16 12 Chemical Shifts in 13 C NMR



- Slides: 40

16. 12 Chemical Shifts in 13 C NMR Spectra • Compared to 1 H, 13 C atoms require a different frequency of energy to excite (resonate) • Compared to the standard TMS, 13 C NMR signals generally appear between 220 and 0 ppm • Each signal on the 13 C spectra represents a carbon with a unique electronic environment • Planes and axes of symmetry can cause carbon signals to overlap if their electronic surroundings are equivalent Copyright 2012 John Wiley & Sons, Inc. 1 Klein, Organic Chemistry 2 e

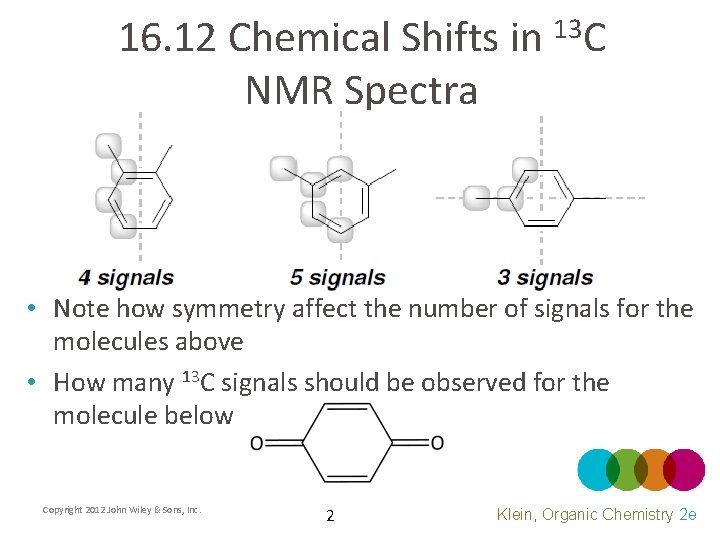
16. 12 Chemical Shifts in 13 C NMR Spectra • Note how symmetry affect the number of signals for the molecules above • How many 13 C signals should be observed for the molecule below Copyright 2012 John Wiley & Sons, Inc. 2 Klein, Organic Chemistry 2 e

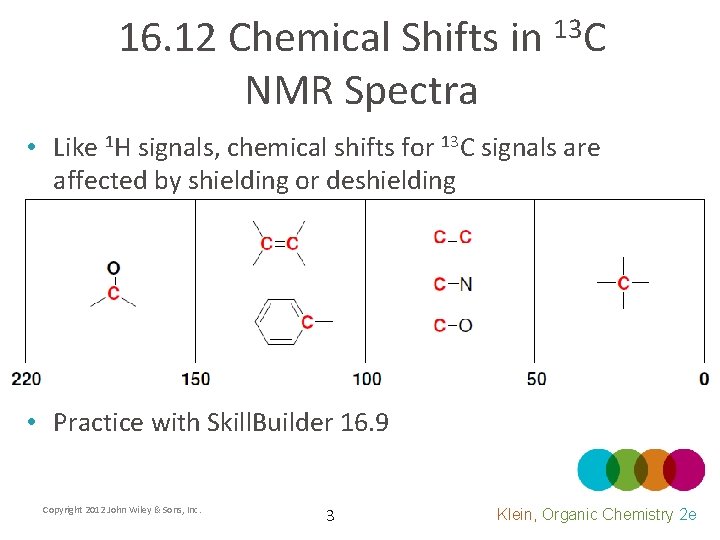
16. 12 Chemical Shifts in 13 C NMR Spectra • Like 1 H signals, chemical shifts for 13 C signals are affected by shielding or deshielding • Practice with Skill. Builder 16. 9 Copyright 2012 John Wiley & Sons, Inc. 3 Klein, Organic Chemistry 2 e

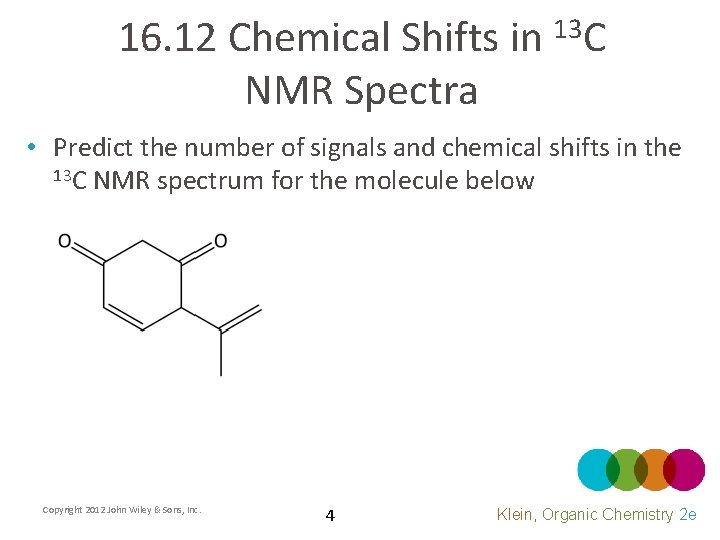
16. 12 Chemical Shifts in 13 C NMR Spectra • Predict the number of signals and chemical shifts in the 13 C NMR spectrum for the molecule below Copyright 2012 John Wiley & Sons, Inc. 4 Klein, Organic Chemistry 2 e

16. 13 DEPT • • • 13 C NMR Spectra 13 C spectra generally give singlets that do not provide information about the number of hydrogen atoms attached to each carbon Distortionless Enhancement by Polarization Transfer (DEPT) 13 C NMR provides information the number of hydrogen atoms attached to each carbon Full decoupled 13 C spectrum: shows all carbon peaks DEPT-90: Only CH signals appear DEPT-135: CH 3 and CH give (+) signals, and CH 2 give (-) signals Copyright 2012 John Wiley & Sons, Inc. 5 Klein, Organic Chemistry 2 e

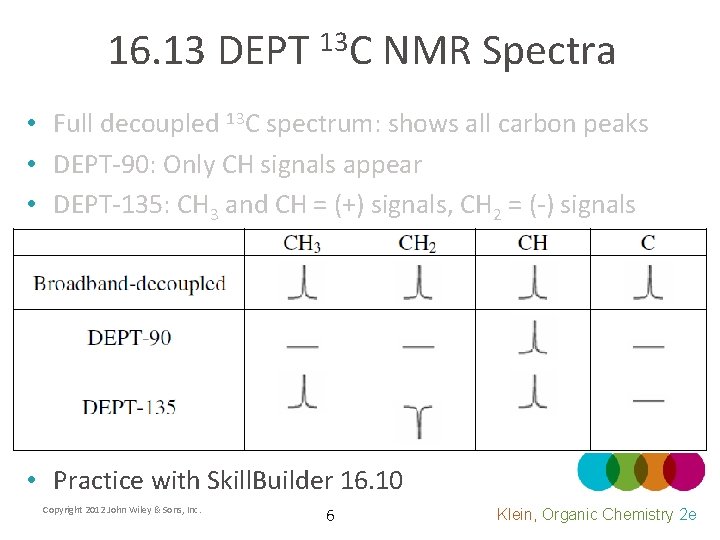
16. 13 DEPT 13 C NMR Spectra • Full decoupled 13 C spectrum: shows all carbon peaks • DEPT-90: Only CH signals appear • DEPT-135: CH 3 and CH = (+) signals, CH 2 = (-) signals • Practice with Skill. Builder 16. 10 Copyright 2012 John Wiley & Sons, Inc. 6 Klein, Organic Chemistry 2 e

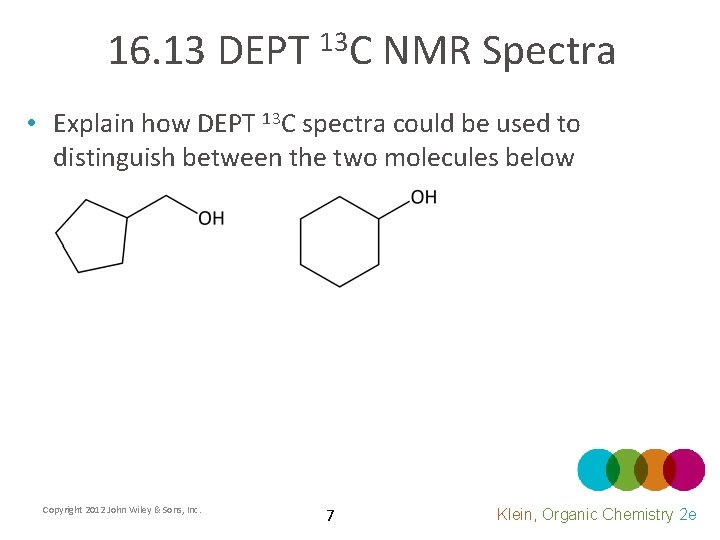
16. 13 DEPT 13 C NMR Spectra • Explain how DEPT 13 C spectra could be used to distinguish between the two molecules below Copyright 2012 John Wiley & Sons, Inc. 7 Klein, Organic Chemistry 2 e

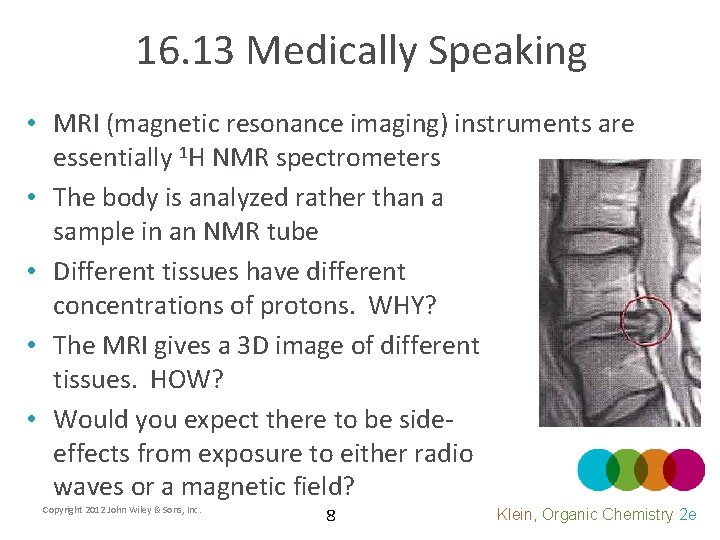
16. 13 Medically Speaking • MRI (magnetic resonance imaging) instruments are essentially 1 H NMR spectrometers • The body is analyzed rather than a sample in an NMR tube • Different tissues have different concentrations of protons. WHY? • The MRI gives a 3 D image of different tissues. HOW? • Would you expect there to be sideeffects from exposure to either radio waves or a magnetic field? Copyright 2012 John Wiley & Sons, Inc. 8 Klein, Organic Chemistry 2 e

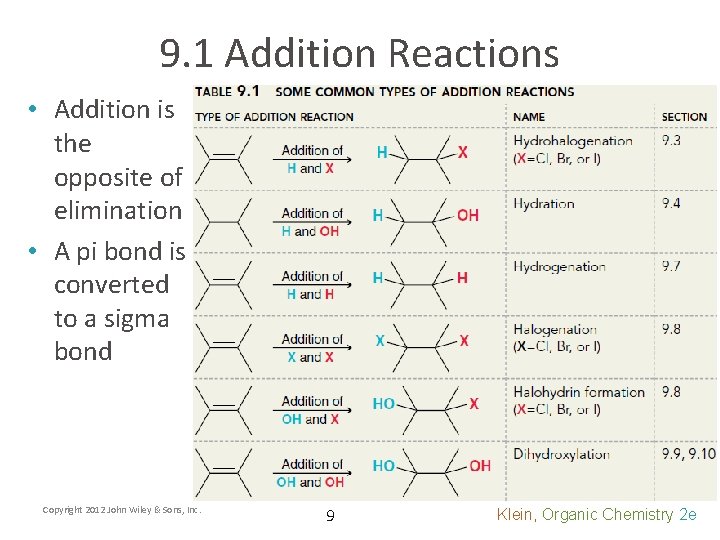
9. 1 Addition Reactions • Addition is the opposite of elimination • A pi bond is converted to a sigma bond Copyright 2012 John Wiley & Sons, Inc. 9 Klein, Organic Chemistry 2 e

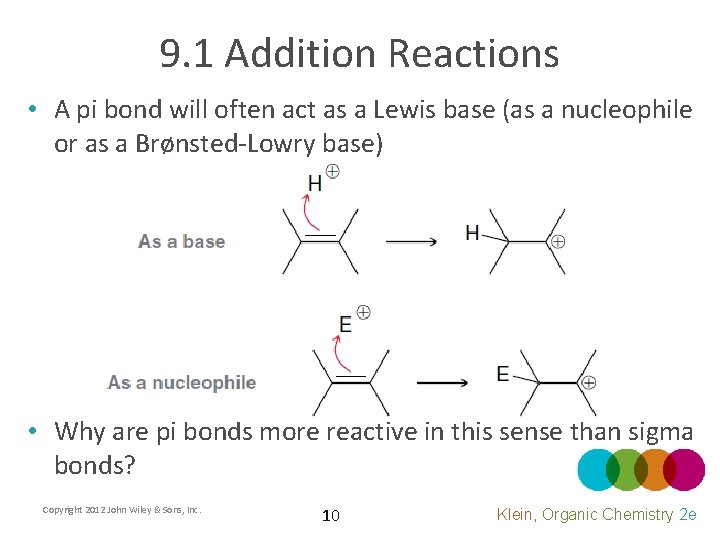
9. 1 Addition Reactions • A pi bond will often act as a Lewis base (as a nucleophile or as a Brønsted-Lowry base) • Why are pi bonds more reactive in this sense than sigma bonds? Copyright 2012 John Wiley & Sons, Inc. 10 Klein, Organic Chemistry 2 e

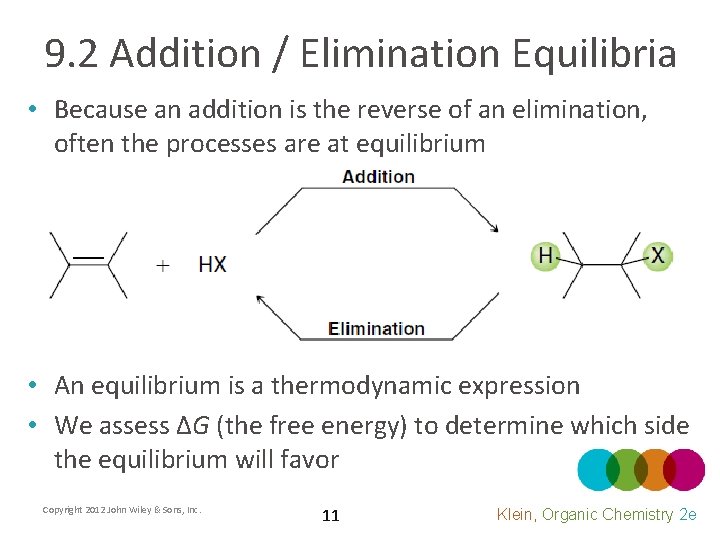
9. 2 Addition / Elimination Equilibria • Because an addition is the reverse of an elimination, often the processes are at equilibrium • An equilibrium is a thermodynamic expression • We assess ΔG (the free energy) to determine which side the equilibrium will favor Copyright 2012 John Wiley & Sons, Inc. 11 Klein, Organic Chemistry 2 e

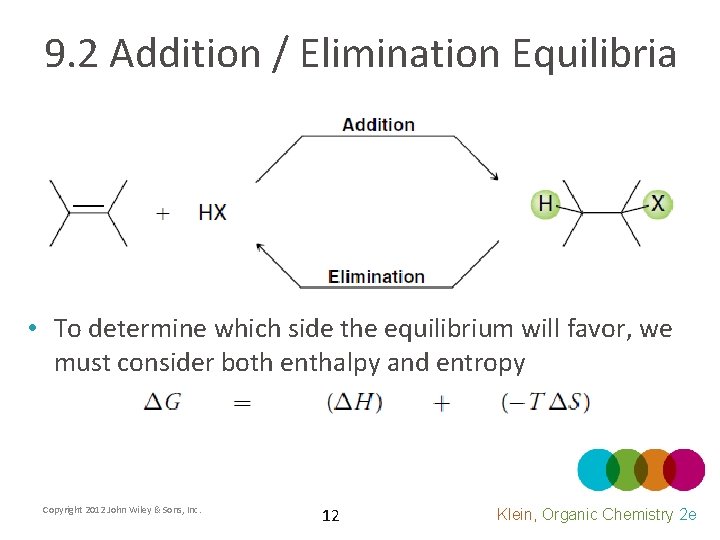
9. 2 Addition / Elimination Equilibria • To determine which side the equilibrium will favor, we must consider both enthalpy and entropy Copyright 2012 John Wiley & Sons, Inc. 12 Klein, Organic Chemistry 2 e

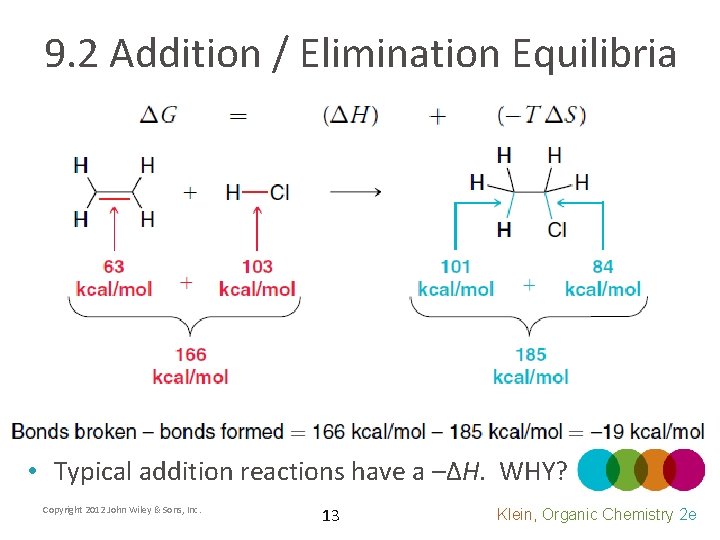
9. 2 Addition / Elimination Equilibria • Typical addition reactions have a –ΔH. WHY? Copyright 2012 John Wiley & Sons, Inc. 13 Klein, Organic Chemistry 2 e

9. 2 Addition / Elimination Equilibria • Typical addition reactions have a –ΔH • Will heat be absorbed by or released into the surroundings? • What will the sign (+/-) be for ΔSsurr? • Will the enthalpy term favor the reactants or products? • The heat change (ΔH) will remain roughly constant regardless of temperature Copyright 2012 John Wiley & Sons, Inc. 14 Klein, Organic Chemistry 2 e

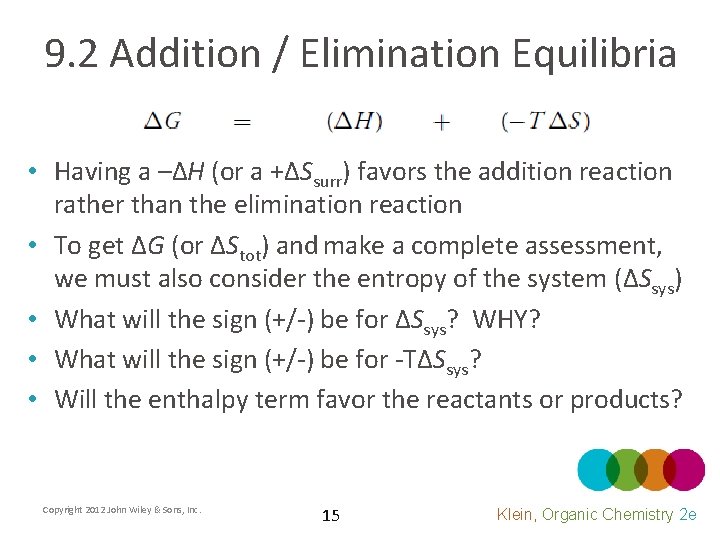
9. 2 Addition / Elimination Equilibria • Having a –ΔH (or a +ΔSsurr) favors the addition reaction rather than the elimination reaction • To get ΔG (or ΔStot) and make a complete assessment, we must also consider the entropy of the system (ΔSsys) • What will the sign (+/-) be for ΔSsys? WHY? • What will the sign (+/-) be for -TΔSsys? • Will the enthalpy term favor the reactants or products? Copyright 2012 John Wiley & Sons, Inc. 15 Klein, Organic Chemistry 2 e

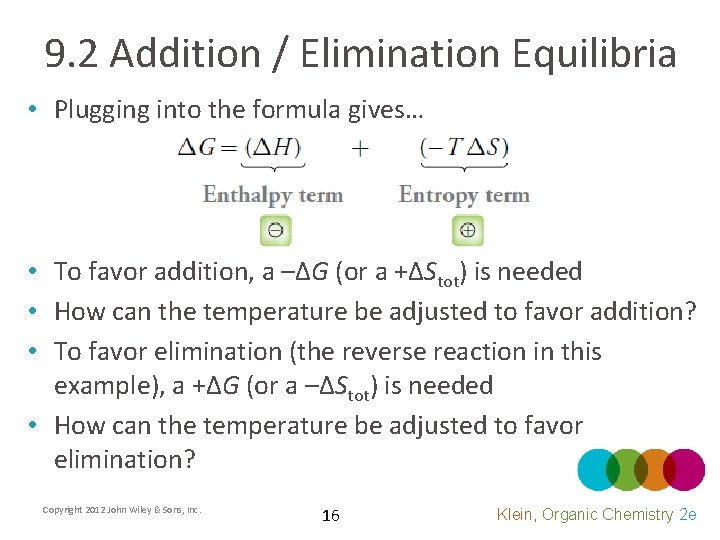
9. 2 Addition / Elimination Equilibria • Plugging into the formula gives… • To favor addition, a –ΔG (or a +ΔStot) is needed • How can the temperature be adjusted to favor addition? • To favor elimination (the reverse reaction in this example), a +ΔG (or a –ΔStot) is needed • How can the temperature be adjusted to favor elimination? Copyright 2012 John Wiley & Sons, Inc. 16 Klein, Organic Chemistry 2 e

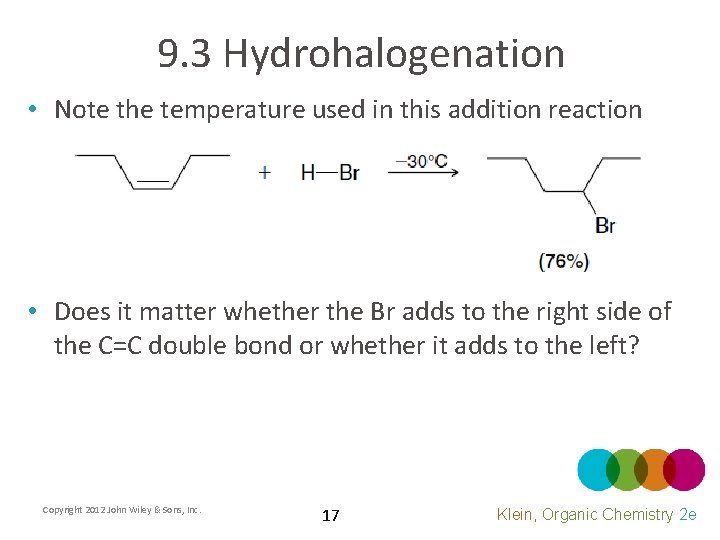
9. 3 Hydrohalogenation • Note the temperature used in this addition reaction • Does it matter whether the Br adds to the right side of the C=C double bond or whether it adds to the left? Copyright 2012 John Wiley & Sons, Inc. 17 Klein, Organic Chemistry 2 e

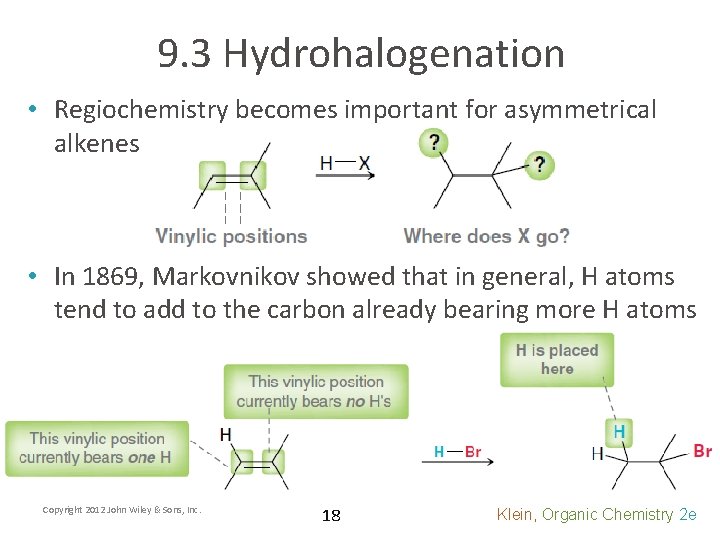
9. 3 Hydrohalogenation • Regiochemistry becomes important for asymmetrical alkenes • In 1869, Markovnikov showed that in general, H atoms tend to add to the carbon already bearing more H atoms Copyright 2012 John Wiley & Sons, Inc. 18 Klein, Organic Chemistry 2 e

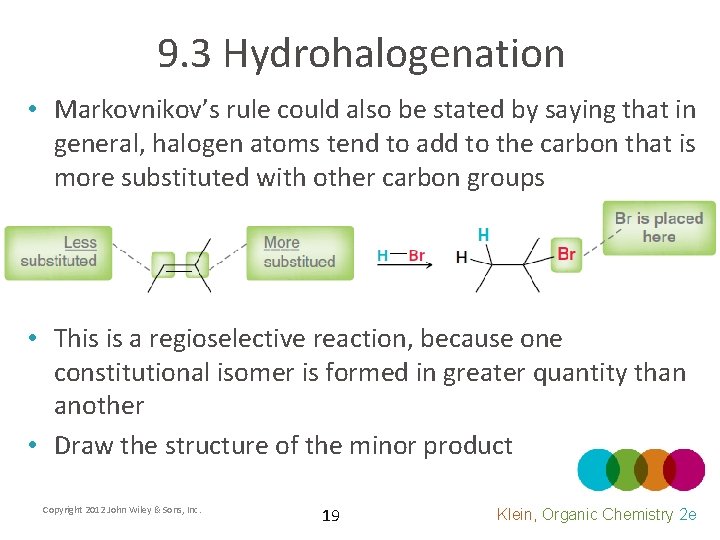
9. 3 Hydrohalogenation • Markovnikov’s rule could also be stated by saying that in general, halogen atoms tend to add to the carbon that is more substituted with other carbon groups • This is a regioselective reaction, because one constitutional isomer is formed in greater quantity than another • Draw the structure of the minor product Copyright 2012 John Wiley & Sons, Inc. 19 Klein, Organic Chemistry 2 e

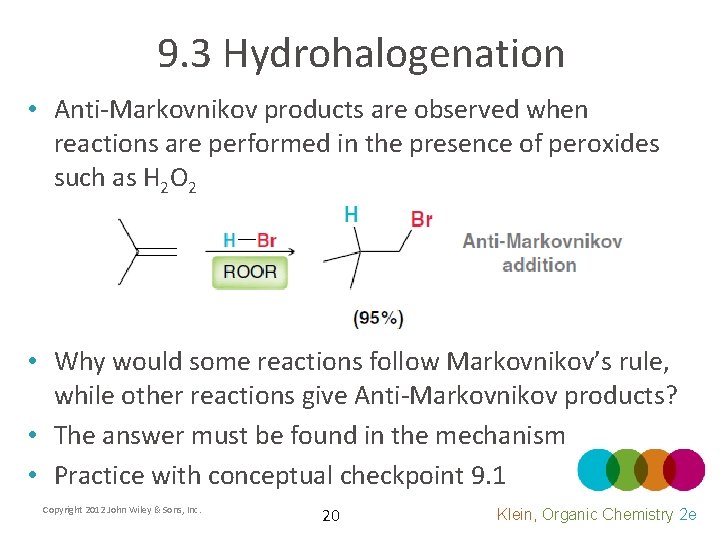
9. 3 Hydrohalogenation • Anti-Markovnikov products are observed when reactions are performed in the presence of peroxides such as H 2 O 2 • Why would some reactions follow Markovnikov’s rule, while other reactions give Anti-Markovnikov products? • The answer must be found in the mechanism • Practice with conceptual checkpoint 9. 1 Copyright 2012 John Wiley & Sons, Inc. 20 Klein, Organic Chemistry 2 e

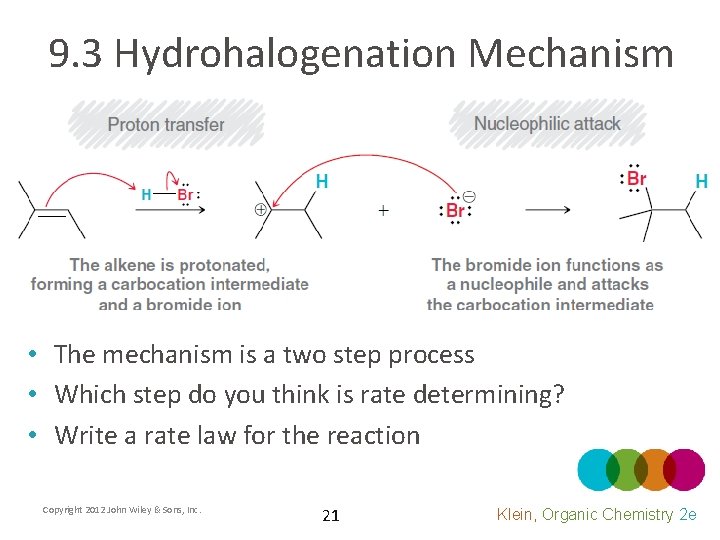
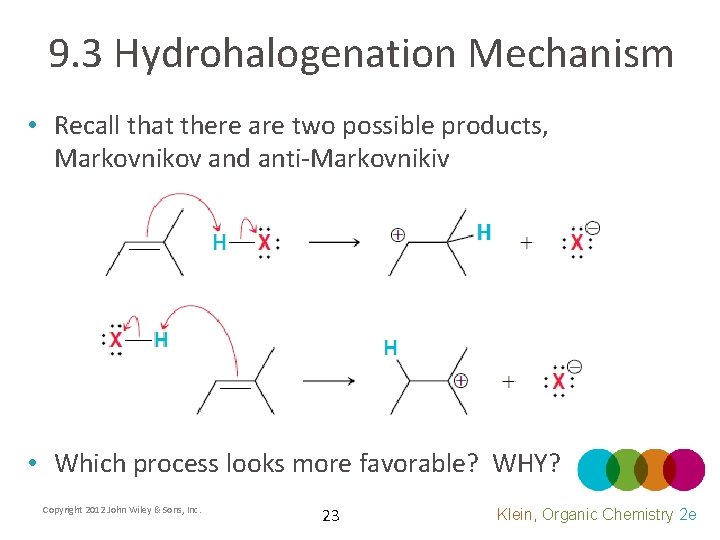
9. 3 Hydrohalogenation Mechanism • The mechanism is a two step process • Which step do you think is rate determining? • Write a rate law for the reaction Copyright 2012 John Wiley & Sons, Inc. 21 Klein, Organic Chemistry 2 e

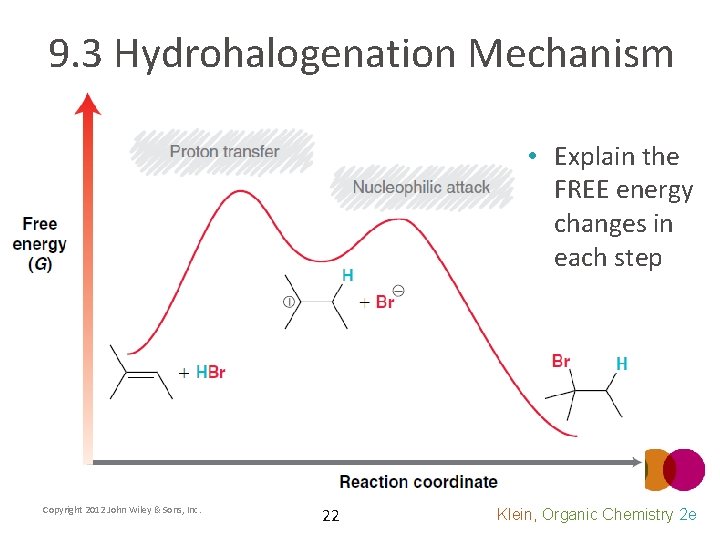
9. 3 Hydrohalogenation Mechanism • Explain the FREE energy changes in each step Copyright 2012 John Wiley & Sons, Inc. 22 Klein, Organic Chemistry 2 e

9. 3 Hydrohalogenation Mechanism • Recall that there are two possible products, Markovnikov and anti-Markovnikiv • Which process looks more favorable? WHY? Copyright 2012 John Wiley & Sons, Inc. 23 Klein, Organic Chemistry 2 e

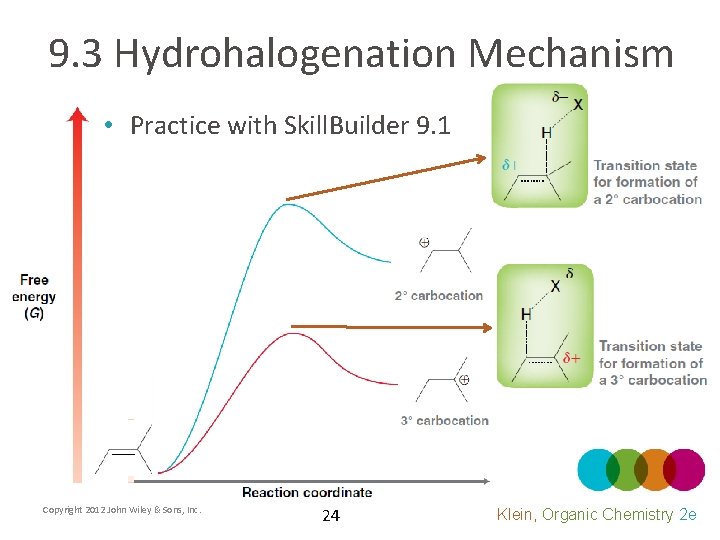
9. 3 Hydrohalogenation Mechanism • Practice with Skill. Builder 9. 1 Copyright 2012 John Wiley & Sons, Inc. 24 Klein, Organic Chemistry 2 e

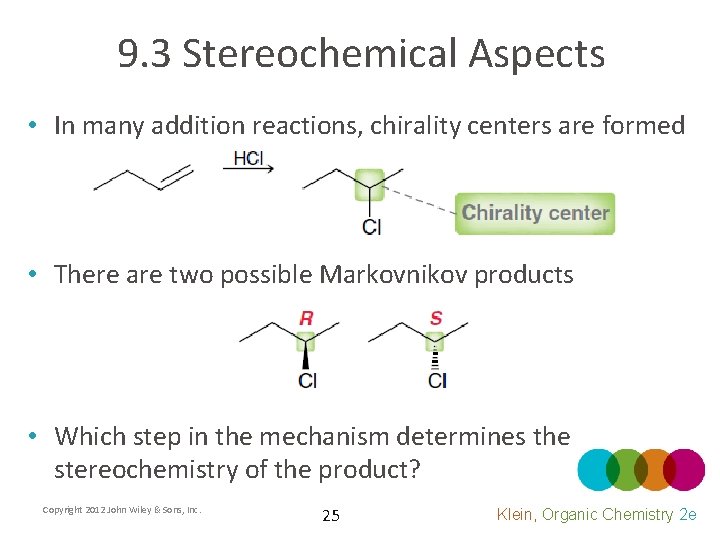
9. 3 Stereochemical Aspects • In many addition reactions, chirality centers are formed • There are two possible Markovnikov products • Which step in the mechanism determines the stereochemistry of the product? Copyright 2012 John Wiley & Sons, Inc. 25 Klein, Organic Chemistry 2 e

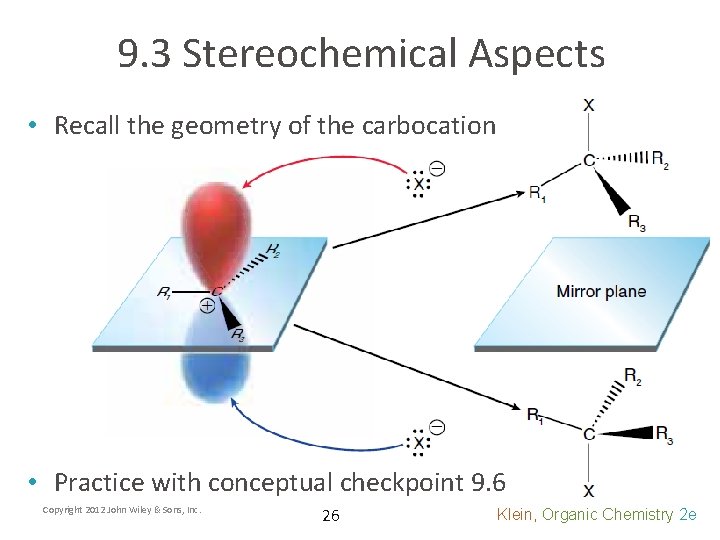
9. 3 Stereochemical Aspects • Recall the geometry of the carbocation • Practice with conceptual checkpoint 9. 6 Copyright 2012 John Wiley & Sons, Inc. 26 Klein, Organic Chemistry 2 e

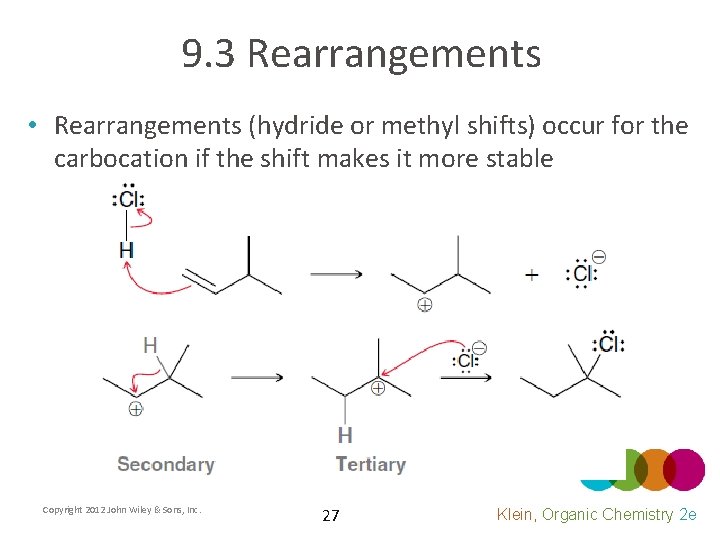
9. 3 Rearrangements • Rearrangements (hydride or methyl shifts) occur for the carbocation if the shift makes it more stable Copyright 2012 John Wiley & Sons, Inc. 27 Klein, Organic Chemistry 2 e

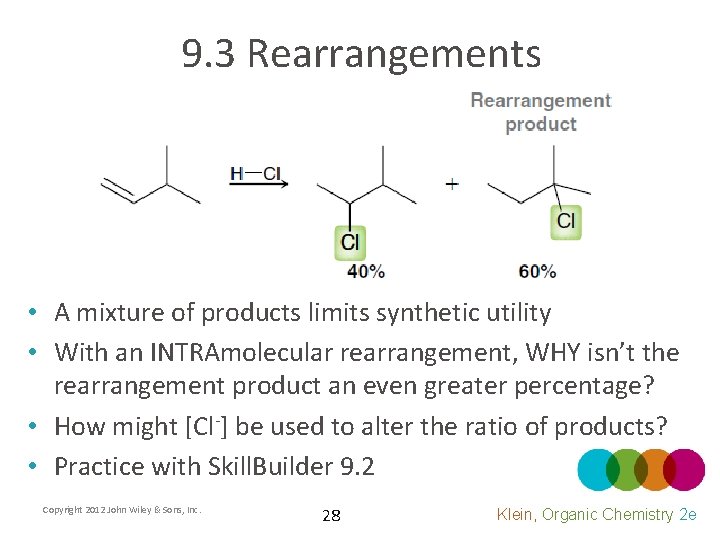
9. 3 Rearrangements • A mixture of products limits synthetic utility • With an INTRAmolecular rearrangement, WHY isn’t the rearrangement product an even greater percentage? • How might [Cl-] be used to alter the ratio of products? • Practice with Skill. Builder 9. 2 Copyright 2012 John Wiley & Sons, Inc. 28 Klein, Organic Chemistry 2 e

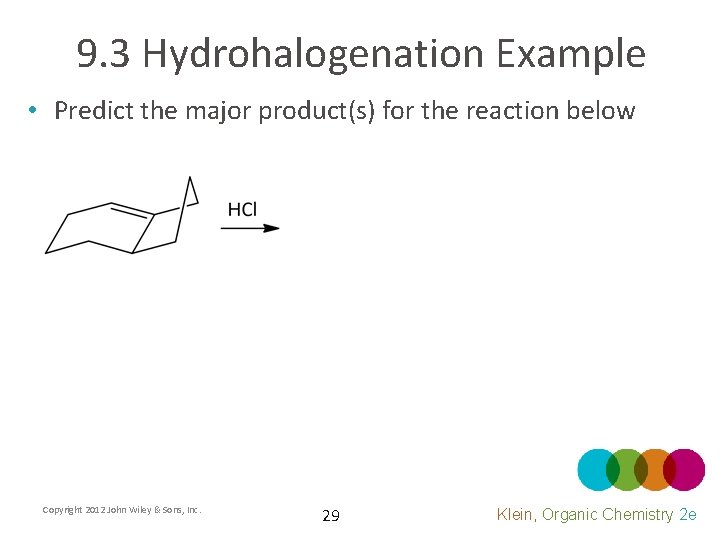
9. 3 Hydrohalogenation Example • Predict the major product(s) for the reaction below Copyright 2012 John Wiley & Sons, Inc. 29 Klein, Organic Chemistry 2 e

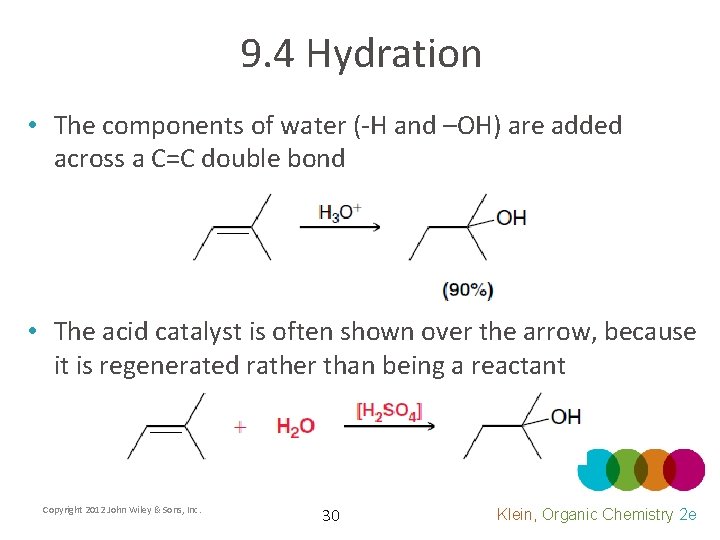
9. 4 Hydration • The components of water (-H and –OH) are added across a C=C double bond • The acid catalyst is often shown over the arrow, because it is regenerated rather than being a reactant Copyright 2012 John Wiley & Sons, Inc. 30 Klein, Organic Chemistry 2 e

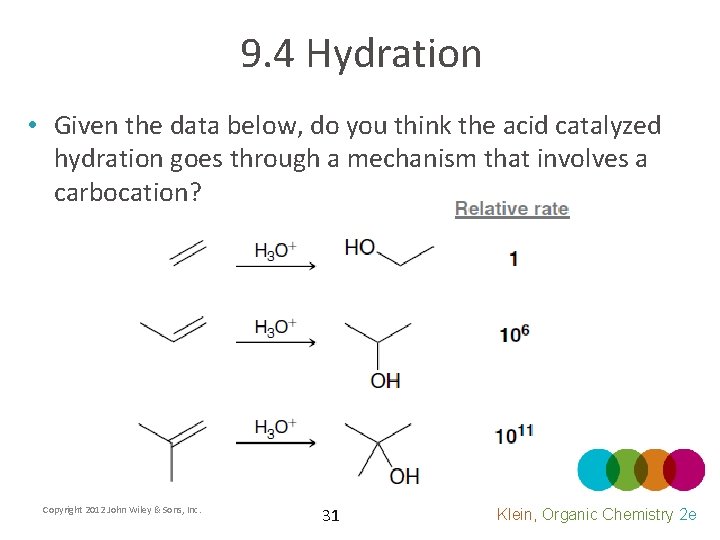
9. 4 Hydration • Given the data below, do you think the acid catalyzed hydration goes through a mechanism that involves a carbocation? Copyright 2012 John Wiley & Sons, Inc. 31 Klein, Organic Chemistry 2 e

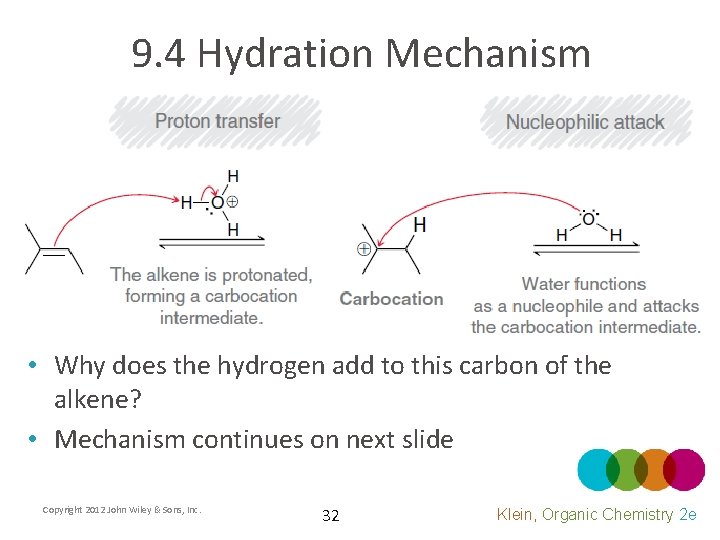
9. 4 Hydration Mechanism • Why does the hydrogen add to this carbon of the alkene? • Mechanism continues on next slide Copyright 2012 John Wiley & Sons, Inc. 32 Klein, Organic Chemistry 2 e

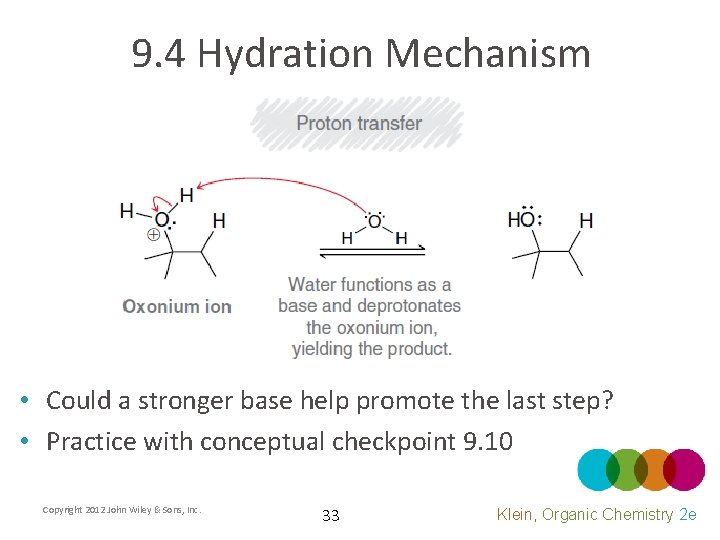
9. 4 Hydration Mechanism • Could a stronger base help promote the last step? • Practice with conceptual checkpoint 9. 10 Copyright 2012 John Wiley & Sons, Inc. 33 Klein, Organic Chemistry 2 e

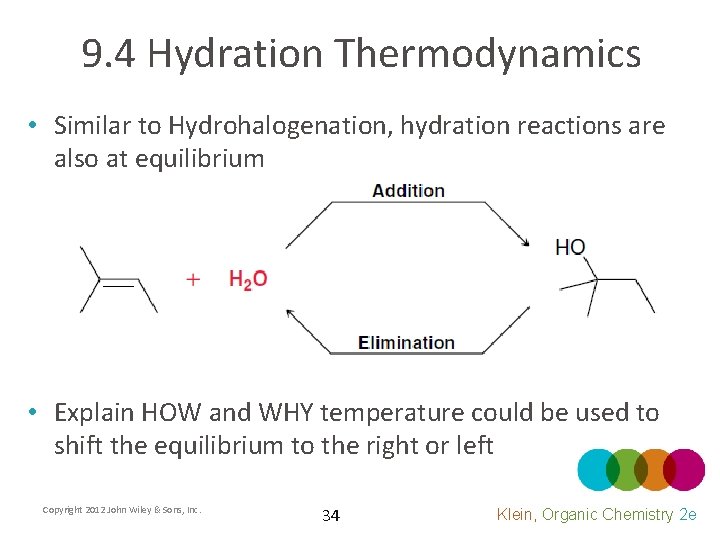
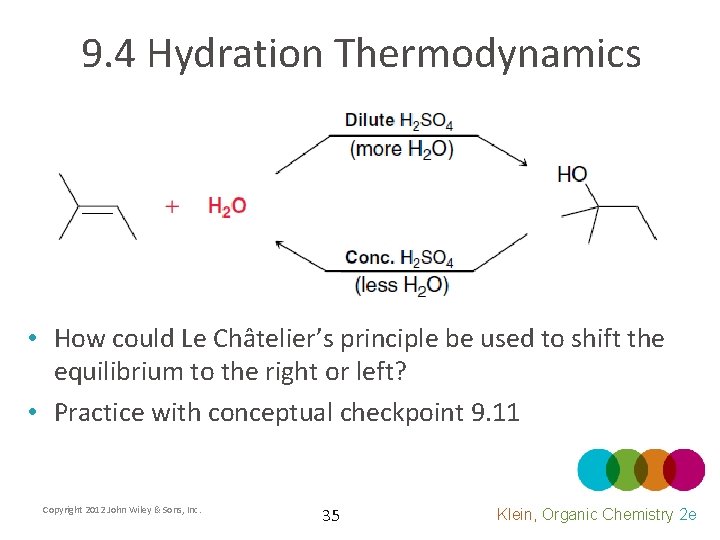
9. 4 Hydration Thermodynamics • Similar to Hydrohalogenation, hydration reactions are also at equilibrium • Explain HOW and WHY temperature could be used to shift the equilibrium to the right or left Copyright 2012 John Wiley & Sons, Inc. 34 Klein, Organic Chemistry 2 e

9. 4 Hydration Thermodynamics • How could Le Châtelier’s principle be used to shift the equilibrium to the right or left? • Practice with conceptual checkpoint 9. 11 Copyright 2012 John Wiley & Sons, Inc. 35 Klein, Organic Chemistry 2 e

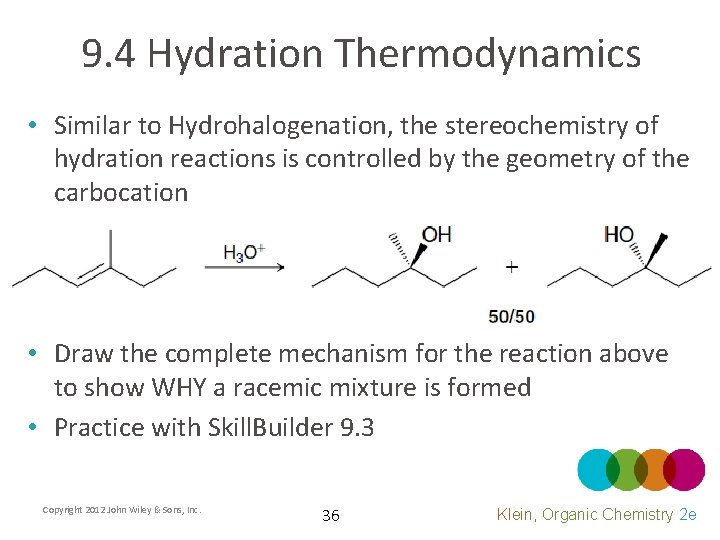
9. 4 Hydration Thermodynamics • Similar to Hydrohalogenation, the stereochemistry of hydration reactions is controlled by the geometry of the carbocation • Draw the complete mechanism for the reaction above to show WHY a racemic mixture is formed • Practice with Skill. Builder 9. 3 Copyright 2012 John Wiley & Sons, Inc. 36 Klein, Organic Chemistry 2 e

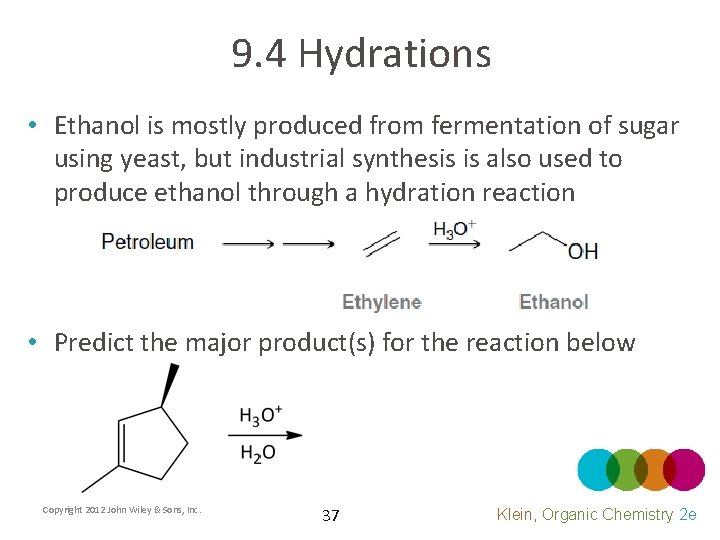
9. 4 Hydrations • Ethanol is mostly produced from fermentation of sugar using yeast, but industrial synthesis is also used to produce ethanol through a hydration reaction • Predict the major product(s) for the reaction below Copyright 2012 John Wiley & Sons, Inc. 37 Klein, Organic Chemistry 2 e

Study Guide for sections 16. 12 -16. 13, 9. 1 -9. 4 DAY 24, Terms to know: Sections 16. 12 -16. 13, 9. 1 -9. 4 Distortionless Enhancement by Polarization Transfer (DEPT), Addition reaction, hydrohalogenation, Markovnikov addition, anti-Markovnikov addition, hydration DAY 24, Specific outcomes and skills that may be tested on exam 4: Sections 16. 12 -16. 13, 9. 1 -9. 4 • Given a molecular structure, be able to predict how many 13 C NMR peaks will be observed and their shifts. • Given any or all of the data: IR, MS, proton NMR 13 C NMR, DEPT, formula, be able to propose a reasonable molecular structure. • Be able to explain how the number of pi and sigma bonds changes during an elimination reaction and identify where in an example reaction the bonds are that are breaking and forming. • Be able to describe the relative reactivity as a base or a nucleophile of pi bonds versus sigma bonds and explain why. • Be able to explain how addition reactions are at equilibrium with the reverse elimination reactions and what factors affect which side of the equilibrium is favored. • Given a reaction and reaction conditions, be able to predict which side of an elim/addn equilibrium reaction is favored. • Be able to predict whether the change in enthalpy for an addition reaction is positive or negative and WHY. • Be able to predict whether the change in entropy for an addition reaction is positive or negative and WHY. • Be able to explain how temperature can be increased or decreased to shift an addition equilibrium toward one side or another. • Be able to predict the regioselectivity of addition reactions to asymmetrical alkenes. • Be able to use the mechanism to explain WHY Markovnikov additions give the observed regioselectivity. • Given addition products, be able to give a set of reactants, reagents, and conditions that will produce the desired product whether it be Markovnikov or anti-Markovnikov. • Be able to predict a reasonable mechanism for Markovnikov addition reactions and explain how the mechanism gives the observed rate law. • Be able to predict a reasonable reaction coordinate diagram for Markovnikov addition reactions and explain the energy changes during each step. • Be able to explain how the stability of the intermediate and transition states during an addition reaction favor Markovnikov rather than anti-Markovnikov. • Be able to predict the stereochemistry of Markovnikov addition products and use the mechanism to explain why products form with the predicted stereochemistry. • Be able to predict when an addition reaction will involve a carbocation rearrangement step in the mechanism. • Be able to give conditions that might favor non-rearrangement products over carbocation rearrangement products in Markovnikov addition reactions. 38 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Practice Problems for sections 16. 12 -16. 13, 9. 1 -9. 4 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 16. 25 16. 26 16. 27 16. 28 16. 35 16. 38 16. 46 16. 62 16. 64 9. 1 9. 2 9. 3 9. 4 9. 6 9. 7 9. 9 9. 10 9. 11 9. 12 9. 48 9. 51 39 Klein, Organic. Chemistry 2 e 2 e Klein, Organic

Prep for Day 25 Must Watch videos: https: //www. youtube. com/watch? v=b 7 Qj-QZR 9 Ck (Oxymercuration-demercuration) https: //www. youtube. com/watch? v=I-pme. Hcj. D 8 M (hydroboration oxidation) https: //www. youtube. com/watch? v=Ercr 5 SCIEn. U (hydrogenation) https: //www. youtube. com/watch? v=5 GQelnlu. Hz. E (halogenation) https: //www. youtube. com/watch? v=S-2 Ca. HDh. Mf. I (halohydrin formation) Other helpful videos: https: //www. youtube. com/watch? v=y. Hm 5 YT-XXi. A (alkoxymercuration-demercuration) http: //ps. uci. edu/content/chem-51 b-organic-chemistry (lecture 8) Read sections 9. 5 -9. 8 40 Klein, Organic. Chemistry 2 e 2 e Klein, Organic
 Hydrohalogenation mechanism
Hydrohalogenation mechanism Factors influencing chemical shift
Factors influencing chemical shift Shift in sras curve
Shift in sras curve Change in supply and change in quantity supplied
Change in supply and change in quantity supplied Tpcastt
Tpcastt I hear america singing tpcastt
I hear america singing tpcastt 7 seismic shifts
7 seismic shifts A reform movement that shifts from the use of customary
A reform movement that shifts from the use of customary Demand curve shifts
Demand curve shifts Tpcastt example
Tpcastt example Ela shifts
Ela shifts Longman
Longman Poetic shift
Poetic shift American hero by essex hemphill analysis
American hero by essex hemphill analysis Society where emphasis shifts from production
Society where emphasis shifts from production Vehicle balance refers to
Vehicle balance refers to What does the phillips curve represent
What does the phillips curve represent Double shifts in demand and supply
Double shifts in demand and supply What shifts the lm curve
What shifts the lm curve Chapter 4 section 2 the demand curve shifts
Chapter 4 section 2 the demand curve shifts Horizontal and vertical shifts
Horizontal and vertical shifts Chapter 5 section 2 the supply curve shifts
Chapter 5 section 2 the supply curve shifts Cultural shifts in a professional learning community
Cultural shifts in a professional learning community Which of the following shifts aggregate demand to the left?
Which of the following shifts aggregate demand to the left? Ad as
Ad as What does tpcastt mean
What does tpcastt mean Translation shifts catford
Translation shifts catford Standard 4 part 1 a: vehicle balance & control
Standard 4 part 1 a: vehicle balance & control Shifts
Shifts Shifters of demand for loanable funds
Shifters of demand for loanable funds Cultural shifts in a professional learning community
Cultural shifts in a professional learning community Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Are kc and kp equal
Are kc and kp equal Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Love formula
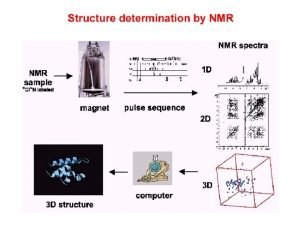
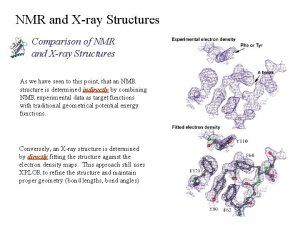
Love formula Nmr spectroscopy
Nmr spectroscopy Evans method magnetic susceptibility
Evans method magnetic susceptibility Nmr integration
Nmr integration Spektra nmr
Spektra nmr