NMR spektroszkpia vegysz mesterkurzus VEMKSI 4312 S 7




![Applications: self-assemblies [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4, 5, 6 tectons: 1 Applications: self-assemblies [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4, 5, 6 tectons: 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-33.jpg)
![Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: pyridine ortho protons, 1 Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: pyridine ortho protons, 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-34.jpg)
![Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: acenaphthane ortho protons 1 Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: acenaphthane ortho protons 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-35.jpg)
![Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 1 H Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 1 H](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-36.jpg)
![Alkalmazások: INEPT-DOSY [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 31 P DOSY Alkalmazások: INEPT-DOSY [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 31 P DOSY](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-38.jpg)
![Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(4, 4’-bpy…)]1, 2, 3, 4 tektonok: 31 P DOSY Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(4, 4’-bpy…)]1, 2, 3, 4 tektonok: 31 P DOSY](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-39.jpg)
![Alkalmazások: önszerveződő rendszerek [(Pd(dppp)(4, 4’-bpy…)]2, 3, 4, 5 tektonok: 1 H DOSY 1 2 Alkalmazások: önszerveződő rendszerek [(Pd(dppp)(4, 4’-bpy…)]2, 3, 4, 5 tektonok: 1 H DOSY 1 2](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-40.jpg)
![Alkalmazások: ruthenium complexes 1 H DOSY dmso 298 K [Ru(diphenylphenantroline)3]+ Dt = 130*10 -12 Alkalmazások: ruthenium complexes 1 H DOSY dmso 298 K [Ru(diphenylphenantroline)3]+ Dt = 130*10 -12](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-41.jpg)
- Slides: 45

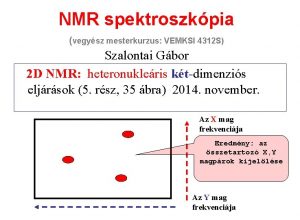
NMR spektroszkópia (vegyész mesterkurzus: VEMKSI 4312 S) 7. rész: Diffúzió-szelektált 2 D NMR spektroszkópia Szalontai Gábor 2014. december (45 ábra) Diffusion Ordered Spectroscop. Y (DOSY) MOBility Ordered NMR Spectroscop. Y (MOBY)

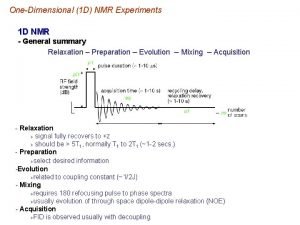
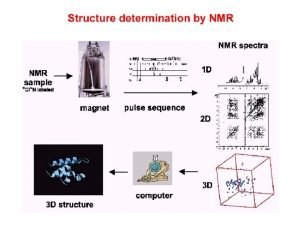
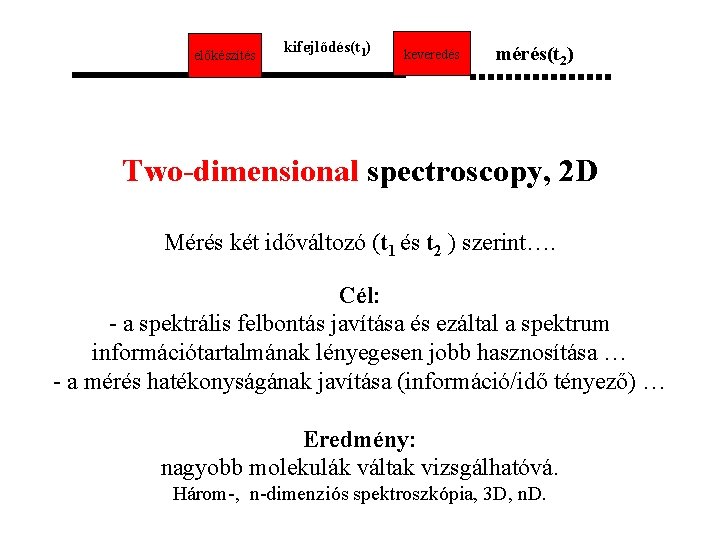
előkészítés kifejlődés(t 1) keveredés mérés(t 2) Two-dimensional spectroscopy, 2 D Mérés két időváltozó (t 1 és t 2 ) szerint…. Cél: - a spektrális felbontás javítása és ezáltal a spektrum információtartalmának lényegesen jobb hasznosítása … - a mérés hatékonyságának javítása (információ/idő tényező) … Eredmény: nagyobb molekulák váltak vizsgálhatóvá. Három-, n-dimenziós spektroszkópia, 3 D, n. D.

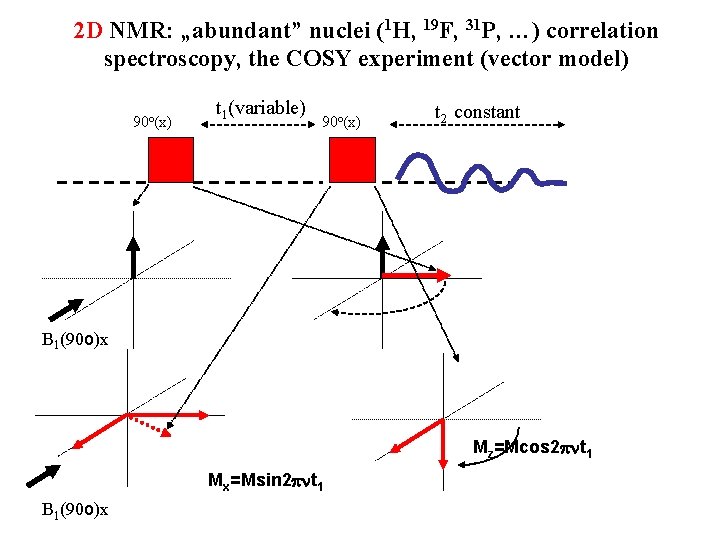
2 D NMR: „abundant” nuclei (1 H, 19 F, 31 P, …) correlation spectroscopy, the COSY experiment (vector model) 90 o(x) t 1(variable) 90 o(x) t 2 constant B 1(90 o)x Mz=Mcos 2 pnt 1 Mx=Msin 2 pnt 1 B 1(90 o)x

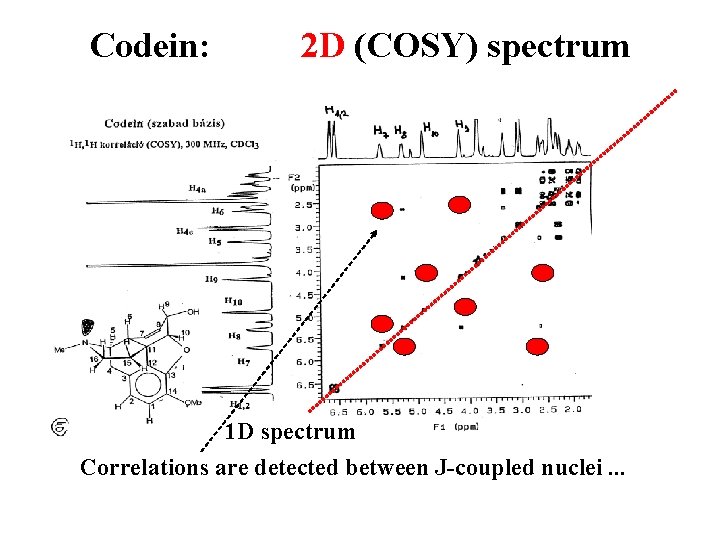
Codein: 2 D (COSY) spectrum 1 D spectrum Correlations are detected between J-coupled nuclei. . .

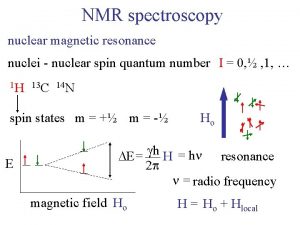
Question: can we use the second dimension (evolution period: t 1 ) to study a molecular property (such as shape, size, diffusion, etc. ) rather than the usual spin Hamiltonian (Jcoupling, chemical shifts, etc, )? Answer: yes, provided these properties have an impact on the spectrum !!.

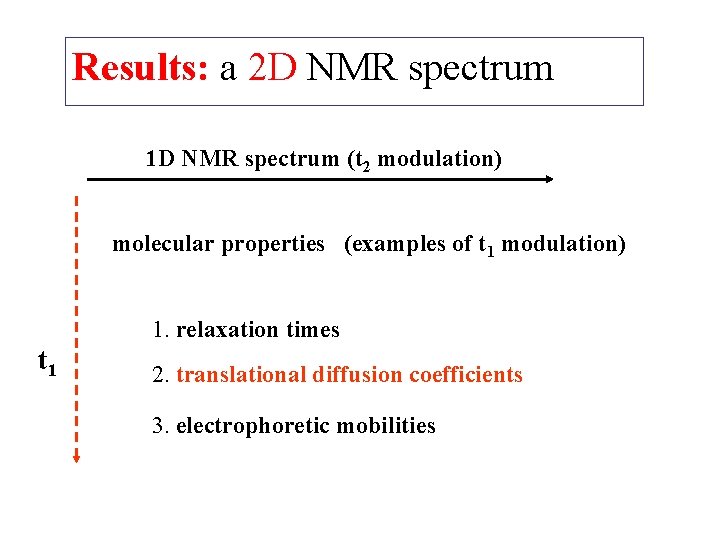
Results: a 2 D NMR spectrum 1 D NMR spectrum (t 2 modulation) molecular properties (examples of t 1 modulation) 1. relaxation times t 1 2. translational diffusion coefficients 3. electrophoretic mobilities

The effect of changing stream gradient on zanders (without diffusion)

The diffusion phenomenon destroys the order!

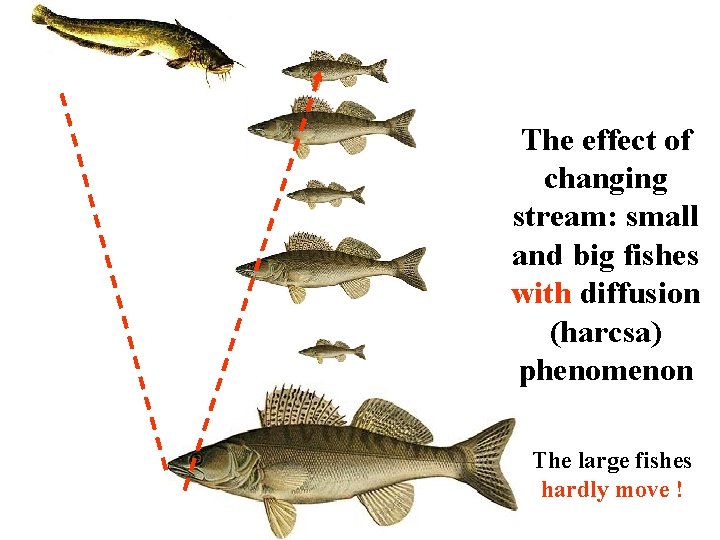
The effect of changing stream: small and big fishes with diffusion (harcsa) phenomenon The large fishes hardly move !

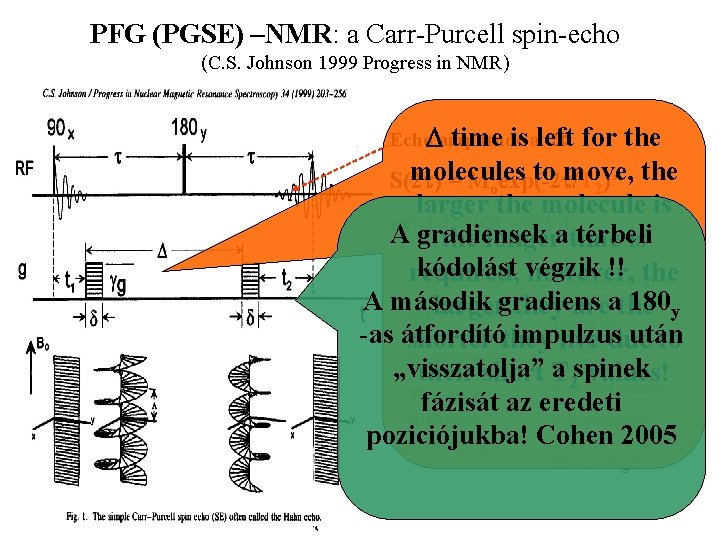
PFG (PGSE) –NMR: a Carr-Purcell spin-echo (C. S. Johnson 1999 Progress in NMR) Echo. Damplitude 2 t: time is atleft for the molecules to move, the S(2 t) = Moexp(-2 t/T )* 2 larger the molecule is 2 exp[-Dq (D - d/3)] A gradiensek térbeli the longeratime is kódolást végzik !! the required, however, Advantages Disadvantages: T 2 avalues A második gradiens 180 y theyshort are thelarger maximum limits the signal strength -as átfordító impulzus után shorter they applications live due toto is recovered, to medium „visszatolja” asmall spinek their short T values! 2 molecules, size The chemical fázisát az eredeti shifts are J-modulation poziciójukba! Cohen 2005 prevent complete refocused! refocusing!

Applications: Pulse (Field) Gradient Spin Echo experiment • Determination of – Translational self-diffusion coefficient, Dt – Hydrodynamic radius, r. H – Hydrodynamic volume, VH

Theory • Diffusion ordered NMR: translational diffusion coefficient (Stokes-Einstein relation) • a = molecular radius, ho= solvent viscosity

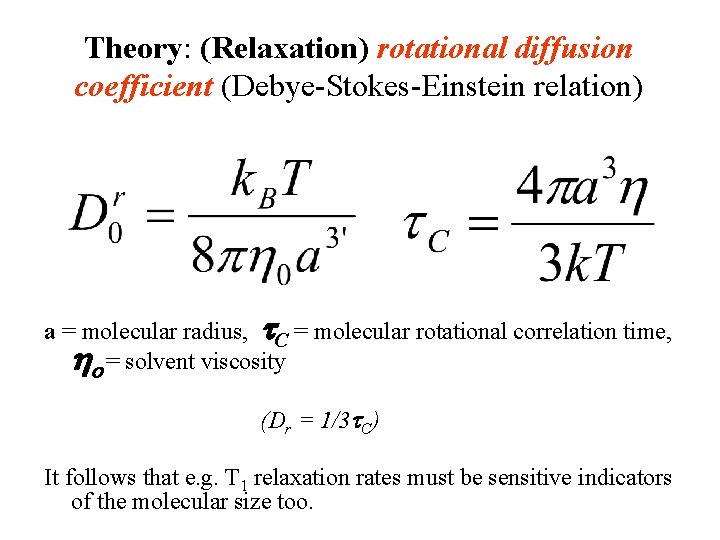
Theory: (Relaxation) rotational diffusion coefficient (Debye-Stokes-Einstein relation) a = molecular radius, t. C = molecular rotational correlation time, ho = solvent viscosity (Dr = 1/3 t. C) It follows that e. g. T 1 relaxation rates must be sensitive indicators of the molecular size too.

Most common experiments • PGSE-STE (STimulated Echo) • PGSE-LED ( Longitudional Eddy current Delay) • BPP-LED (Bipolar Pulse Pairs)

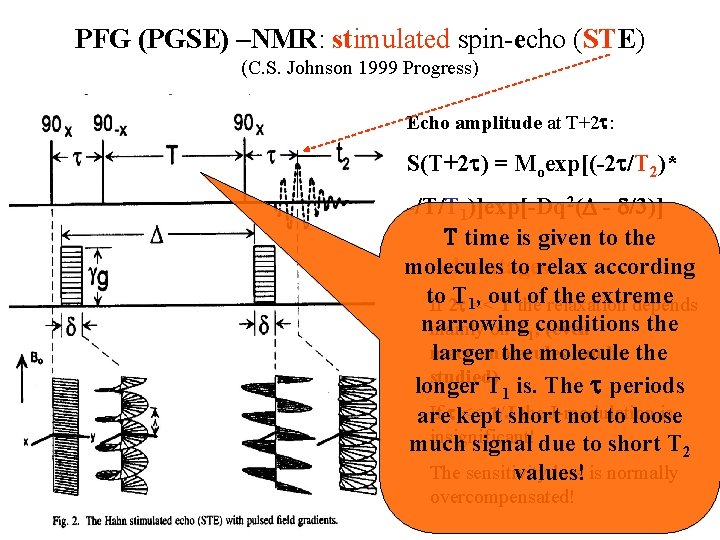
PFG (PGSE) –NMR: stimulated spin-echo (STE) (C. S. Johnson 1999 Progress) Echo amplitude at T+2 t: S(T+2 t) = Moexp[(-2 t/T 2)* -/T/T 1)]exp[-Dq 2(D - d/3)] T time is given to the molecules to relax according Advantages: to T 1<< , out ofrelaxation the extreme If 2 t T the depends narrowing the mainly on T 1, conditions (even macromolecules can be the larger the molecule studied) longer T 1 is. The t periods If tkept << 1/Jshort the J-modulation is are not to loose insignificant! much signal due to short T 2 The sensitivity loss is normally values! overcompensated!

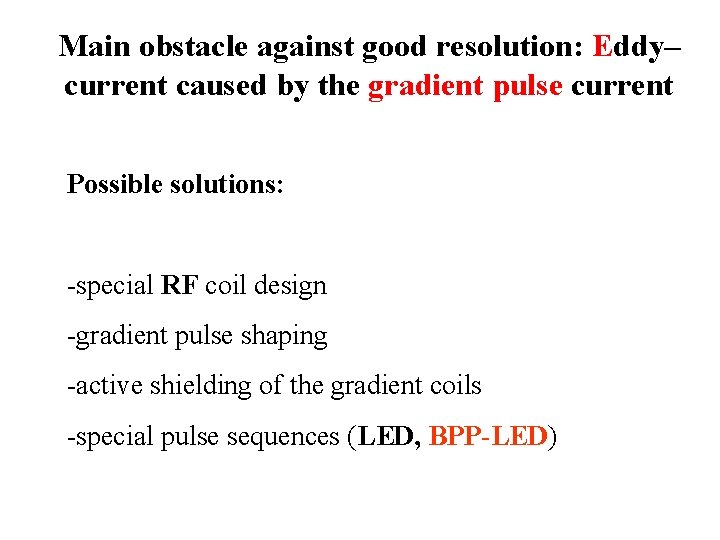
Main obstacle against good resolution: Eddy– current caused by the gradient pulse current Possible solutions: -special RF coil design -gradient pulse shaping -active shielding of the gradient coils -special pulse sequences (LED, BPP-LED)

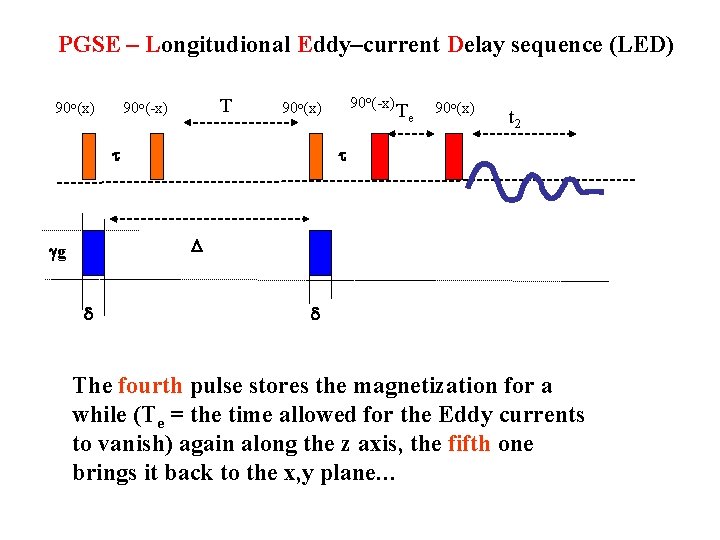
PGSE – Longitudional Eddy–current Delay sequence (LED) 90 o(x) T 90 o(-x) 90 o(x) t Te 90 o(x) t 2 t D gg d d The fourth pulse stores the magnetization for a while (Te = the time allowed for the Eddy currents to vanish) again along the z axis, the fifth one brings it back to the x, y plane…

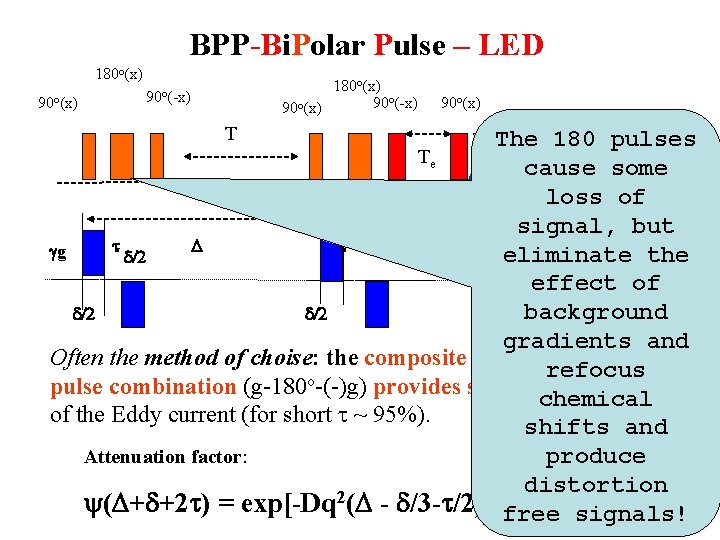
BPP-Bi. Polar Pulse – LED 180 o(x) 90 o(x) 180 o(x) 90 o(-x) T 90 o(x) t 2 The 180 pulses Te cause some loss of signal, but t D t gg eliminate the d/2 effect of background d/2 gradients and Often the method of choise: the composite bipolarrefocus gradient pulse combination (g-180 o-(-)g) provides self-compensation chemical of the Eddy current (for short t ~ 95%). shifts and Attenuation factor: produce distortion 2 y(D+d+2 t) = exp[-Dq (D - d/3 -t/2)] free signals!

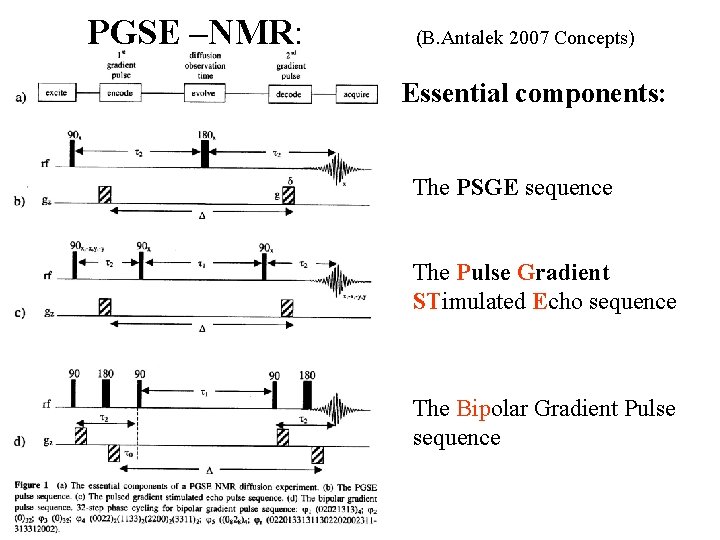
PGSE –NMR: (B. Antalek 2007 Concepts) Essential components: The PSGE sequence The Pulse Gradient STimulated Echo sequence The Bipolar Gradient Pulse sequence

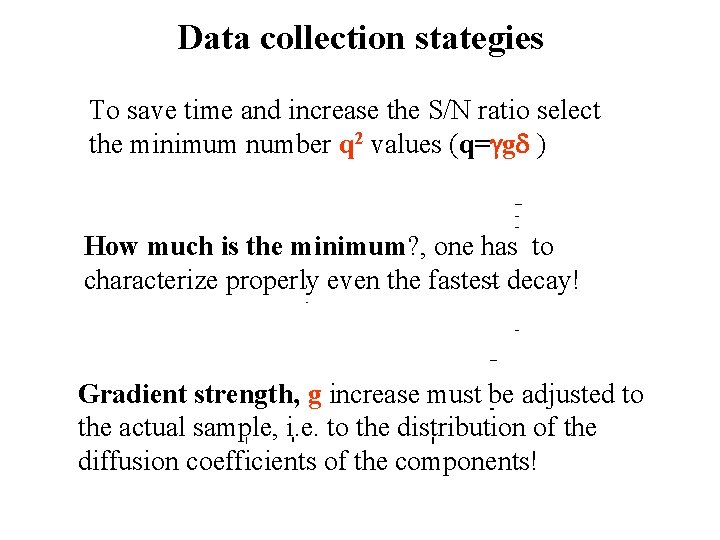
Data collection stategies To save time and increase the S/N ratio select the minimum number q 2 values (q=ggd ) How much is the minimum? , one has to characterize properly even the fastest decay! Gradient strength, g increase must be adjusted to the actual sample, i. e. to the distribution of the diffusion coefficients of the components!

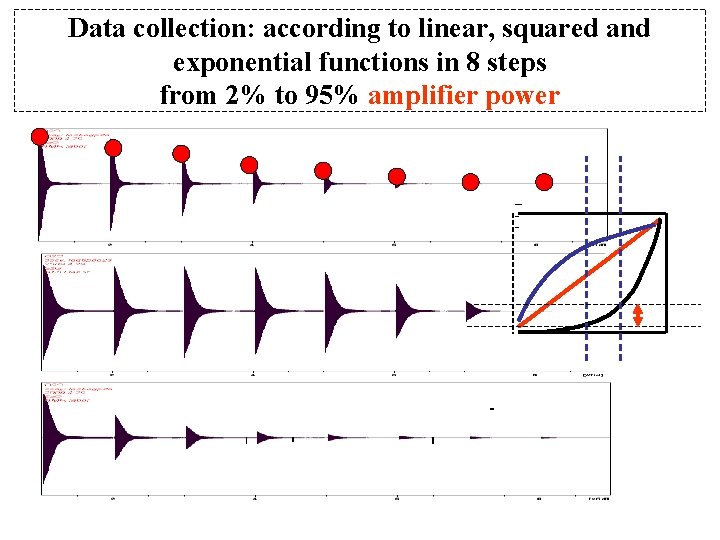
Data collection: according to linear, squared and exponential functions in 8 steps from 2% to 95% amplifier power

Applications: separation of mixture based on the components diffusion constants (Diffusion Ordered Spectroscopy) • DOSY: not much more then a convenient 2 D data processing and displaying method (Johnson 1996) • In comparison with P(F)GSE it can separate components of a mixture along the diffusion dimension if their translational diffusion differ.

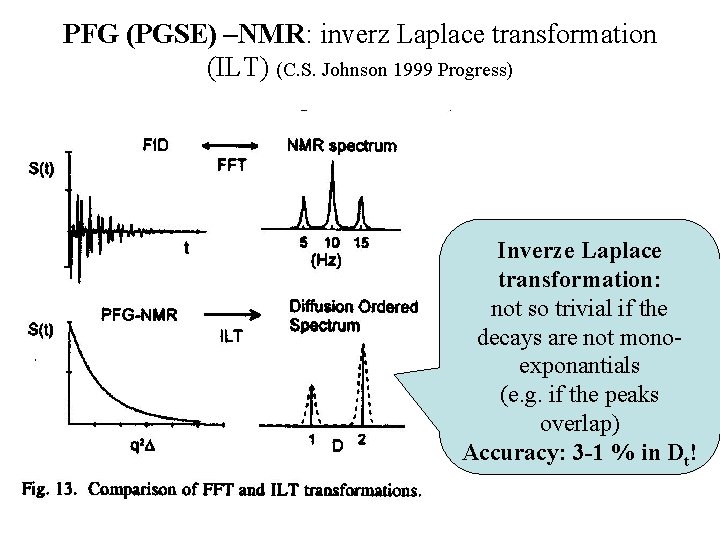
PFG (PGSE) –NMR: inverz Laplace transformation (ILT) (C. S. Johnson 1999 Progress) Inverze Laplace transformation: not so trivial if the decays are not monoexponantials (e. g. if the peaks overlap) Accuracy: 3 -1 % in Dt!

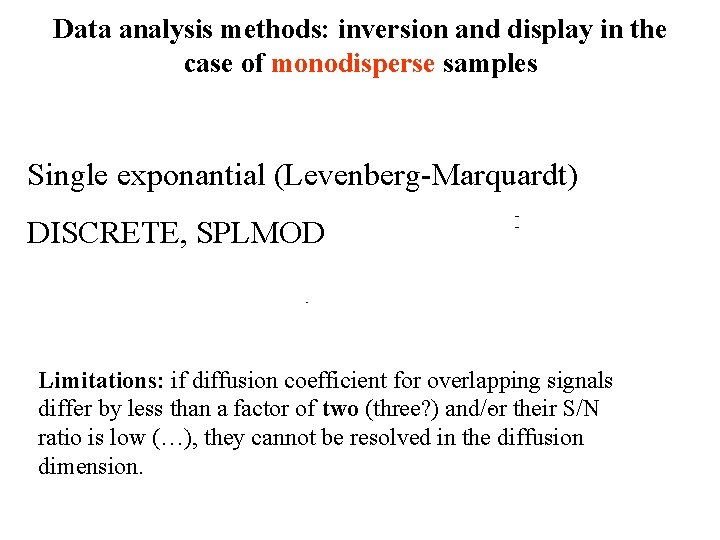
Data analysis methods: inversion and display in the case of monodisperse samples Single exponantial (Levenberg-Marquardt) DISCRETE, SPLMOD Limitations: if diffusion coefficient for overlapping signals differ by less than a factor of two (three? ) and/or their S/N ratio is low (…), they cannot be resolved in the diffusion dimension.

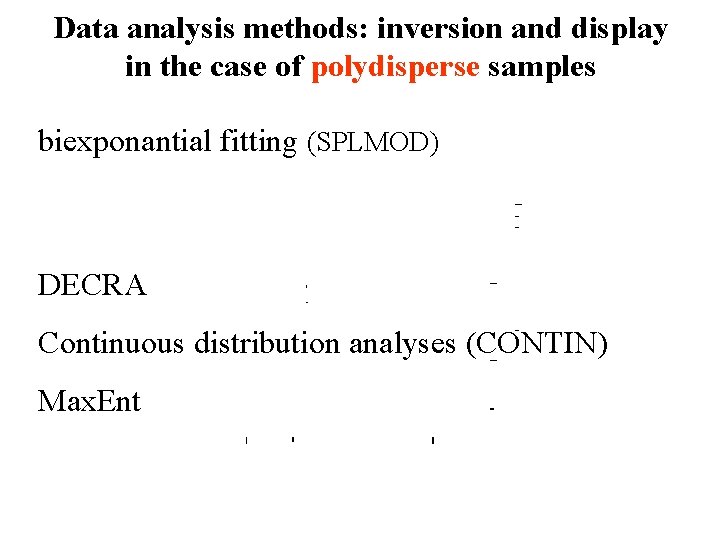
Data analysis methods: inversion and display in the case of polydisperse samples biexponantial fitting (SPLMOD) DECRA Continuous distribution analyses (CONTIN) Max. Ent

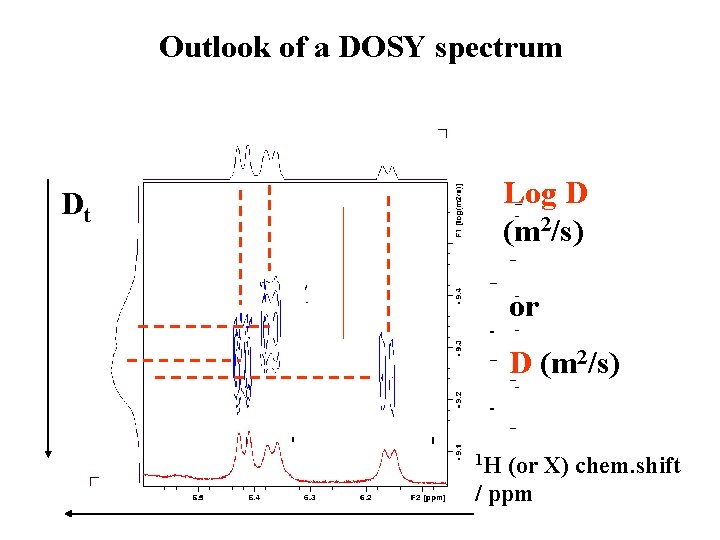
Outlook of a DOSY spectrum Log D (m 2/s) Dt or D (m 2/s) 1 H (or X) chem. shift / ppm

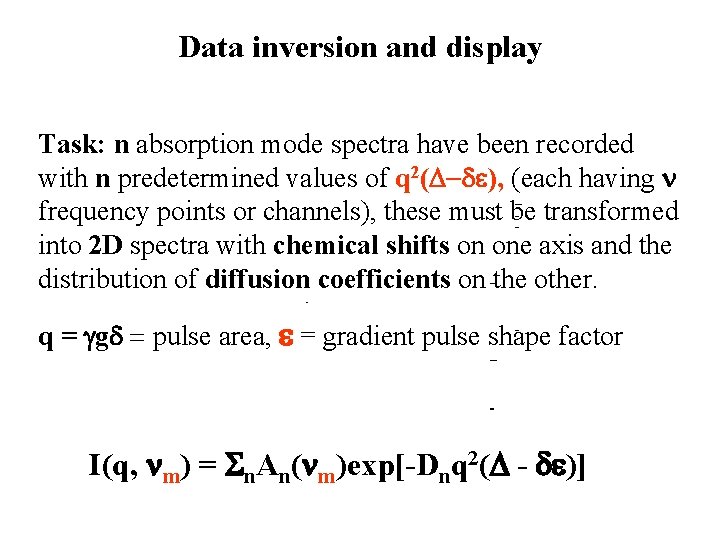
Data inversion and display Task: n absorption mode spectra have been recorded with n predetermined values of q 2(D-de), (each having n frequency points or channels), these must be transformed into 2 D spectra with chemical shifts on one axis and the distribution of diffusion coefficients on the other. q = ggd = pulse area, e = gradient pulse shape factor I(q, nm) = Sn. An(nm)exp[-Dnq 2(D - de)]

The stronger the gradient Ideal conditions: basic requirements Higher the use is thefields largerormolecules • • • of other nuclei suchlonger as This means much can be considered, but 19 F, 31 13 13 or Eddy C will help. C, acq. for e. g. also. Ptime the current lot! may be isa it proportionally signalseffectbut worthwhile!! larger! Complete separation of Good signal to noise ratio Strong and linear Bo pulse gradients Low constant background gradient Low internal magnetic field gradient caused by susceptibility changes over the sample • No heat convection in the sample ! • Low to medium solvent viscosity (? )

Artifacts and pitfalls (Ref. : Aksnes MRC 40 (2002) S 139) • • • Calibration of the gradient strength Eddy current effects Constant background gradients Unwanted flow within the sample Correction of effective diffusion times

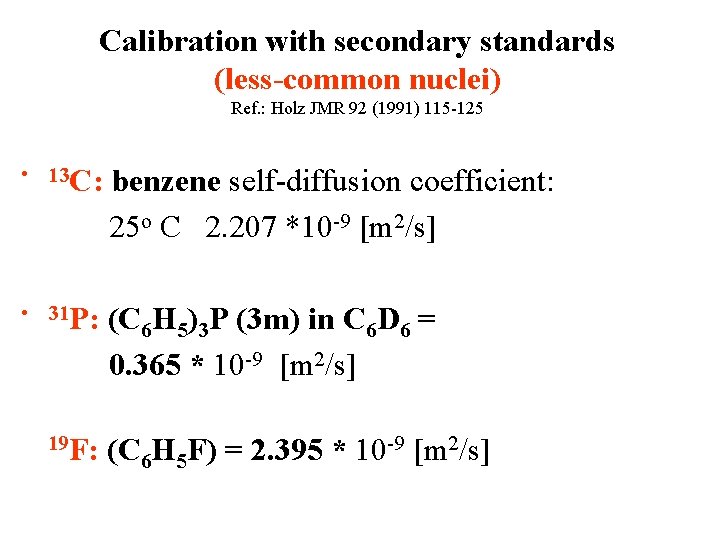
Calibration of the gradient strength Ref. : Holz JMR 92 (1991) 115 -125 • • The usual high. We need absolute values in G/cm or T/m resolution Gradient coil factor = gradient spectrometers can calibration constant? produce gradients of about G/cm what Shape factor 50 -60 = int(shape)/int(rectang. ) iscalibration sufficient to Direct with secondary standards analyze molecules up to 50 k. Ga.

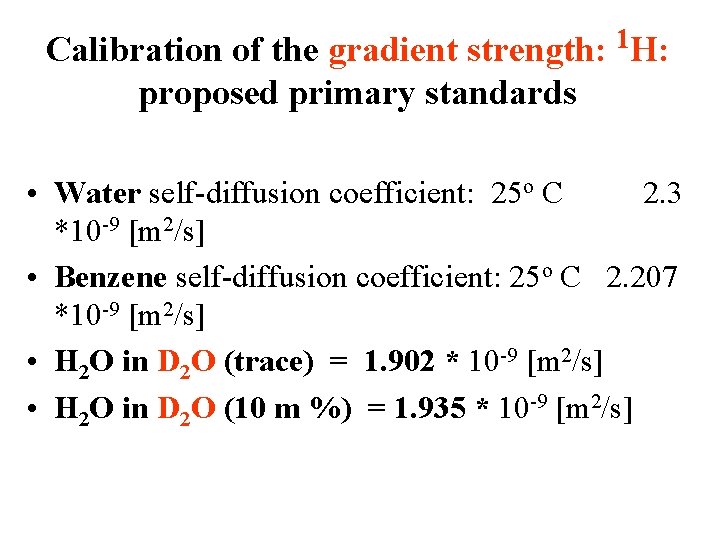
Calibration of the gradient strength: proposed primary standards 1 H: • Water self-diffusion coefficient: 25 o C 2. 3 *10 -9 [m 2/s] • Benzene self-diffusion coefficient: 25 o C 2. 207 *10 -9 [m 2/s] • H 2 O in D 2 O (trace) = 1. 902 * 10 -9 [m 2/s] • H 2 O in D 2 O (10 m %) = 1. 935 * 10 -9 [m 2/s]

Calibration with secondary standards (less-common nuclei) Ref. : Holz JMR 92 (1991) 115 -125 • 13 C: benzene self-diffusion coefficient: 25 o C 2. 207 *10 -9 [m 2/s] • 31 P: (C 6 H 5)3 P (3 m) in C 6 D 6 = 0. 365 * 10 -9 [m 2/s] 19 F: (C 6 H 5 F) = 2. 395 * 10 -9 [m 2/s]
![Applications selfassemblies Pdbifosz N N1 2 3 4 5 6 tectons 1 Applications: self-assemblies [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4, 5, 6 tectons: 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-33.jpg)
Applications: self-assemblies [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4, 5, 6 tectons: 1 H DOSY 2+ ; [Pd(dppp)] L 1 + + + 1: 1 2 a + 3 a 4 a 5 a 2: 1 + 3 aa + 4 aa + 5 aa 6 aa
![Applications PddpppN N1 2 3 4 5 tectons pyridine ortho protons 1 Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: pyridine ortho protons, 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-34.jpg)
Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: pyridine ortho protons, 1 H DOSY in CD 2 Cl 2 Dt H H 1 H chem. shift/ppm
![Applications PddpppN N1 2 3 4 5 tectons acenaphthane ortho protons 1 Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: acenaphthane ortho protons 1](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-35.jpg)
Applications: [(Pd(dppp)(N …. . N)]1, 2, 3, 4, 5 tectons: acenaphthane ortho protons 1 H DOSY in CD 2 Cl 2 Dt H H H 1 H chem. shift/ppm
![Alkalmazások önszerveződő rendszerek Pdbifosz N N1 2 3 4 tektonok 1 H Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 1 H](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-36.jpg)
Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 1 H DOSY

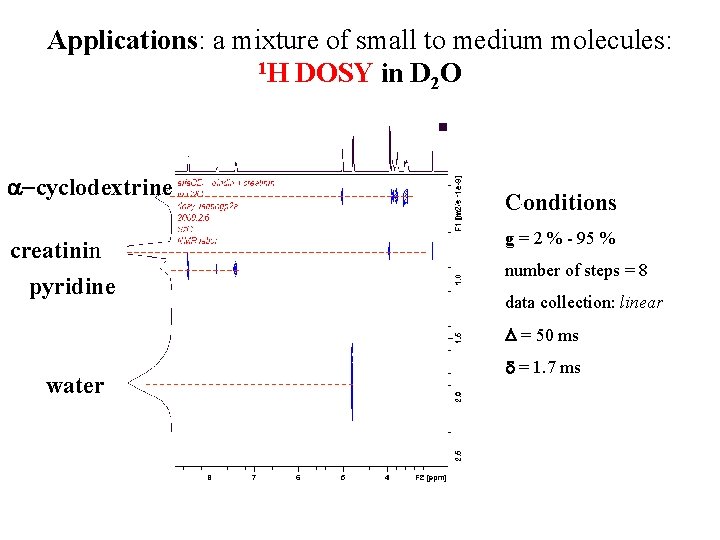
Applications: a mixture of small to medium molecules: 1 H DOSY in D O 2 a-cyclodextrine creatinin pyridine Conditions g = 2 % - 95 % number of steps = 8 data collection: linear D = 50 ms water d = 1. 7 ms
![Alkalmazások INEPTDOSY Pdbifosz N N1 2 3 4 tektonok 31 P DOSY Alkalmazások: INEPT-DOSY [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 31 P DOSY](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-38.jpg)
Alkalmazások: INEPT-DOSY [(Pd(bifosz. )(N …. . N)]1, 2, 3, 4 tektonok: 31 P DOSY
![Alkalmazások önszerveződő rendszerek Pdbifosz 4 4bpy1 2 3 4 tektonok 31 P DOSY Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(4, 4’-bpy…)]1, 2, 3, 4 tektonok: 31 P DOSY](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-39.jpg)
Alkalmazások: önszerveződő rendszerek [(Pd(bifosz. )(4, 4’-bpy…)]1, 2, 3, 4 tektonok: 31 P DOSY
![Alkalmazások önszerveződő rendszerek Pddppp4 4bpy2 3 4 5 tektonok 1 H DOSY 1 2 Alkalmazások: önszerveződő rendszerek [(Pd(dppp)(4, 4’-bpy…)]2, 3, 4, 5 tektonok: 1 H DOSY 1 2](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-40.jpg)
Alkalmazások: önszerveződő rendszerek [(Pd(dppp)(4, 4’-bpy…)]2, 3, 4, 5 tektonok: 1 H DOSY 1 2 7 6 8 5 4 3 N 9 N N N
![Alkalmazások ruthenium complexes 1 H DOSY dmso 298 K Rudiphenylphenantroline3 Dt 13010 12 Alkalmazások: ruthenium complexes 1 H DOSY dmso 298 K [Ru(diphenylphenantroline)3]+ Dt = 130*10 -12](https://slidetodoc.com/presentation_image/4fa200192ad2cb597f4e4300504bcc3f/image-41.jpg)
Alkalmazások: ruthenium complexes 1 H DOSY dmso 298 K [Ru(diphenylphenantroline)3]+ Dt = 130*10 -12 m 2/s [Ru(methylbpy)3]+ Dt = 150*10 -12 m 2/s [Ru(phenantroline)3]+ Dt = 165*10 -12 m 2/s [Ru(bpy)3]+ Dt = 180*10 -12 m 2/s

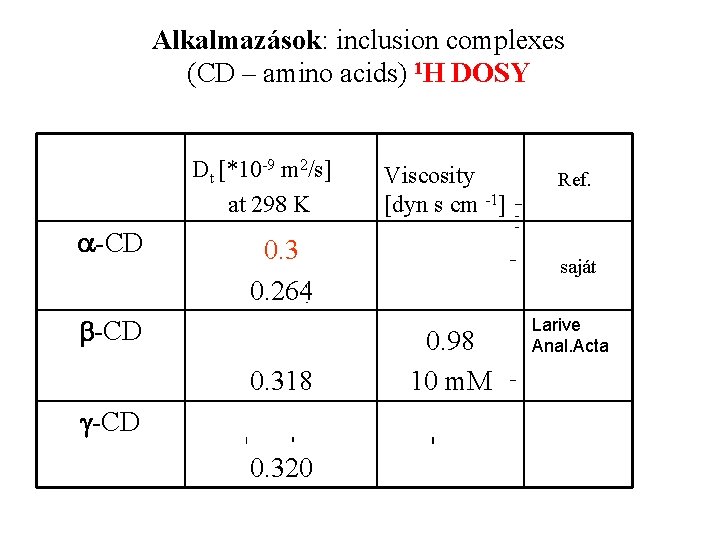
Alkalmazások: inclusion complexes (CD – amino acids) 1 H DOSY Dt [*10 -9 m 2/s] at 298 K a-CD Viscosity [dyn s cm -1] 0. 3 0. 264 b-CD 0. 318 g-CD 0. 320 Ref. saját 0. 98 10 m. M Larive Anal. Acta

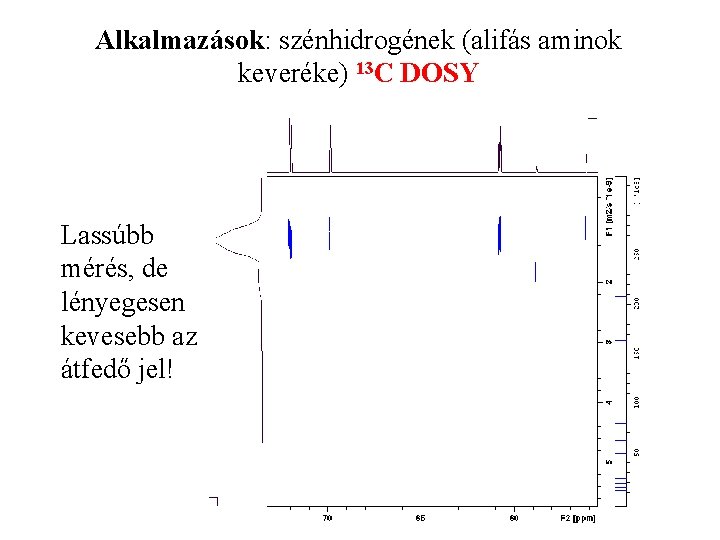
Alkalmazások: szénhidrogének (alifás aminok keveréke) 13 C DOSY Lassúbb mérés, de lényegesen kevesebb az átfedő jel!

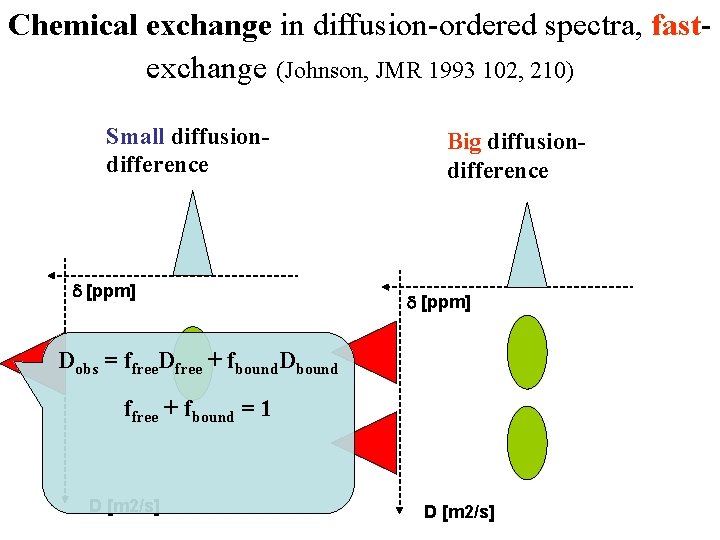
Chemical exchange in diffusion-ordered spectra, fastexchange (Johnson, JMR 1993 102, 210) Small diffusiondifference d [ppm] Big diffusiondifference d [ppm] Dobs = ffree. Dfree + fbound. Dbound ffree + fbound = 1 D [m 2/s]

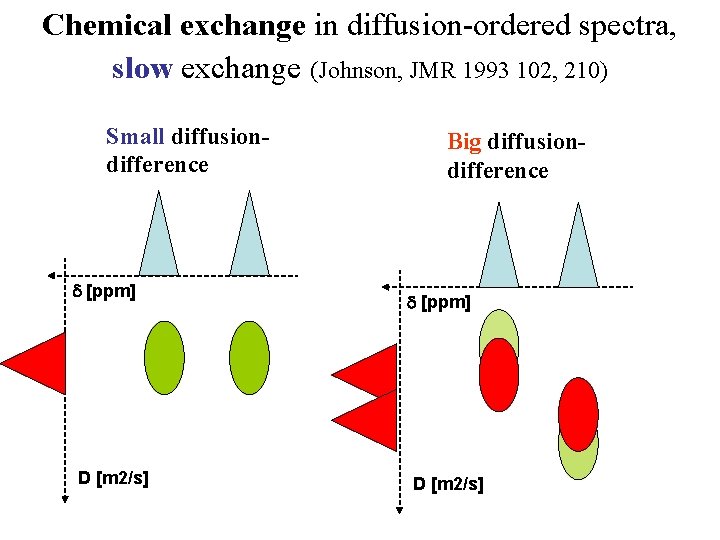
Chemical exchange in diffusion-ordered spectra, slow exchange (Johnson, JMR 1993 102, 210) Small diffusiondifference d [ppm] D [m 2/s] Big diffusiondifference d [ppm] D [m 2/s]
 St olaf nmr
St olaf nmr Nmr and esr
Nmr and esr Nmr lipoprofile
Nmr lipoprofile Alkyne carbon nmr
Alkyne carbon nmr Enantiotopic protons
Enantiotopic protons Gyromagnetic ratio of 1h
Gyromagnetic ratio of 1h Traº
Traº Nmr polymer
Nmr polymer Function of nmr
Function of nmr Susceptibility
Susceptibility Nmr spectrum of 1 1 2-tribromoethane
Nmr spectrum of 1 1 2-tribromoethane Ketone nmr
Ketone nmr Michael sattler nmr
Michael sattler nmr Gyromagnetic ratio
Gyromagnetic ratio St olaf nmr
St olaf nmr Nmr sample tube
Nmr sample tube Perch nmr software
Perch nmr software Nuts nmr
Nuts nmr Haddock nmr
Haddock nmr H nmr
H nmr Virstatin nmr
Virstatin nmr Sübstitüent nedir
Sübstitüent nedir Acetyl ferrocene nmr
Acetyl ferrocene nmr Nmr integration
Nmr integration Casper wu
Casper wu Nmr splitting patterns names
Nmr splitting patterns names Stała sprzężenia nmr
Stała sprzężenia nmr Nmr structure calculator
Nmr structure calculator Evans method magnetic susceptibility
Evans method magnetic susceptibility Carbon nmr chart
Carbon nmr chart Nmr lipoprofile
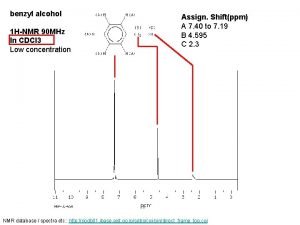
Nmr lipoprofile Benzyl alcohol h nmr
Benzyl alcohol h nmr Spektra nmr
Spektra nmr Mannose nmr
Mannose nmr Angle tip
Angle tip Grover algorithm
Grover algorithm Kostana modrica
Kostana modrica 1 bromopropane nmr
1 bromopropane nmr Cyclohexane nmr splitting
Cyclohexane nmr splitting Hydrohalogenation mechanism
Hydrohalogenation mechanism Advantages of nmr spectroscopy
Advantages of nmr spectroscopy Integration in nmr
Integration in nmr Tabella nmr
Tabella nmr Doublet triplet quartet
Doublet triplet quartet Multiplisitas nmr adalah
Multiplisitas nmr adalah Ch3cb
Ch3cb