brain damage. Ammonia is formed when animal waste is decomposed by bacteria.

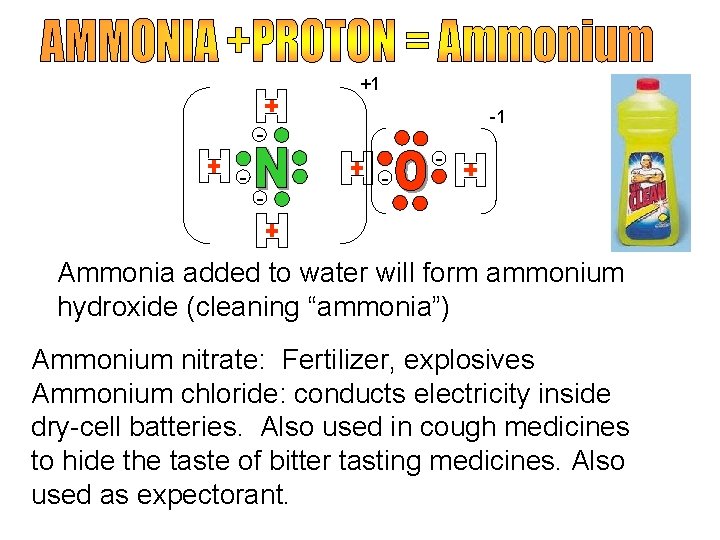
+1 -1 - - Ammonia added to water will form ammonium hydroxide (cleaning “ammonia”) Ammonium nitrate: Fertilizer, explosives Ammonium chloride: conducts electricity inside dry-cell batteries. Also used in cough medicines to hide the taste of bitter tasting medicines. Also used as expectorant.


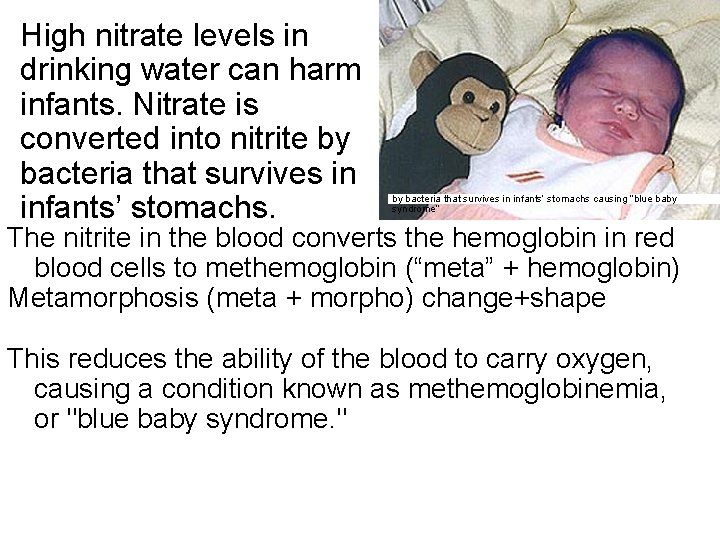
High nitrate levels in drinking water can harm infants. Nitrate is converted into nitrite by bacteria that survives in infants’ stomachs causing “blue baby syndrome” The nitrite in the blood converts the hemoglobin in red blood cells to methemoglobin (“meta” + hemoglobin) Metamorphosis (meta + morpho) change+shape This reduces the ability of the blood to carry oxygen, causing a condition known as methemoglobinemia, or "blue baby syndrome. "

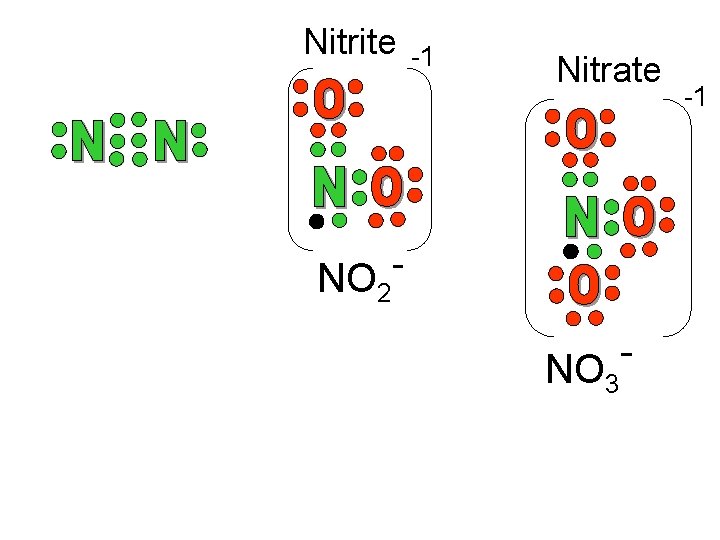
Nitrite -1 Nitrate NO 2 NO 3 -1

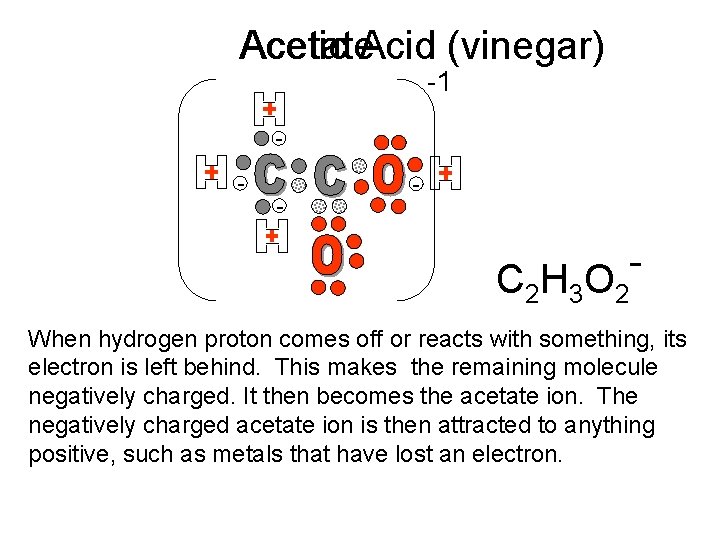
Acetic Acetate. Acid (vinegar) -1 - - - C 2 H 3 O 2 When hydrogen proton comes off or reacts with something, its electron is left behind. This makes the remaining molecule negatively charged. It then becomes the acetate ion. The negatively charged acetate ion is then attracted to anything positive, such as metals that have lost an electron.

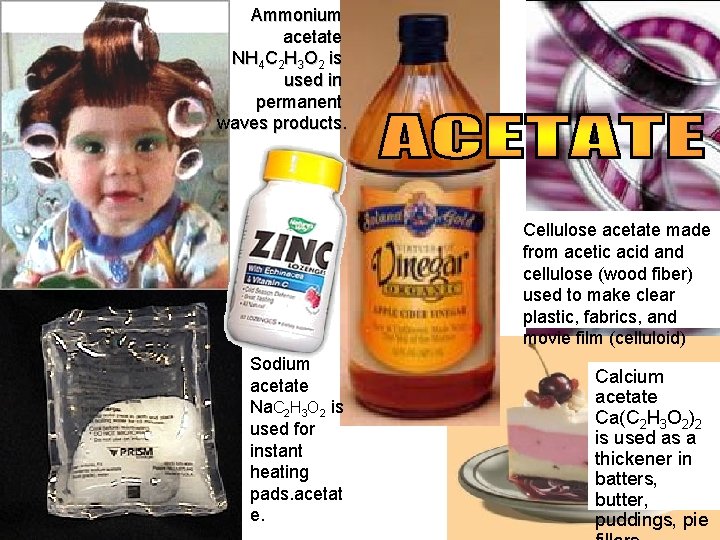
Ammonium acetate NH 4 C 2 H 3 O 2 is used in permanent waves products. Cellulose acetate made from acetic acid and cellulose (wood fiber) used to make clear plastic, fabrics, and movie film (celluloid) Sodium acetate Na. C 2 H 3 O 2 is used for instant heating pads. acetat e. Calcium acetate Ca(C 2 H 3 O 2)2 is used as a thickener in batters, butter, puddings, pie

Cyanide CN- is found in solution. Hydrogen cyanide HCN is the gas. Gas chambers used a pesticide called Zyklon B, which decomposed to HCN. First used in camps to delouse and for Typhus. The seeds and pits of apricots, cherries, almonds, peaches, and apples contain amygdalin. Inside the intestine bacteria can convert this to cyanide. Under the name of Laetrile, amygdalin has been proposed as a treatment for cancer, but the medical community has rejected this claim.

- hydrogen cyanide HCN Cassava is an important food source for 500 million people, but the roots contain a substance that, when eaten, can trigger the production of cyanide. Only proper cooking can neutralize the cyanide CN- -
 Antigentest åre
Antigentest åre Cn functional group
Cn functional group Nomenclature of coordination compounds
Nomenclature of coordination compounds Heterocyclic compounds nomenclature
Heterocyclic compounds nomenclature Nomenclature of heterocyclic compounds
Nomenclature of heterocyclic compounds Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Naming metallic compounds
Naming metallic compounds Ni ionic charge
Ni ionic charge Chemical
Chemical Phosphorus pentafluoride covalent compound formula
Phosphorus pentafluoride covalent compound formula How to write chemical formulas for ionic compounds
How to write chemical formulas for ionic compounds Naming compounds and writing formulas
Naming compounds and writing formulas Carbon monoxide formual
Carbon monoxide formual Naming ionic compounds
Naming ionic compounds When to use prefixes for naming compounds
When to use prefixes for naming compounds Naming binary compounds ionic
Naming binary compounds ionic Formula of binary compound
Formula of binary compound Ionic compounds containing polyatomic ions
Ionic compounds containing polyatomic ions Concept 2 notes naming ionic compounds
Concept 2 notes naming ionic compounds Writing formulas and naming compounds section 3
Writing formulas and naming compounds section 3 Writing formulas criss cross method
Writing formulas criss cross method Pentaboron nonahydride formula
Pentaboron nonahydride formula Systematic name
Systematic name Binary molecule
Binary molecule Monatomic ion
Monatomic ion Naming ionic compounds flowchart
Naming ionic compounds flowchart Flowchart for naming binary compounds
Flowchart for naming binary compounds Chemistry meth eth prop but
Chemistry meth eth prop but How to name covalent compounds
How to name covalent compounds Name of so4
Name of so4 Naming molecular compounds
Naming molecular compounds Dinitrogen hexacarbide
Dinitrogen hexacarbide Tetraiodine nonaoxide formula
Tetraiodine nonaoxide formula Is sugar ionic or molecular
Is sugar ionic or molecular