POWER POINT LECTURE ON NAMING COMPOUNDS Naming Compounds















- Slides: 18

POWER POINT LECTURE ON NAMING COMPOUNDS

Naming Compounds There are two types of compounds that we can name:

Naming Compounds What is a chemical formula? A representation of the # of atoms of each kind in a compound using atomic symbols and subscripts (So there!!!)

Binary Ionic Compounds Ionic compounds that contain two different atoms: Examples: Na. Cl, Mg. F 2, Li 2 O, Al. Br 3 Now let’s learn to name them!

Binary Ionic Compounds The metal (or the positive component) is named 1 st. The nonmetal (or negative component) is added to the name. Then the letters - ide are added to the ending Example: Na. Cl Na is sodium and Cl is chlorine. Therefore Na. Cl is Sodium Chloride

Binary Ionic Compounds Now try these: Mg. Br 2 Mg is Magnesium and Br is Bromine. Therefore Mg. Br 2 is Magnesium Bromide Li 2 O Li is Lithium and O is Oxygen. Therefore Li 2 O is Lithium Oxide

Binary Ionic Compounds Now why is it that some formulas have subscripts? Each atom has a different oxidation #. This tells us in what proportions, the atoms must combine in. For example: Mg has a +2 oxidation # and Br has a -1 oxidation # therefore it would take 1 Mg+2 to cancel out 2 Br-1 The molecular formula is therefore Mg. Br 2

Binary Ionic Compounds Or you could look at it like this: When you want to know what subscripts to use, simply switch the oxidation #s and turn them into subscripts. Mg +2 Br -1 becomes Mg. Br 2 Get it! Good!
Binary Ionic Compounds Now some metals have more then one oxidation state. Examples: Cu could be +1 or +2 Fe could be +2 or +3 Now what do we do?

Binary Ionic Compounds We use the same system, but now we insert a Roman Numeral after the metal to designate the oxidation state of the metal. Example: For Cu. Cl 2 we would call this…. Copper (II) chloride How do we know it was Copper (II) and not (I) ? Remember in order to get Cu. Cl 2 we had to switch the oxidation #s, so copper must have been +2.

Binary Ionic Compounds Now you try. (You will need your Reference Table) Name these: Fe 2 O 3 Fe. S Cu. Cl Iron (III) oxide Iron (II) sulfide Copper (I) chloride

Binary Ionic Compounds Still a little unsure? Let’s try to explain further. . . Fe 2 O 3 was made from Fe+3 and O-2 Once we switch the oxidation #s, we get. . . Fe 2 O 3 - Iron (III) oxide because Fe was +3. Fe. S was made from Fe+2 and S-2 Once we switch the oxidation #s, we get… Fe. S - Iron (II) sulfide because Fe was +2.

Polyatomic Ionic Compounds Naming Polyatomic ionic compounds are almost as easy as naming binary ionic compounds. Name the positive component 1 st. Name the negative component 2 nd (No -ide ending this time) Example: Na. OH (You will need Reference Table F) Na is Sodium and OH- (from table F) is hydroxide therefore this compound is sodium hydroxide

Polyatomic Ionic Compounds Now you try some… (and don’t let the subscripts throw you): Li. NO 3 Mg. CO 3 Na 3 PO 4 lithium nitrate magnesium carbonate sodium phosphate

Polyatomic Ionic Compounds Now you try some harder ones: Ca 3(PO 4)2 (NH 4)2 CO 3 Al(OH)3 calcium phosphate ammonium carbonate aluminum hydroxide

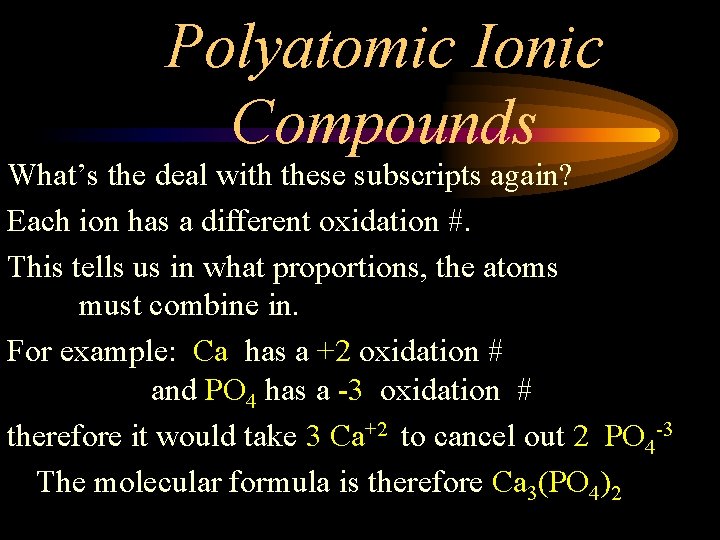
Polyatomic Ionic Compounds What’s the deal with these subscripts again? Each ion has a different oxidation #. This tells us in what proportions, the atoms must combine in. For example: Ca has a +2 oxidation # and PO 4 has a -3 oxidation # therefore it would take 3 Ca+2 to cancel out 2 PO 4 -3 The molecular formula is therefore Ca 3(PO 4)2

Polyatomic Ionic Compounds Or you could look at it like this: When you want to know what subscripts to use, simply switch the oxidation #s and turn them into subscripts. Ca +2 PO 4 -3 becomes Ca 3(PO 4)2 Remember! We learned this already! I knew you’d get it!

Polyatomic Ionic Compounds I think you are ready to do some work on your own now… What do you think? You see… this is why you are my favorite class! Hey wait doesn’t he show this to all his classes? Mr. Baruch - that’s not right!