NMR and Xray Structures Comparison of NMR and
- Slides: 24

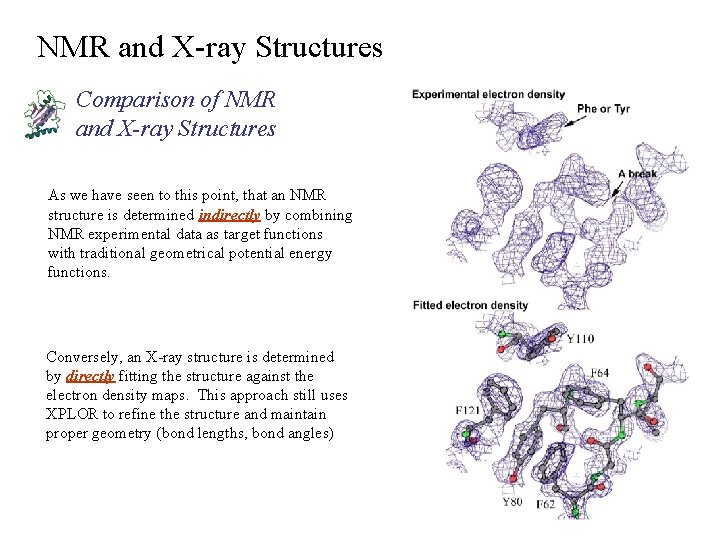
NMR and X-ray Structures Comparison of NMR and X-ray Structures As we have seen to this point, that an NMR structure is determined indirectly by combining NMR experimental data as target functions with traditional geometrical potential energy functions. Conversely, an X-ray structure is determined by directly fitting the structure against the electron density maps. This approach still uses XPLOR to refine the structure and maintain proper geometry (bond lengths, bond angles)

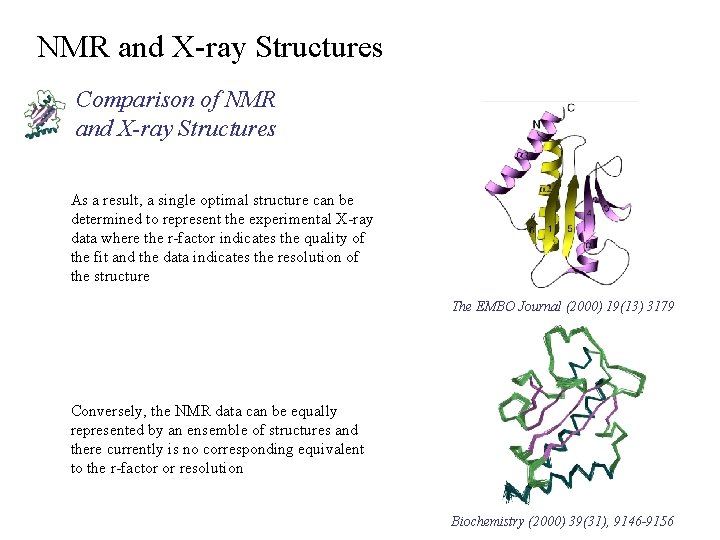
NMR and X-ray Structures Comparison of NMR and X-ray Structures As a result, a single optimal structure can be determined to represent the experimental X-ray data where the r-factor indicates the quality of the fit and the data indicates the resolution of the structure The EMBO Journal (2000) 19(13) 3179 Conversely, the NMR data can be equally represented by an ensemble of structures and there currently is no corresponding equivalent to the r-factor or resolution Biochemistry (2000) 39(31), 9146 -9156

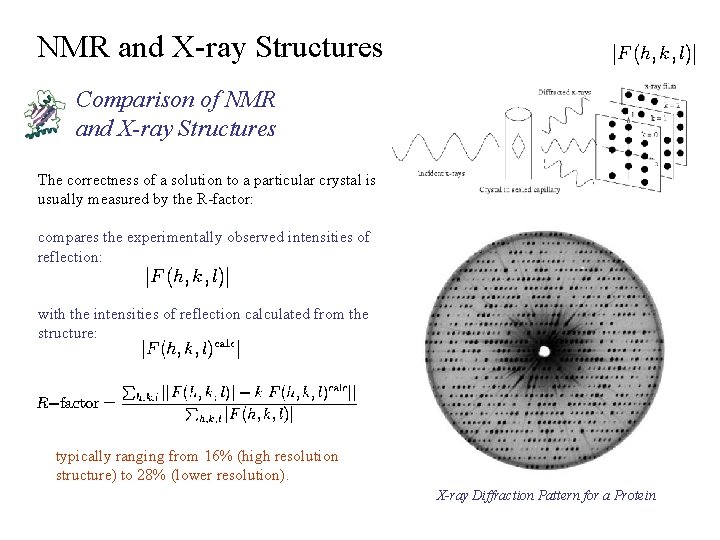
NMR and X-ray Structures Comparison of NMR and X-ray Structures The correctness of a solution to a particular crystal is usually measured by the R-factor: compares the experimentally observed intensities of reflection: with the intensities of reflection calculated from the structure: typically ranging from 16% (high resolution structure) to 28% (lower resolution). X-ray Diffraction Pattern for a Protein

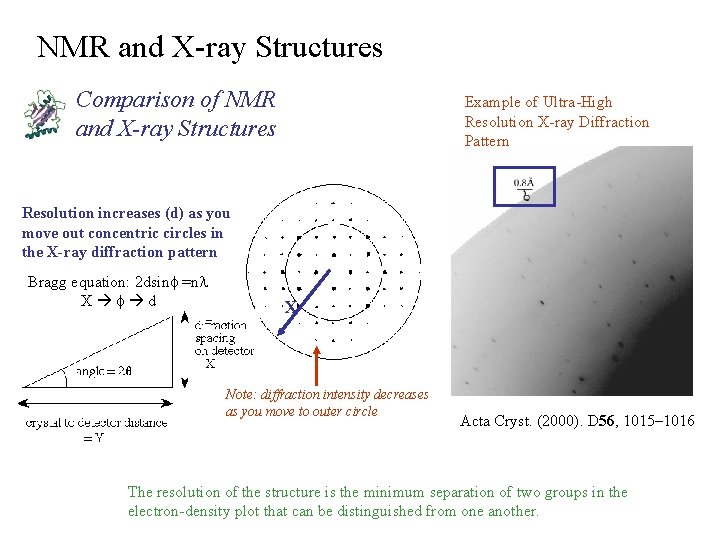
NMR and X-ray Structures Comparison of NMR and X-ray Structures Example of Ultra-High Resolution X-ray Diffraction Pattern Resolution increases (d) as you move out concentric circles in the X-ray diffraction pattern Bragg equation: 2 dsinf =nl X f d X Note: diffraction intensity decreases as you move to outer circle Acta Cryst. (2000). D 56, 1015– 1016 The resolution of the structure is the minimum separation of two groups in the electron-density plot that can be distinguished from one another.

NMR and X-ray Structures Comparison of NMR and X-ray Structures NMR and X-ray structures generally exhibit the same fold Local differences may be attributed to: 1) dynamics 2) crystal-packing interactions 3) solid vs. solution state - solvent is present in crystals - lowest energy conformer in crystal? 4) Resolution/experimental error Nevertheless, there are some examples where distinct functional differences are observed between the NMR and X-ray structures Protein Science (1996). 5: 2391 -2398.

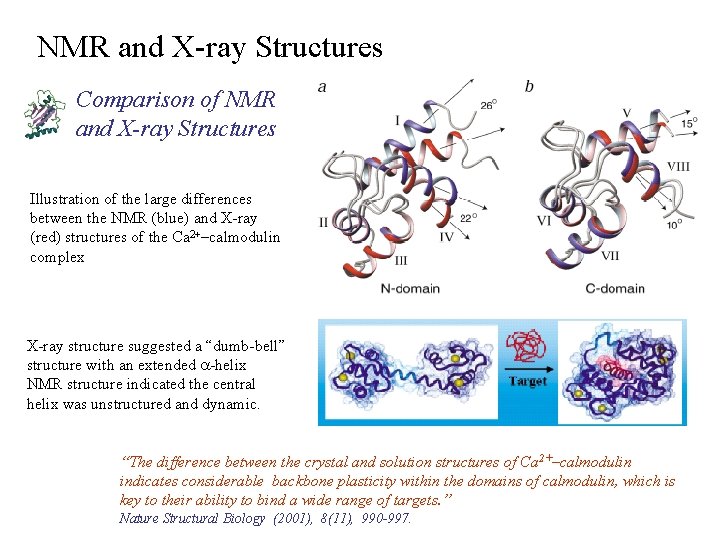
NMR and X-ray Structures Comparison of NMR and X-ray Structures Illustration of the large differences between the NMR (blue) and X-ray (red) structures of the Ca 2+–calmodulin complex X-ray structure suggested a “dumb-bell” structure with an extended a-helix NMR structure indicated the central helix was unstructured and dynamic. “The difference between the crystal and solution structures of Ca 2+–calmodulin indicates considerable backbone plasticity within the domains of calmodulin, which is key to their ability to bind a wide range of targets. ” Nature Structural Biology (2001), 8(11), 990 -997.

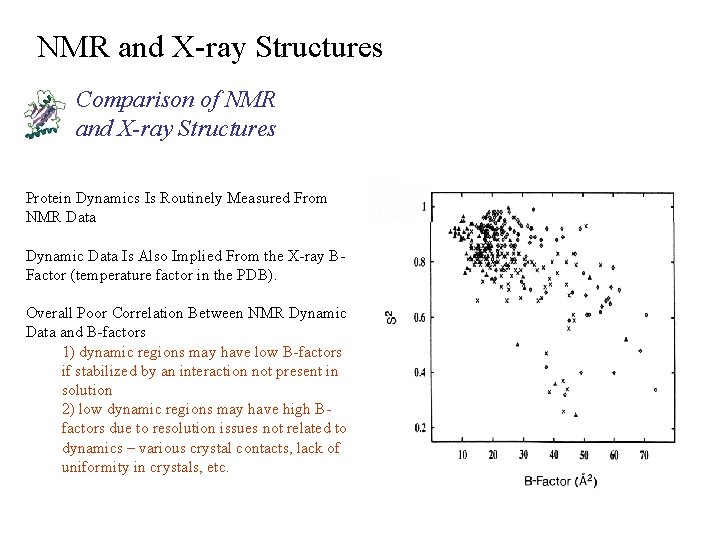
NMR and X-ray Structures Comparison of NMR and X-ray Structures Protein Dynamics Is Routinely Measured From NMR Data Dynamic Data Is Also Implied From the X-ray BFactor (temperature factor in the PDB). Overall Poor Correlation Between NMR Dynamic Data and B-factors 1) dynamic regions may have low B-factors if stabilized by an interaction not present in solution 2) low dynamic regions may have high Bfactors due to resolution issues not related to dynamics – various crystal contacts, lack of uniformity in crystals, etc.

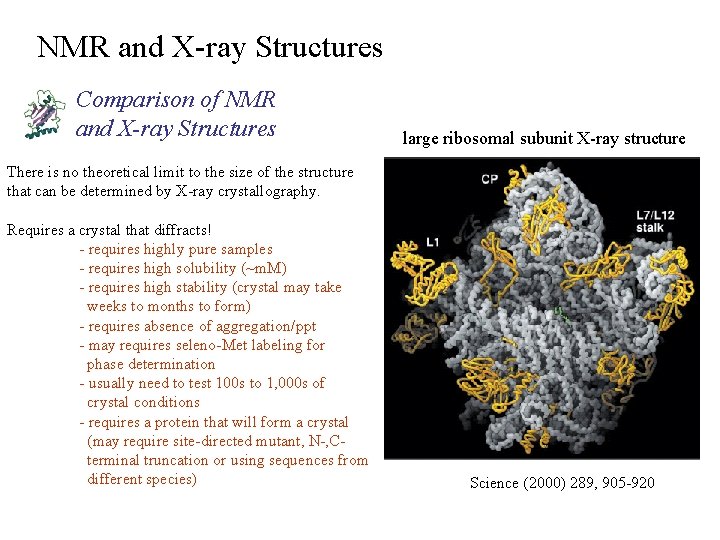
NMR and X-ray Structures Comparison of NMR and X-ray Structures large ribosomal subunit X-ray structure There is no theoretical limit to the size of the structure that can be determined by X-ray crystallography. Requires a crystal that diffracts! - requires highly pure samples - requires high solubility (~m. M) - requires high stability (crystal may take weeks to months to form) - requires absence of aggregation/ppt - may requires seleno-Met labeling for phase determination - usually need to test 100 s to 1, 000 s of crystal conditions - requires a protein that will form a crystal (may require site-directed mutant, N-, C terminal truncation or using sequences from different species) Science (2000) 289, 905 -920

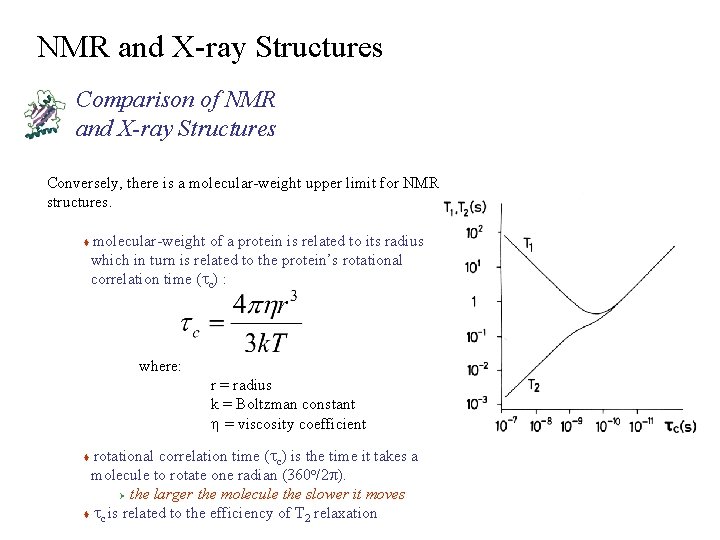
NMR and X-ray Structures Comparison of NMR and X-ray Structures Conversely, there is a molecular-weight upper limit for NMR structures. molecular-weight of a protein is related to its radius which in turn is related to the protein’s rotational correlation time (tc) : t where: r = radius k = Boltzman constant h = viscosity coefficient rotational correlation time (tc) is the time it takes a molecule to rotate one radian (360 o/2 p). Ø the larger the molecule the slower it moves t tc is related to the efficiency of T 2 relaxation t

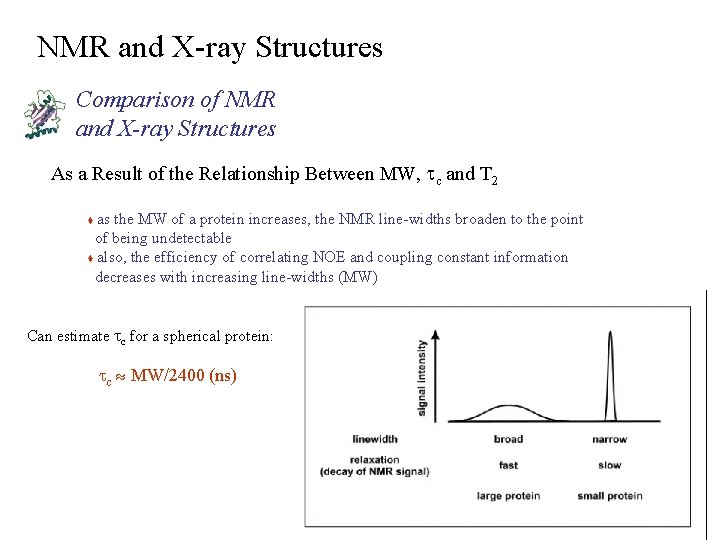
NMR and X-ray Structures Comparison of NMR and X-ray Structures As a Result of the Relationship Between MW, tc and T 2 as the MW of a protein increases, the NMR line-widths broaden to the point of being undetectable t also, the efficiency of correlating NOE and coupling constant information decreases with increasing line-widths (MW) t Can estimate tc for a spherical protein: tc » MW/2400 (ns)

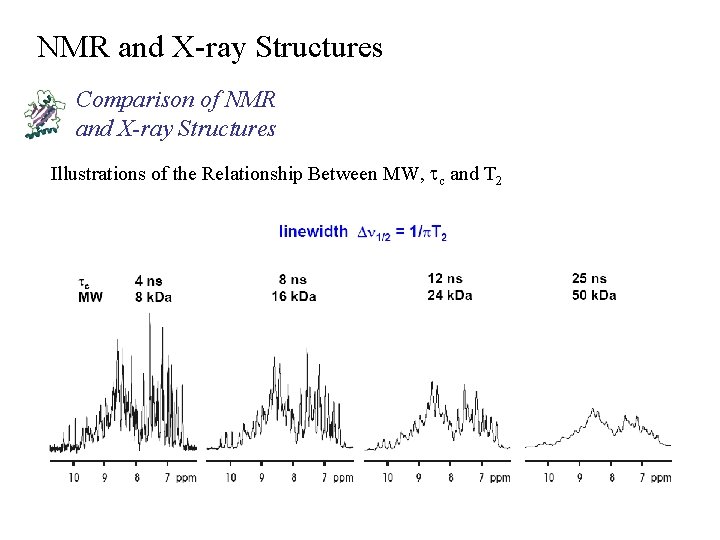
NMR and X-ray Structures Comparison of NMR and X-ray Structures Illustrations of the Relationship Between MW, tc and T 2

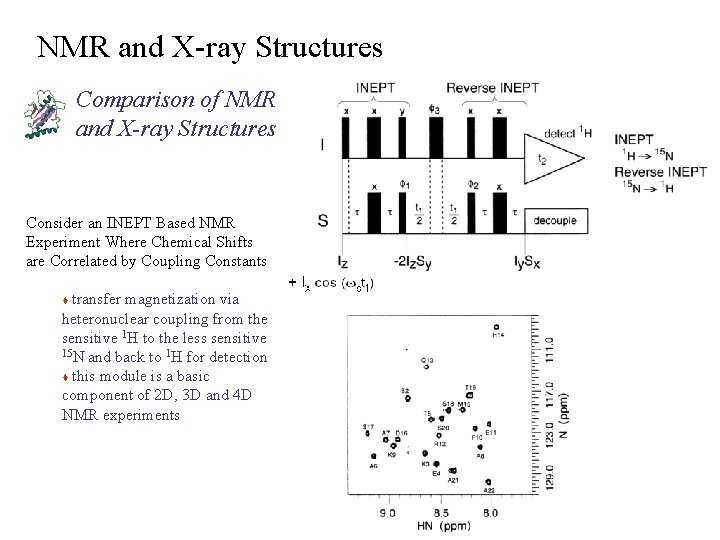
NMR and X-ray Structures Comparison of NMR and X-ray Structures Consider an INEPT Based NMR Experiment Where Chemical Shifts are Correlated by Coupling Constants transfer magnetization via heteronuclear coupling from the sensitive 1 H to the less sensitive 15 N and back to 1 H for detection t this module is a basic component of 2 D, 3 D and 4 D NMR experiments t

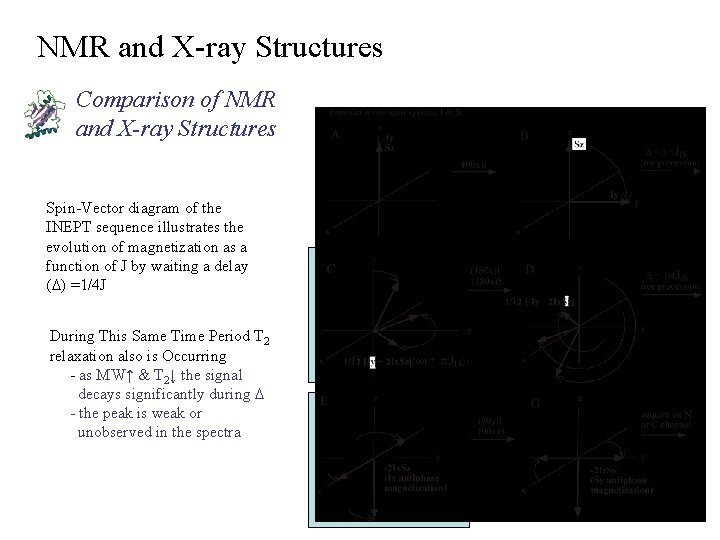
NMR and X-ray Structures Comparison of NMR and X-ray Structures Spin-Vector diagram of the INEPT sequence illustrates the evolution of magnetization as a function of J by waiting a delay (D) =1/4 J During This Same Time Period T 2 relaxation also is Occurring - as MW↑ & T 2↓ the signal decays significantly during D - the peak is weak or unobserved in the spectra

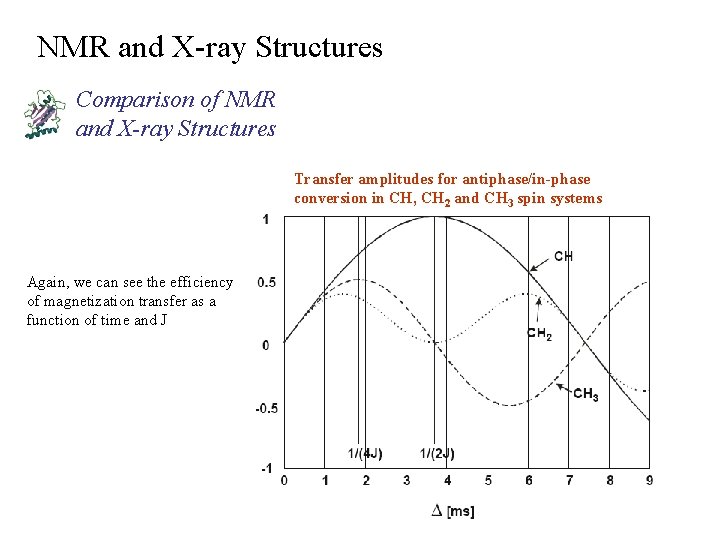
NMR and X-ray Structures Comparison of NMR and X-ray Structures Transfer amplitudes for antiphase/in-phase conversion in CH, CH 2 and CH 3 spin systems Again, we can see the efficiency of magnetization transfer as a function of time and J

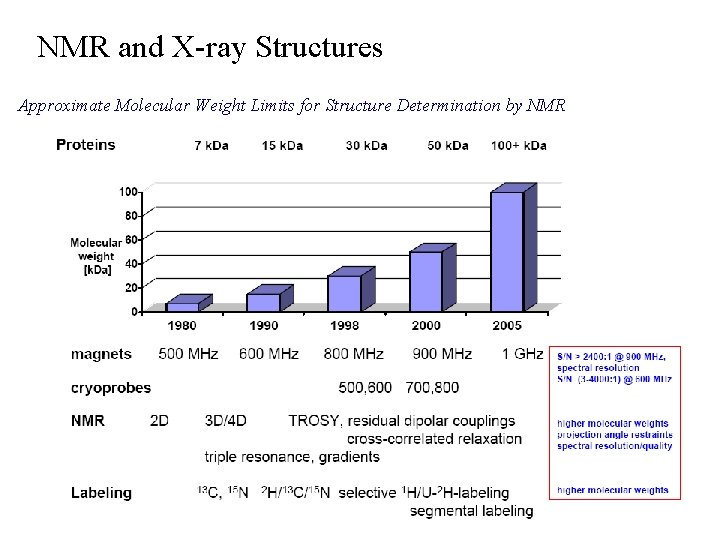
NMR and X-ray Structures Approximate Molecular Weight Limits for Structure Determination by NMR

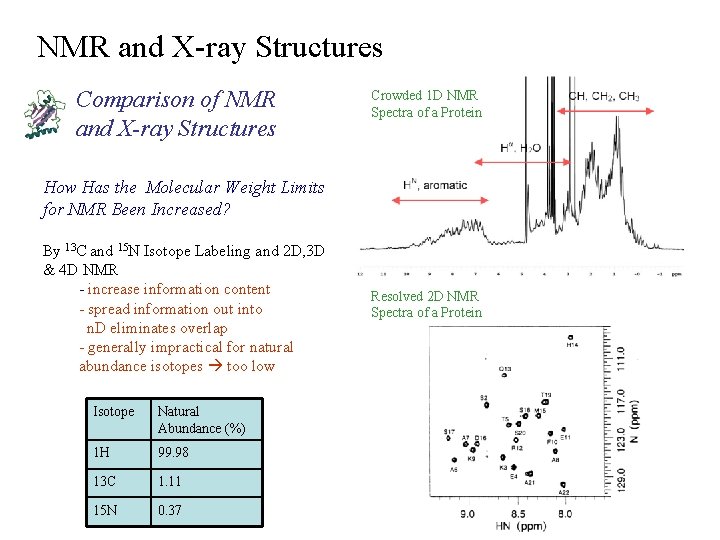
NMR and X-ray Structures Comparison of NMR and X-ray Structures Crowded 1 D NMR Spectra of a Protein How Has the Molecular Weight Limits for NMR Been Increased? By 13 C and 15 N Isotope Labeling and 2 D, 3 D & 4 D NMR - increase information content - spread information out into n. D eliminates overlap - generally impractical for natural abundance isotopes too low Isotope Natural Abundance (%) 1 H 99. 98 13 C 1. 11 15 N 0. 37 Resolved 2 D NMR Spectra of a Protein

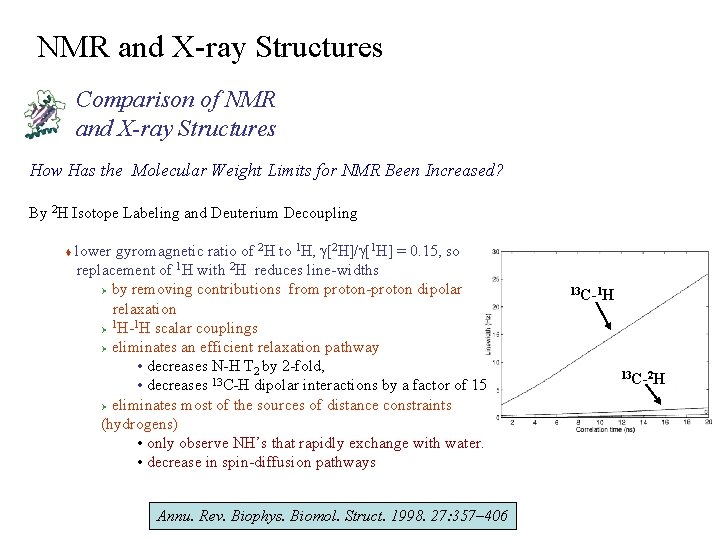
NMR and X-ray Structures Comparison of NMR and X-ray Structures How Has the Molecular Weight Limits for NMR Been Increased? By 2 H Isotope Labeling and Deuterium Decoupling lower gyromagnetic ratio of 2 H to 1 H, g[2 H]/g[1 H] = 0. 15, so replacement of 1 H with 2 H reduces line-widths Ø by removing contributions from proton-proton dipolar relaxation 1 1 Ø H- H scalar couplings Ø eliminates an efficient relaxation pathway • decreases N-H T 2 by 2 -fold, • decreases 13 C-H dipolar interactions by a factor of 15 Ø eliminates most of the sources of distance constraints (hydrogens) • only observe NH’s that rapidly exchange with water. • decrease in spin-diffusion pathways t Annu. Rev. Biophys. Biomol. Struct. 1998. 27: 357– 406 13 C-1 H 13 C-2 H

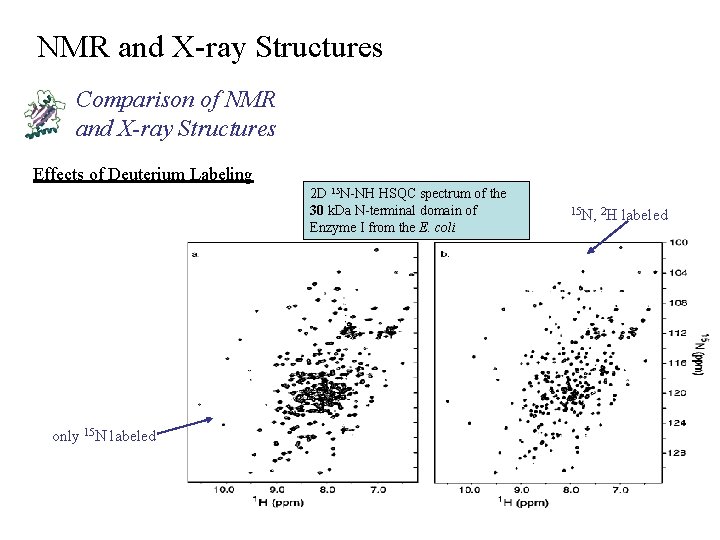
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of Deuterium Labeling 2 D 15 N-NH HSQC spectrum of the 30 k. Da N-terminal domain of Enzyme I from the E. coli only 15 N labeled 15 N, 2 H labeled

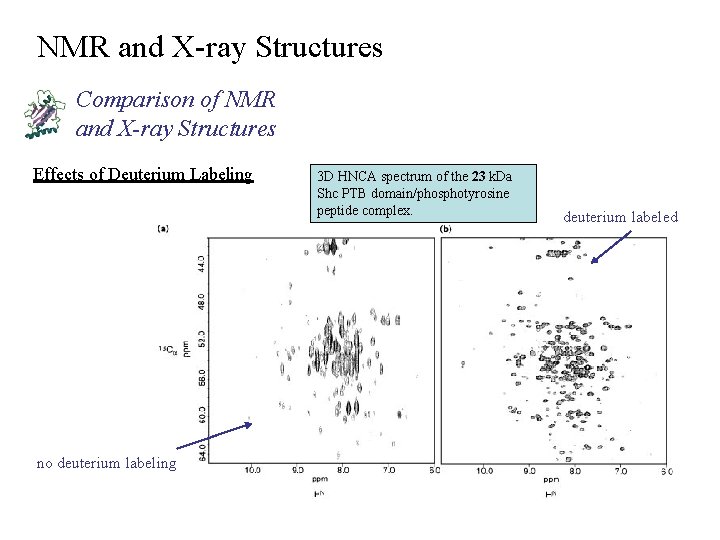
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of Deuterium Labeling no deuterium labeling 3 D HNCA spectrum of the 23 k. Da Shc PTB domain/phosphotyrosine peptide complex. deuterium labeled

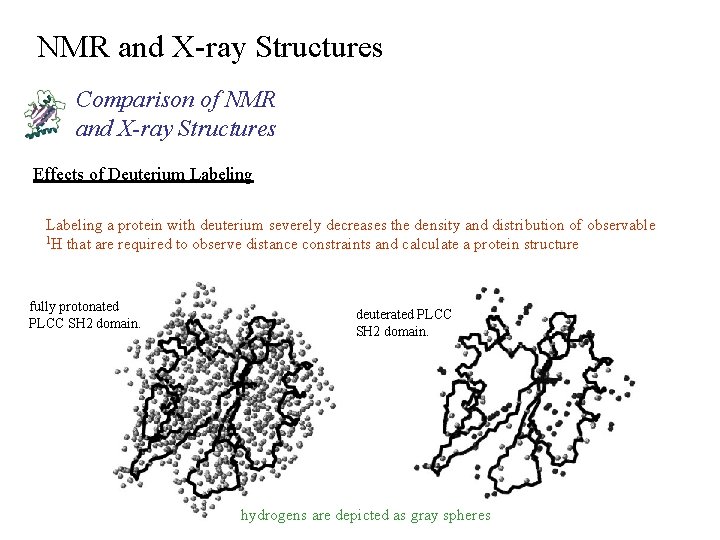
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of Deuterium Labeling a protein with deuterium severely decreases the density and distribution of observable 1 H that are required to observe distance constraints and calculate a protein structure fully protonated PLCC SH 2 domain. deuterated PLCC SH 2 domain. hydrogens are depicted as gray spheres

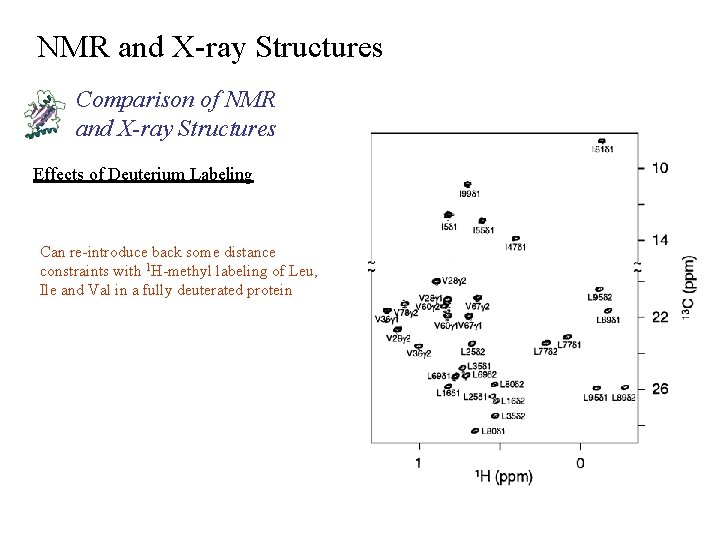
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of Deuterium Labeling Can re-introduce back some distance constraints with 1 H-methyl labeling of Leu, Ile and Val in a fully deuterated protein

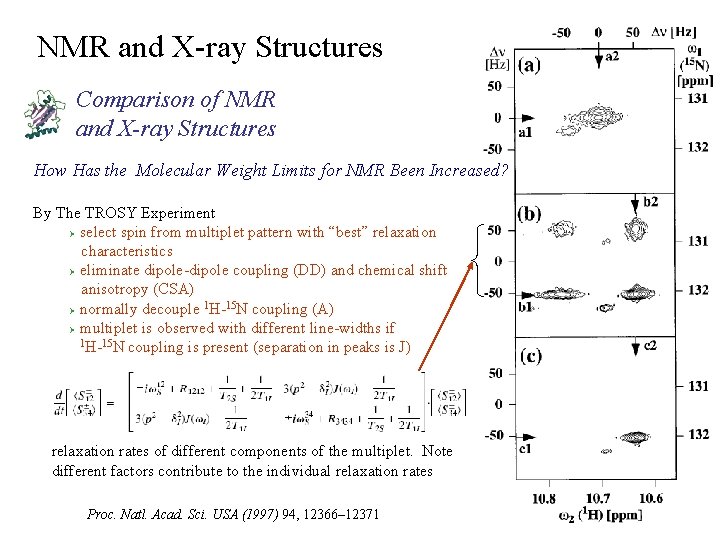
NMR and X-ray Structures Comparison of NMR and X-ray Structures How Has the Molecular Weight Limits for NMR Been Increased? By The TROSY Experiment Ø select spin from multiplet pattern with “best” relaxation characteristics Ø eliminate dipole-dipole coupling (DD) and chemical shift anisotropy (CSA) 1 15 Ø normally decouple H- N coupling (A) Ø multiplet is observed with different line-widths if 1 H-15 N coupling is present (separation in peaks is J) relaxation rates of different components of the multiplet. Note different factors contribute to the individual relaxation rates Proc. Natl. Acad. Sci. USA (1997) 94, 12366– 12371

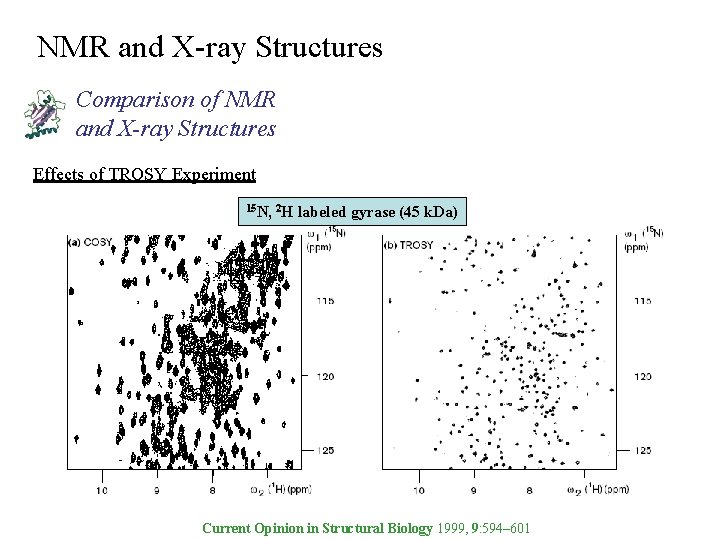
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of TROSY Experiment 15 N, 2 H labeled gyrase (45 k. Da) Current Opinion in Structural Biology 1999, 9: 594– 601

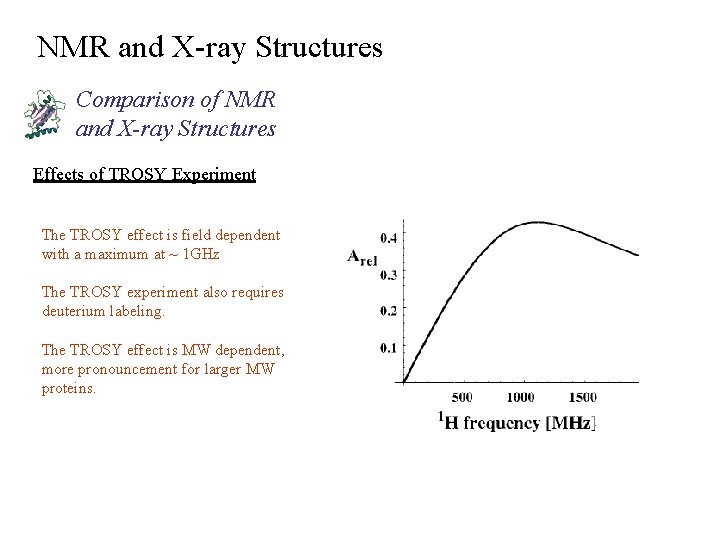
NMR and X-ray Structures Comparison of NMR and X-ray Structures Effects of TROSY Experiment The TROSY effect is field dependent with a maximum at ~ 1 GHz The TROSY experiment also requires deuterium labeling. The TROSY effect is MW dependent, more pronouncement for larger MW proteins.