NMR Assignments What is the NMR Assignment Issue






























- Slides: 87

NMR Assignments What is the NMR Assignment Issue? • Each observable NMR resonance needs to be assigned or associated with the atom in the protein structure. NMR spectra of proteins are complex, where the complexity increases with the size or number of residues of the protein 13 C & 15 N isotope enrichment to simplify the NMR spectra need to assign these NMR Use resonances 1 13 C and 15 N NMR resonances to assign a typical protein will have hundreds of H, 1 H NMR Spectra Protein PDB File

NMR Assignments Again, as illustrated here, the goal is to explicitly assign each H, C, & N in the protein’s primary sequence with its corresponding NMR resonance 15 N 119. 3 ppm HN 7. 76 ppm 15 N 114. 8 ppm HN 7. 08 ppm 13 Ca 55. 5 ppm Ha 3. 76 ppm 13 CO 13 Cb 17. 5 ppm Hb 1. 45 ppm 171. 9 ppm 13 Cb 64. 8 ppm Hb 3. 73 ppm 15 N 125. 6 ppm HN 8. 20 ppm 13 CO 178. 1 ppm 13 Ca 59. 9 ppm Ha 4. 35 ppm 13 Ca 58. 6 ppm Ha 4. 09 ppm 13 CO 170. 9 ppm 13 Cb 42. 9 ppm Hb 1. 52 ppm 13 Cd 13 Cg 27. 9 ppm Hg 1. 65 ppm 25. 4 ppm; 25. 7 ppm Hd 0. 82 ppm; 0. 98 ppm

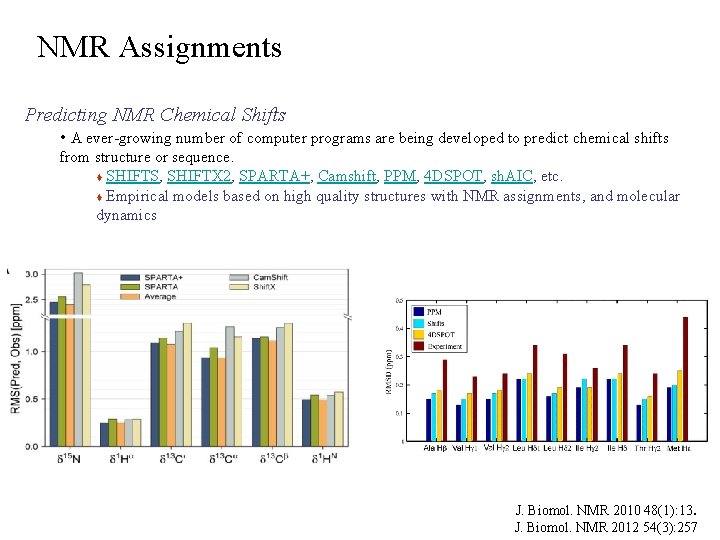
NMR Assignments Predicting NMR Chemical Shifts • A ever-growing number of computer programs are being developed to predict chemical shifts from structure or sequence. SHIFTS, SHIFTX 2, SPARTA+, Camshift, PPM, 4 DSPOT, sh. AIC, etc. Empirical models based on high quality structures with NMR assignments, and molecular dynamics J. Biomol. NMR 2010 48(1): 13. J. Biomol. NMR 2012 54(3): 257

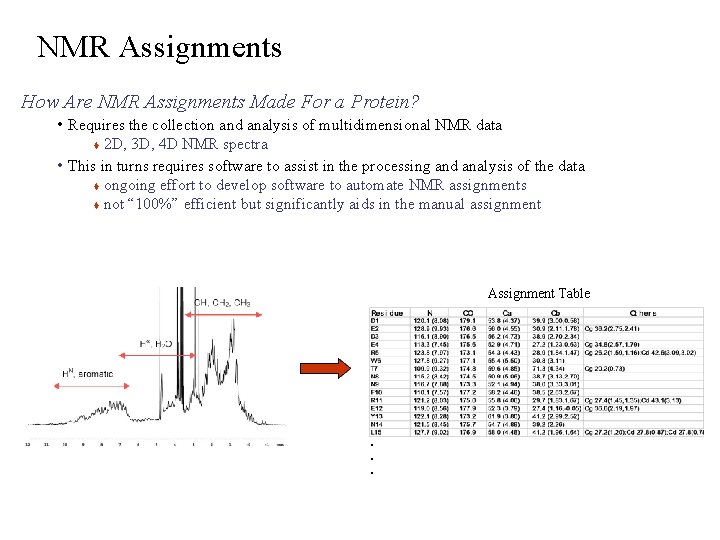
NMR Assignments How Are NMR Assignments Made For a Protein? • Requires the collection and analysis of multidimensional NMR data 2 D, 3 D, 4 D NMR spectra • This in turns requires software to assist in the processing and analysis of the data ongoing effort to develop software to automate NMR assignments not “ 100%” efficient but significantly aids in the manual assignment Assignment Table . . .

NMR Assignments NMR Data Processing Software • Needs to specifically handle format of multidimensional NMR data 2 D, 3 D, 4 D NMR spectra • NMRPipe, Felix, ACD and others all have similar functions and capability all handle common instrument data formats (Bruker, Varian) choice is primarily based on personal preference NMRpipe: - UNIX/LINUX - simple script to process NMR data - mimics flow of processing steps - uses UNIX pipe functionality to pass data between one function to the next

NMR Assignments NMR Data Processing Software • Main steps in the processing process include: window function (SP), zero fill (ZF), Fourier transform (FT), phase (PS), transpose (TP) • Other steps include removing solvent (SOL), linear prediction (LP) and data extraction (EXT) • These steps are simply repeated for each dimension of the NMR data Standard Processing Script for 3 D NMR Data X Processing steps for X, Y, Z dimensions of 3 D spectra Y Z

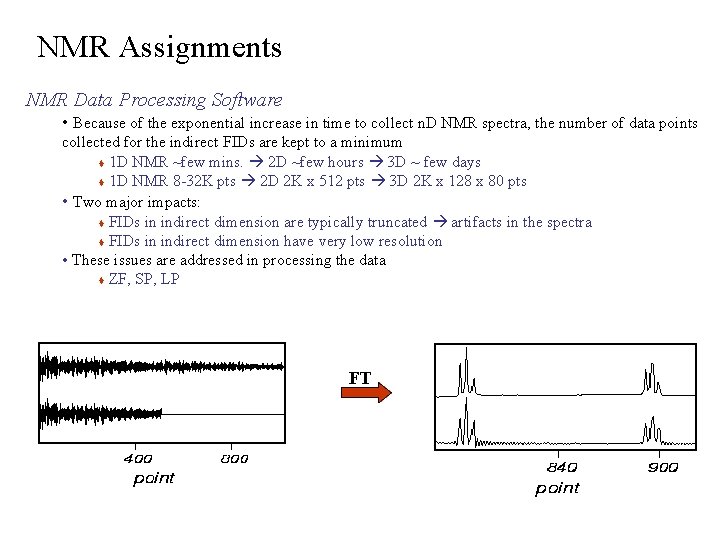
NMR Assignments NMR Data Processing Software • Because of the exponential increase in time to collect n. D NMR spectra, the number of data points collected for the indirect FIDs are kept to a minimum 1 D NMR ~few mins. 2 D ~few hours 3 D ~ few days 1 D NMR 8 -32 K pts 2 D 2 K x 512 pts 3 D 2 K x 128 x 80 pts • Two major impacts: FIDs in indirect dimension are typically truncated artifacts in the spectra FIDs in indirect dimension have very low resolution • These issues are addressed in processing the data ZF, SP, LP FT

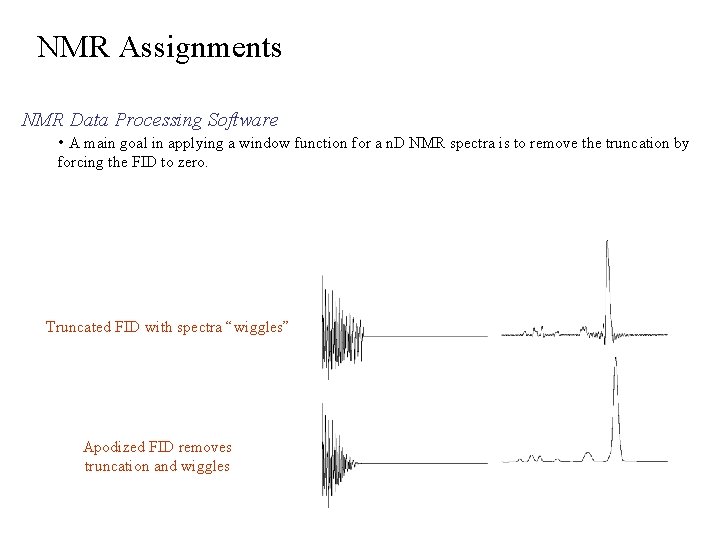
NMR Assignments NMR Data Processing Software • A main goal in applying a window function for a n. D NMR spectra is to remove the truncation by forcing the FID to zero. Truncated FID with spectra “wiggles” Apodized FID removes truncation and wiggles

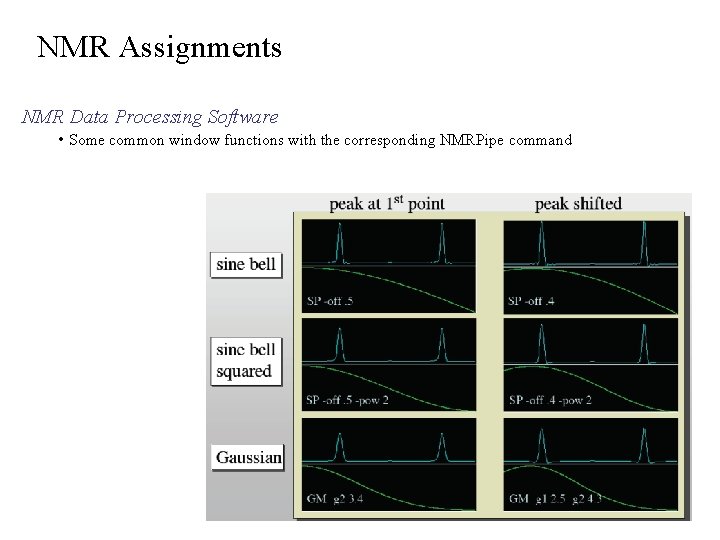
NMR Assignments NMR Data Processing Software • Some common window functions with the corresponding NMRPipe command

NMR Assignments NMR Data Processing Software • Want to maximize digital resolution, number of data points in each dimension time constraints are a practical limitation for n. D NMR data

NMR Assignments NMR Data Processing Software • Improve digital resolution by adding zero data points at end of FID essential for n. D NMR data no significant gain after one ZF, just interpolation between points 8 K data 8 K FID No zero-filling 8 K zero-fill 16 K FID 8 K zero-filling

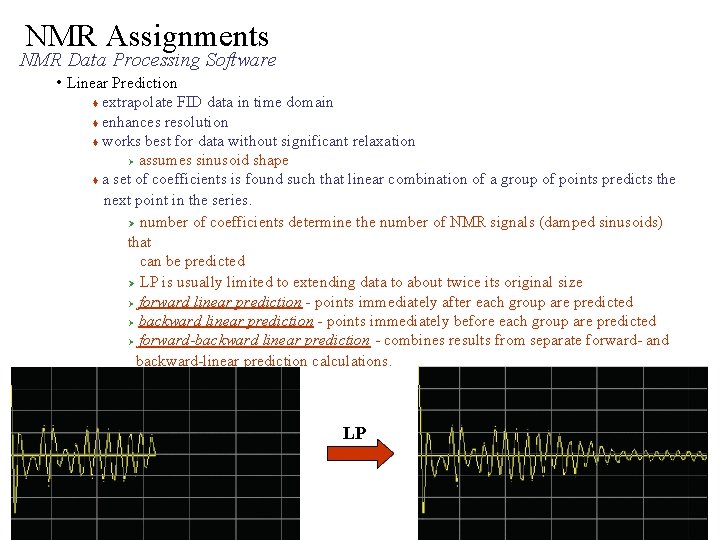
NMR Assignments NMR Data Processing Software • Linear Prediction extrapolate FID data in time domain enhances resolution works best for data without significant relaxation Ø assumes sinusoid shape a set of coefficients is found such that linear combination of a group of points predicts the next point in the series. Ø number of coefficients determine the number of NMR signals (damped sinusoids) that can be predicted Ø LP is usually limited to extending data to about twice its original size Ø forward linear prediction - points immediately after each group are predicted Ø backward linear prediction - points immediately before each group are predicted Ø forward-backward linear prediction - combines results from separate forward- and backward-linear prediction calculations. LP

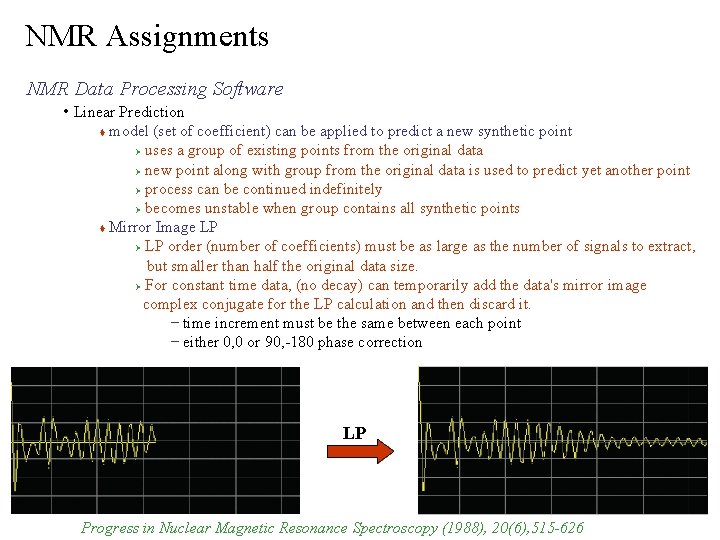
NMR Assignments NMR Data Processing Software • Linear Prediction model (set of coefficient) can be applied to predict a new synthetic point Ø uses a group of existing points from the original data Ø new point along with group from the original data is used to predict yet another point Ø process can be continued indefinitely Ø becomes unstable when group contains all synthetic points Mirror Image LP Ø LP order (number of coefficients) must be as large as the number of signals to extract, but smaller than half the original data size. Ø For constant time data, (no decay) can temporarily add the data's mirror image complex conjugate for the LP calculation and then discard it. – time increment must be the same between each point – either 0, 0 or 90, -180 phase correction LP Progress in Nuclear Magnetic Resonance Spectroscopy (1988), 20(6), 515 -626

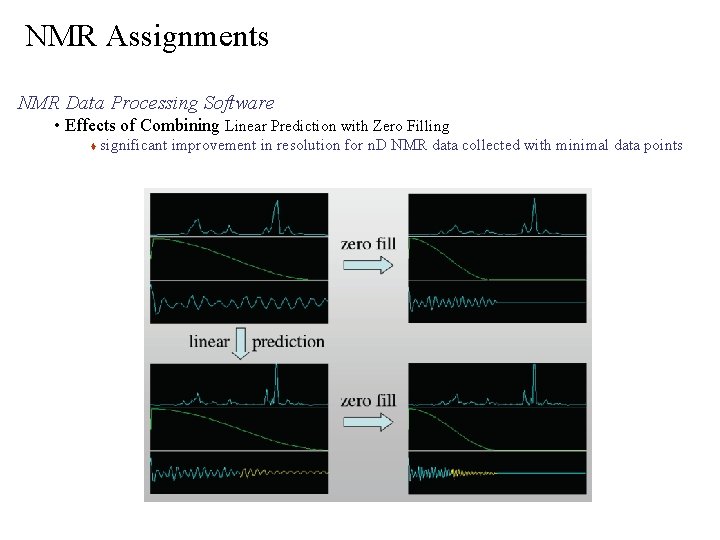
NMR Assignments NMR Data Processing Software • Effects of Combining Linear Prediction with Zero Filling significant improvement in resolution for n. D NMR data collected with minimal data points

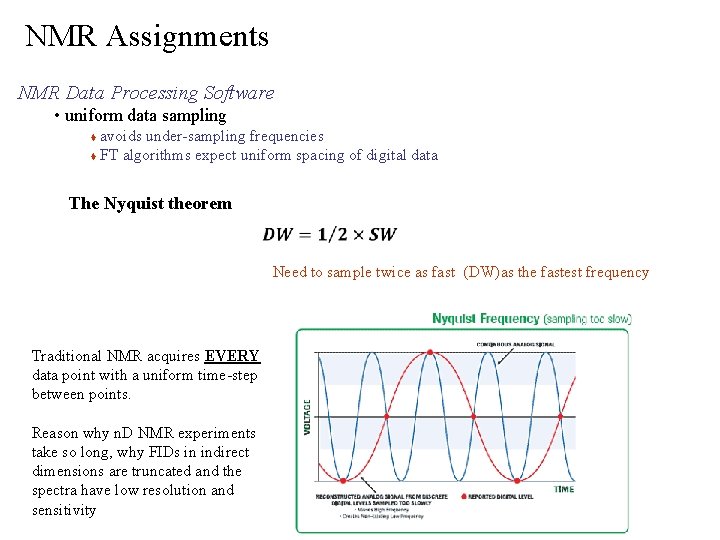
NMR Assignments NMR Data Processing Software • uniform data sampling avoids under-sampling frequencies FT algorithms expect uniform spacing of digital data The Nyquist theorem Need to sample twice as fast (DW)as the fastest frequency Traditional NMR acquires EVERY data point with a uniform time-step between points. Reason why n. D NMR experiments take so long, why FIDs in indirect dimensions are truncated and the spectra have low resolution and sensitivity

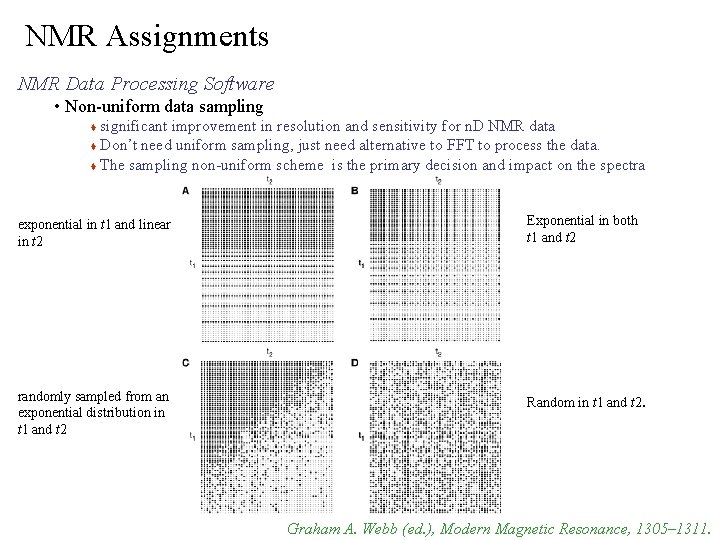
NMR Assignments NMR Data Processing Software • Non-uniform data sampling significant improvement in resolution and sensitivity for n. D NMR data Don’t need uniform sampling, just need alternative to FFT to process the data. The sampling non-uniform scheme is the primary decision and impact on the spectra exponential in t 1 and linear in t 2 Exponential in both t 1 and t 2 randomly sampled from an exponential distribution in t 1 and t 2 Random in t 1 and t 2. Graham A. Webb (ed. ), Modern Magnetic Resonance, 1305– 1311.

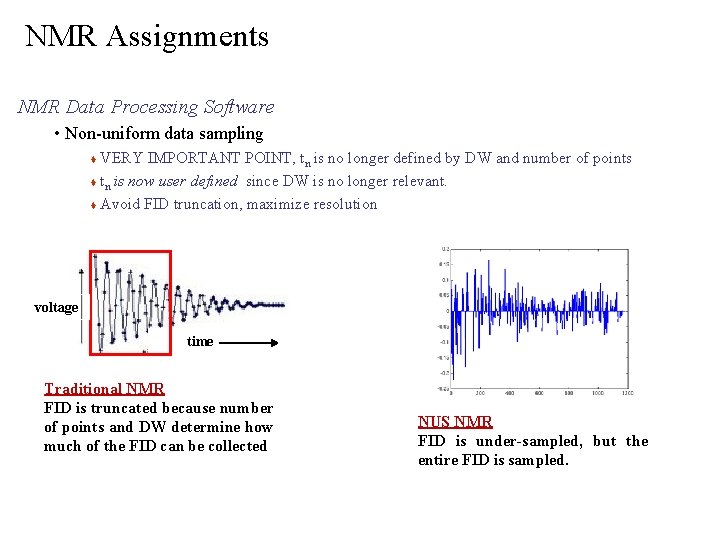
NMR Assignments NMR Data Processing Software • Non-uniform data sampling VERY IMPORTANT POINT, tn is no longer defined by DW and number of points tn is now user defined since DW is no longer relevant. Avoid FID truncation, maximize resolution voltage time Traditional NMR FID is truncated because number of points and DW determine how much of the FID can be collected NUS NMR FID is under-sampled, but the entire FID is sampled.

NMR Assignments NMR Data Processing Software • Non-uniform data sampling Both noise (N) and signal to noise (SNR) are proportional to the total evolution time Optimal setting is 1. 3 T 2 of the evolving coherence Maximize sensitivity Magn. Reson. Chem. 2011, 49, 483– 491

NMR Assignments NMR Data Processing Software • Non-uniform data sampling What is the optimal sampling density? Increase enhancement by increase exponential bias, eventually regenerate truncated FID Highly resolved spectra is p. T 2 TSMP – time constant for the exponential weighting of the sampling. h - enhancement lw – line width Magn. Reson. Chem. 2011, 49, 483– 491

NMR Assignments NMR Data Processing Software • Non-uniform data sampling A 1. 5 to 2. 0 bias to early data points and a 4 x reduction yields a 2 x enhancement Or a 3 T 2 with a 3 x reduction yields a 1. 7 enhancement Truncated FID Sampling Density/LW = TSMP/T 2 Magn. Reson. Chem. 2011, 49, 483– 491

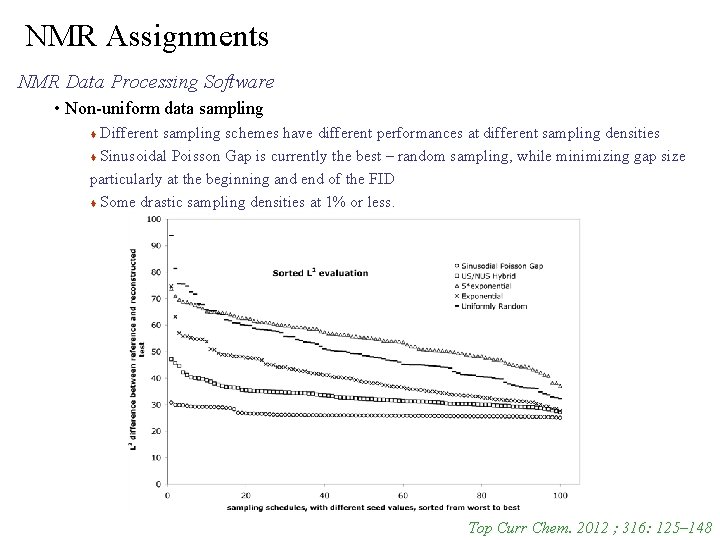
NMR Assignments NMR Data Processing Software • Non-uniform data sampling Different sampling schemes have different performances at different sampling densities Sinusoidal Poisson Gap is currently the best – random sampling, while minimizing gap size particularly at the beginning and end of the FID Some drastic sampling densities at 1% or less. Top Curr Chem. 2012 ; 316: 125– 148

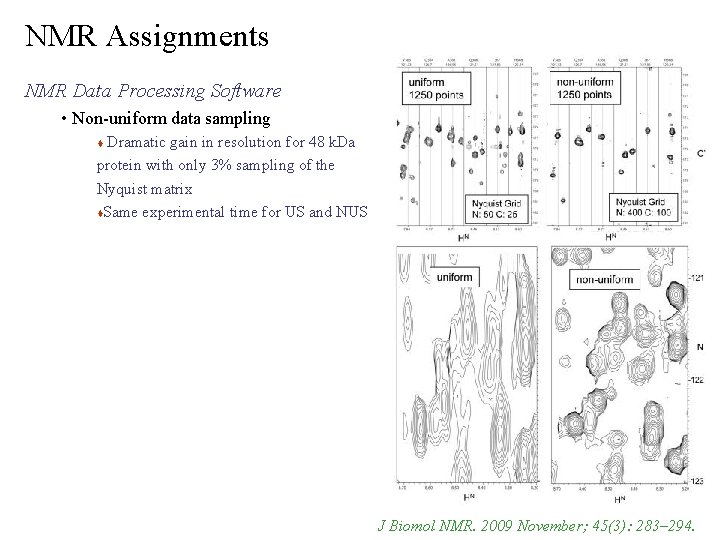
NMR Assignments NMR Data Processing Software • Non-uniform data sampling Dramatic gain in resolution for 48 k. Da protein with only 3% sampling of the Nyquist matrix Same experimental time for US and NUS J Biomol NMR. 2009 November; 45(3): 283– 294.

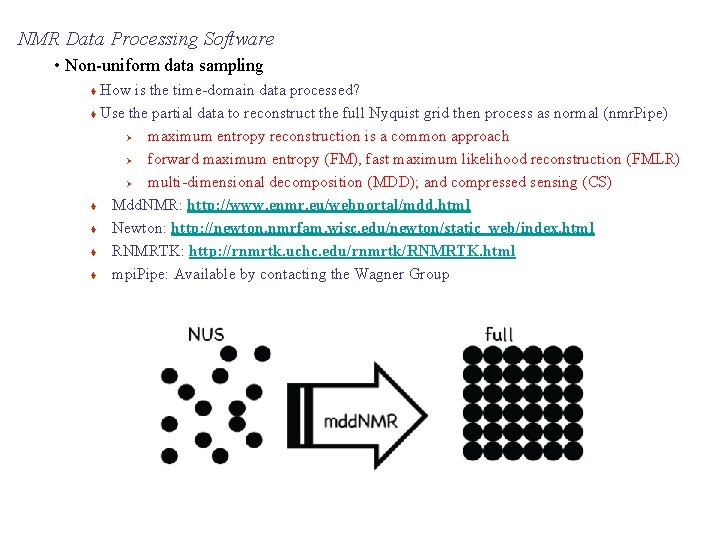
NMR Data Processing Software • Non-uniform data sampling How is the time-domain data processed? Use the partial data to reconstruct the full Nyquist grid then process as normal (nmr. Pipe) Ø maximum entropy reconstruction is a common approach Ø forward maximum entropy (FM), fast maximum likelihood reconstruction (FMLR) Ø multi-dimensional decomposition (MDD); and compressed sensing (CS) Mdd. NMR: http: //www. enmr. eu/webportal/mdd. html Newton: http: //newton. nmrfam. wisc. edu/newton/static_web/index. html RNMRTK: http: //rnmrtk. uchc. edu/rnmrtk/RNMRTK. html mpi. Pipe: Available by contacting the Wagner Group

NMR Assignments NMR Data Processing Software • Solvent Removal (SOL) protein NMR spectra are typical collected in water the large solvent signal can interfere with the interpretation of the NMR data Carrier frequency is usually centered on the water signal the signal associated with the water resonance can be filtered or subtracted from the time domain of the FID SOL

NMR Assignments NMR Data Processing Software • Solvent Removal (SOL) with Solvent Subtraction without Solvent Subtraction

NMR Assignments NMR Data Processing Software • Phase Correction (PS) Because of the challenges of phasing n. D NMR data and the baseline artifacts that firstorder phase corrections are known to cause, typically phase corrections are set to 0, 0 or 90180 by proper delays in the pulse sequence A number of methods of data collection are used to obtain phase correction in the indirect dimensions Ø Fourier transformed data contains a real part that is an absorption lorentzian and an imaginary part which is a dispersion lorentzian Ø we want to maintain the real absorption mode line-shape Ø done by applying a phase factor (exp(i. Q)) to set F to zero Ø this is what we are doing when we phase the spectra

NMR Assignments NMR Data Processing Software • Phase Correction (PS) Phase of the peak is determined by the relative phase of the pulse and the receiver to obtain correct phasing in the indirect dimension, we need to collect both sine and cosine modulated data alternate both the phase of the pulse relative to the receiver and the storage of this data between real (sine) and imaginary (cosine)

NMR Assignments NMR Data Processing Software • Phase Correction (PS) Phase of the peak is determined by the relative phase of the pulse and the receiver Also determines the order in which the data is stored. Some Common Phase Cycle Schemes: o 0 STATES – phase cycles the 90 -pulses prior to t 1 incrimination by 90 TPPI – phase cycles both the receiver and the 90 o-pulses prior to t 1 by 90 o for each t 1 increment o o States-TPPI – phase cycles both the receiver and the 90 -pulses prior to t 1 by 180 for each t 1 increment Echo-antiecho – uses gradients to reduce the number of phase cycling steps and combines N (echo) and P(antiecho) coherence selection

NMR Assignments NMR Data Processing Software • Phase Correction (PS) Experiment Increment Pulse Phase Receiver Phase TPPI (4 k + 1) t 1(0) + (4 k)D x x (4 k + 2) t 1(0) + (4 k + 1)D y x (4 k + 3) t 1(0) + (4 k + 2)D -x x (4 k + 4) t 1(0) + (4 k + 3)D -y x STATES (4 k + 1) t 1(0) + (4 k)2 D x x (4 k + 2) t 1(0) + (4 k)2 D y x (4 k + 3) t 1(0) + (4 k + 1)2 D x x (4 k + 4) t 1(0) + (4 k + 1)2 D y x States-TPPI (4 k + 1) t 1(0) + (4 k)2 D x x (4 k + 2) t 1(0) + (4 k)2 D y x (4 k + 3) t 1(0) + (4 k + 1)2 D -x -x (4 k + 4) t 1(0) + (4 k + 1)2 D -y -x

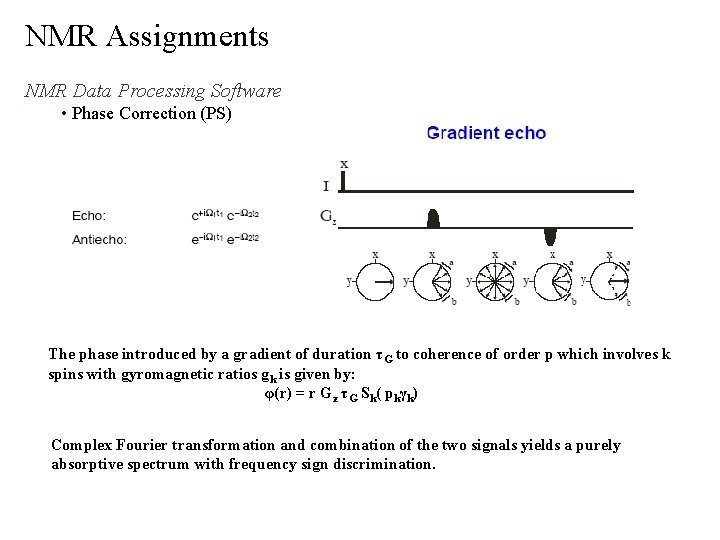
NMR Assignments NMR Data Processing Software • Phase Correction (PS) The phase introduced by a gradient of duration τG to coherence of order p which involves k spins with gyromagnetic ratios gk is given by: φ(r) = r Gz τG Sk( pkγk) Complex Fourier transformation and combination of the two signals yields a purely absorptive spectrum with frequency sign discrimination.

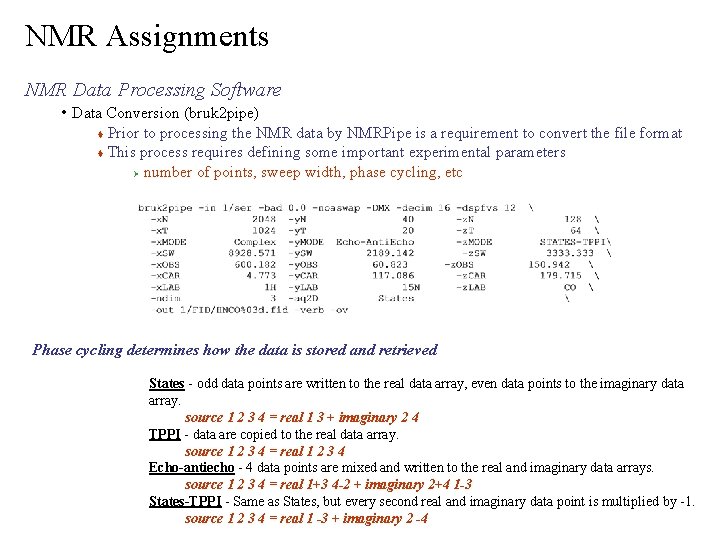
NMR Assignments NMR Data Processing Software • Data Conversion (bruk 2 pipe) Prior to processing the NMR data by NMRPipe is a requirement to convert the file format This process requires defining some important experimental parameters Ø number of points, sweep width, phase cycling, etc Phase cycling determines how the data is stored and retrieved States - odd data points are written to the real data array, even data points to the imaginary data array. source 1 2 3 4 = real 1 3 + imaginary 2 4 TPPI - data are copied to the real data array. source 1 2 3 4 = real 1 2 3 4 Echo-antiecho - 4 data points are mixed and written to the real and imaginary data arrays. source 1 2 3 4 = real 1+3 4 -2 + imaginary 2+4 1 -3 States-TPPI - Same as States, but every second real and imaginary data point is multiplied by -1. source 1 2 3 4 = real 1 -3 + imaginary 2 -4

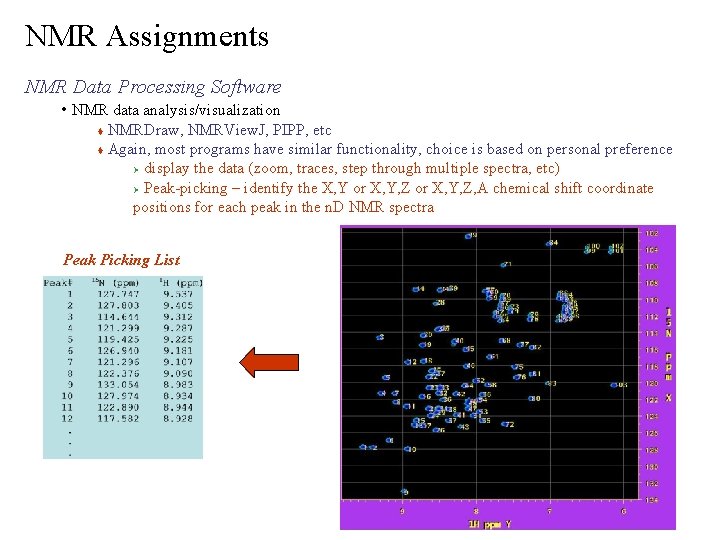
NMR Assignments NMR Data Processing Software • NMR data analysis/visualization NMRDraw, NMRView. J, PIPP, etc Again, most programs have similar functionality, choice is based on personal preference Ø display the data (zoom, traces, step through multiple spectra, etc) Ø Peak-picking – identify the X, Y or X, Y, Z, A chemical shift coordinate positions for each peak in the n. D NMR spectra Peak Picking List

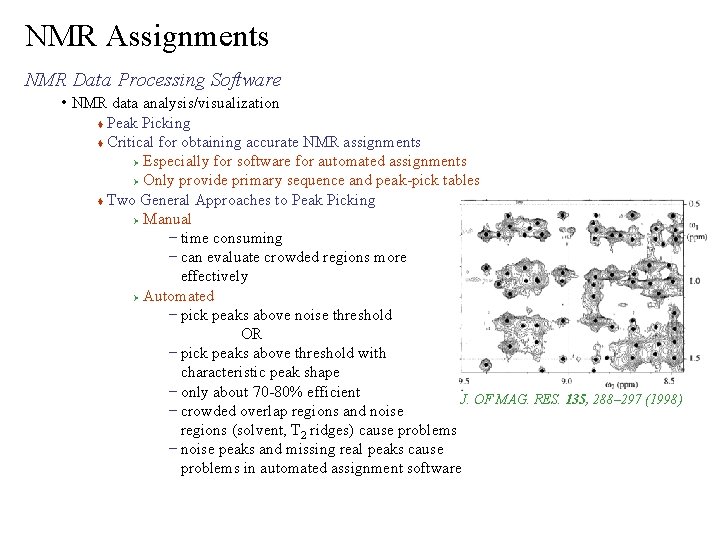
NMR Assignments NMR Data Processing Software • NMR data analysis/visualization Peak Picking Critical for obtaining accurate NMR assignments Ø Especially for software for automated assignments Ø Only provide primary sequence and peak-pick tables Two General Approaches to Peak Picking Ø Manual – time consuming – can evaluate crowded regions more effectively Ø Automated – pick peaks above noise threshold OR – pick peaks above threshold with characteristic peak shape – only about 70 -80% efficient J. OF MAG. RES. 135, 288– 297 (1998) – crowded overlap regions and noise regions (solvent, T 2 ridges) cause problems – noise peaks and missing real peaks cause problems in automated assignment software

NMR Assignments NMR Data Processing Software • NMR data analysis/visualization What is the Statistical likelihood that a signal is a peak? 100 simulated spectra containing a single peak with random noise. A successful identification occurred if the known peak has the highest intensity that is at least 1. 414 times greater than the next intense peak. A signal intensity of 1 corresponds to a SNR of 80. J Biomol NMR (2013) 55: 167– 178.

NMR Assignments NMR Data Processing Software • Automated NMR assignments Auto. Assign, CONTRAST, GARANT, PASTA, etc Ø uses peak lists, primary protein sequence, details of NMR experiments Ø tries to mimic “skilled user”, uses databases of previous assignments, etc Automated analysis of NOESY data is a sub-set of the NMR assignment issue with programs designed to specifically address this need Ø Auto. Structure, CANDID, ARIA, ROSSETTA, etc From, peak-lists and protein sequence, software attempts to make the assignment. Not 100% success rate, still need user intervention to complete/correct assignments. Most problems arise from quality of peak-list: noise, missing peaks, etc. Need to Know How Assignments are Made!

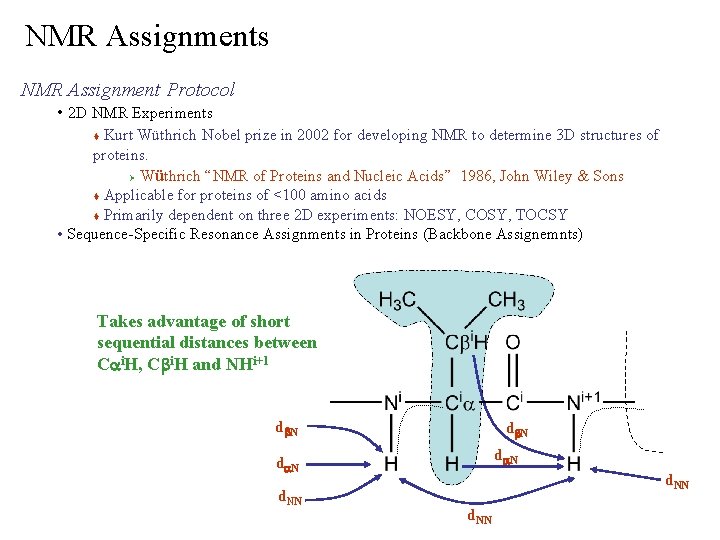
NMR Assignments NMR Assignment Protocol • 2 D NMR Experiments Kurt Wüthrich Nobel prize in 2002 for developing NMR to determine 3 D structures of proteins. Ø Wüthrich “NMR of Proteins and Nucleic Acids” 1986, John Wiley & Sons Applicable for proteins of <100 amino acids Primarily dependent on three 2 D experiments: NOESY, COSY, TOCSY • Sequence-Specific Resonance Assignments in Proteins (Backbone Assignemnts) Takes advantage of short sequential distances between Cai. H, Cbi. H and NHi+1 db. N da. N d. NN

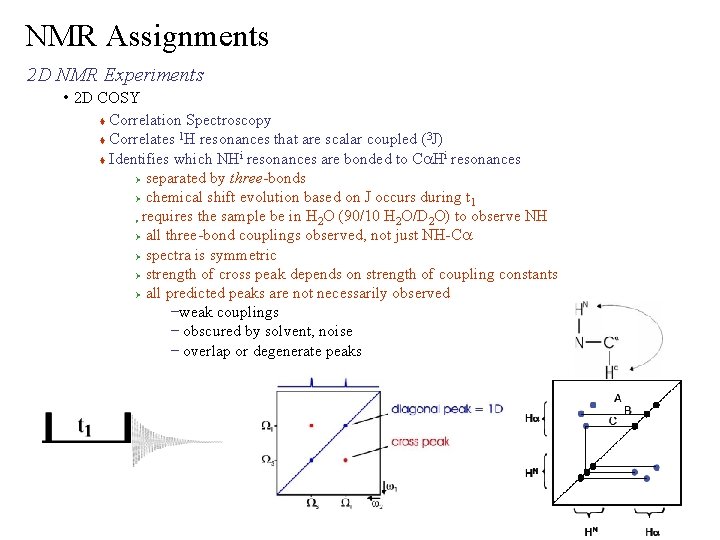
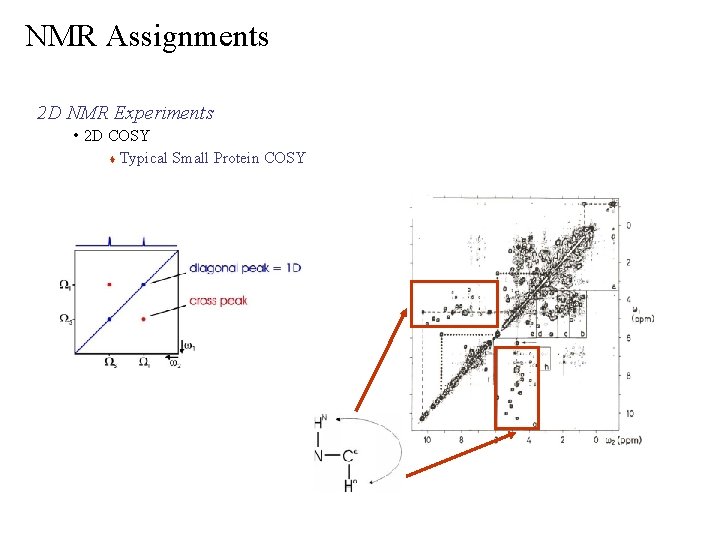
NMR Assignments 2 D NMR Experiments • 2 D COSY Correlation Spectroscopy 1 3 Correlates H resonances that are scalar coupled ( J) i i Identifies which NH resonances are bonded to Ca. H resonances Ø separated by three-bonds Ø chemical shift evolution based on J occurs during t 1 requires the sample be in H 2 O (90/10 H 2 O/D 2 O) to observe NH Ø all three-bond couplings observed, not just NH-Ca Ø spectra is symmetric Ø strength of cross peak depends on strength of coupling constants Ø all predicted peaks are not necessarily observed –weak couplings – obscured by solvent, noise – overlap or degenerate peaks Ø

NMR Assignments 2 D NMR Experiments • 2 D COSY Typical Small Protein COSY

NMR Assignments 2 D NMR Experiments • 2 D NOESY Nuclear Overhauser Spectroscopy 1 Correlates H resonances that close in space (≤ 5Å) Ø also contains COSY peaks Ø NOE intensity builds up during mixing time (tm), ususally 100 -150 ms i+1 resonances with Ca. Hi resonances Correlates NH

NMR Assignments 2 D NMR Experiments • 2 D NOESY Typical Protein NOESY (Lysozyme) Both NHi-Cai and NHi+1 -Cai are present

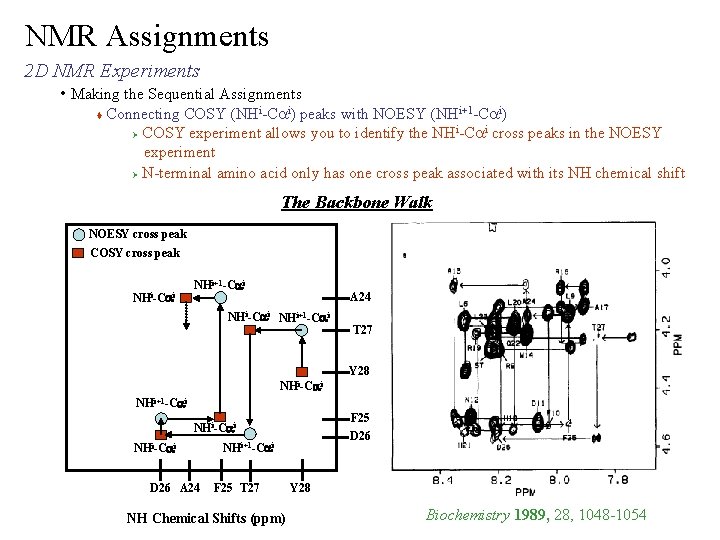
NMR Assignments 2 D NMR Experiments • Making the Sequential Assignments Connecting COSY (NHi-Cai) peaks with NOESY (NHi+1 -Cai) i i Ø COSY experiment allows you to identify the NH -Ca cross peaks in the NOESY experiment Ø N-terminal amino acid only has one cross peak associated with its NH chemical shift The Backbone Walk NOESY cross peak COSY cross peak NHi-Cai NHi+1 -Cai A 24 NHi-Cai NHi+1 -Cai T 27 Y 28 NHi-Cai NHi+1 -Cai F 25 D 26 NHi-Cai D 26 A 24 NHi+1 -Cai F 25 T 27 NH Chemical Shifts (ppm) Y 28 Biochemistry 1989, 28, 1048 -1054

NMR Assignments 2 D NMR Experiments • Verifying the Sequential Assignments and Side-Chain Assignments The accuracy of the backbone assignments from connecting COSY (NHi-Cai) peaks with NOESY (NHi+1 -Cai) can be verified by proper assignment of the side-chain with the backbone assignments. Ø know the primary sequence of the protein Ø therefore, know what amino acid is residue (i) and what amino-acid should be (i+1) Ø amino acid type indicates the number and type or chemical shifts that should be observed for the residue As example: Gly – no side chain Ala – single methyl (1. 39 ppm) Val – two g methlys (0. 97 & 0. 94 ppm) one Hb (2. 13 ppm)

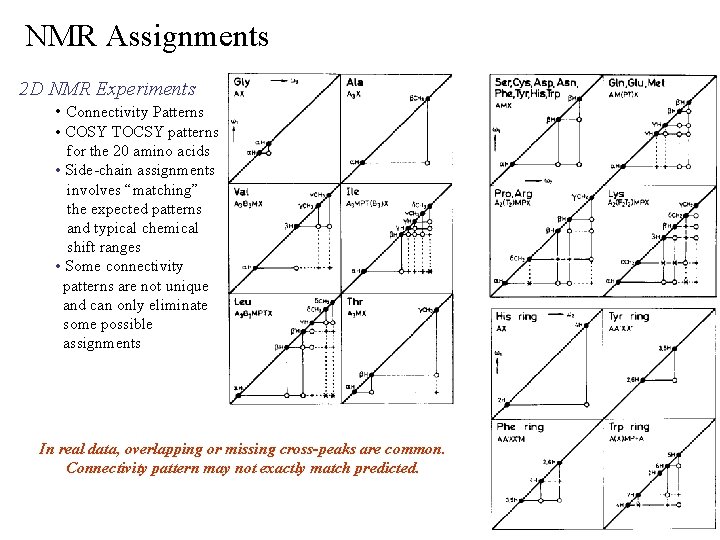
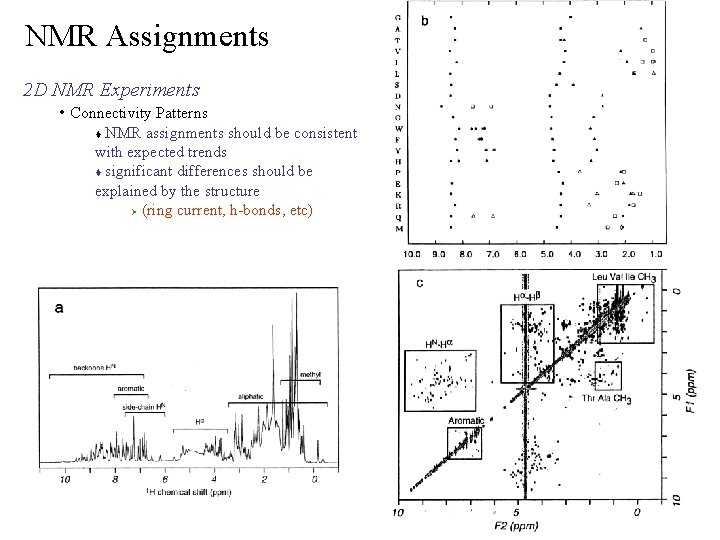
NMR Assignments 2 D NMR Experiments • Connectivity Patterns • COSY TOCSY patterns for the 20 amino acids • Side-chain assignments involves “matching” the expected patterns and typical chemical shift ranges • Some connectivity patterns are not unique and can only eliminate some possible assignments In real data, overlapping or missing cross-peaks are common. Connectivity pattern may not exactly match predicted.

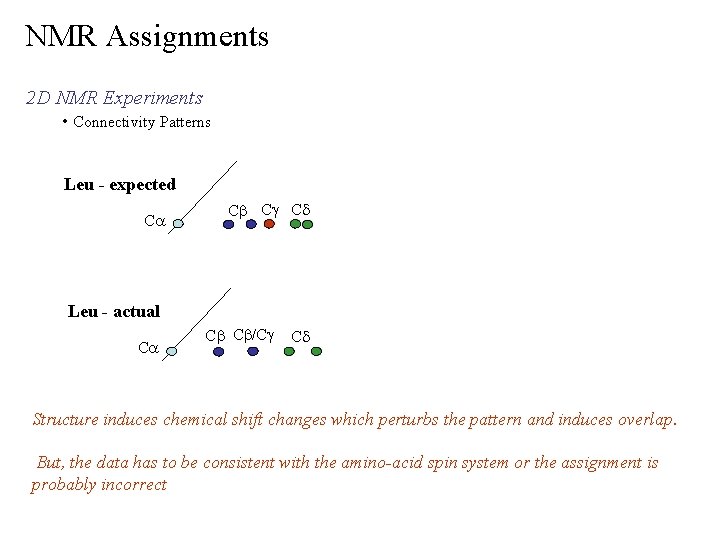
NMR Assignments 2 D NMR Experiments • Connectivity Patterns Leu - expected Ca Cb Cg Cd Leu - actual Ca Cb Cb/Cg Cd Structure induces chemical shift changes which perturbs the pattern and induces overlap. But, the data has to be consistent with the amino-acid spin system or the assignment is probably incorrect

NMR Assignments 2 D NMR Experiments • Connectivity Patterns NMR assignments should be consistent with expected trends significant differences should be explained by the structure Ø (ring current, h-bonds, etc)

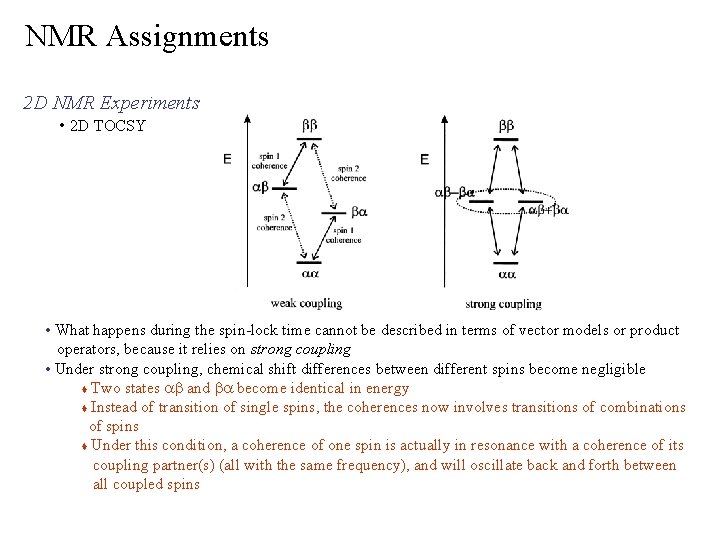
NMR Assignments 2 D NMR Experiments • 2 D TOCSY TOtal Correlation Spectroscop. Y Ø cross peaks are generated between all members of a coupled spin network – NMR resonances for the complete side-chain spin systems is obtained Ø coherence transfer period occurs during a multi-pulse spin-lock period Ø length of spin-lock determines how “far” the spin coupling network will be probed Ø 1/(10 JHH) should be used for each transfer step Ø not all correlations are observed COSY TOCSY Spin-Lock Pulse (~14 ms)

NMR Assignments 2 D NMR Experiments • 2 D TOCSY • What happens during the spin-lock time cannot be described in terms of vector models or product operators, because it relies on strong coupling • Under strong coupling, chemical shift differences between different spins become negligible Two states ab and ba become identical in energy Instead of transition of single spins, the coherences now involves transitions of combinations of spins Under this condition, a coherence of one spin is actually in resonance with a coherence of its coupling partner(s) (all with the same frequency), and will oscillate back and forth between all coupled spins

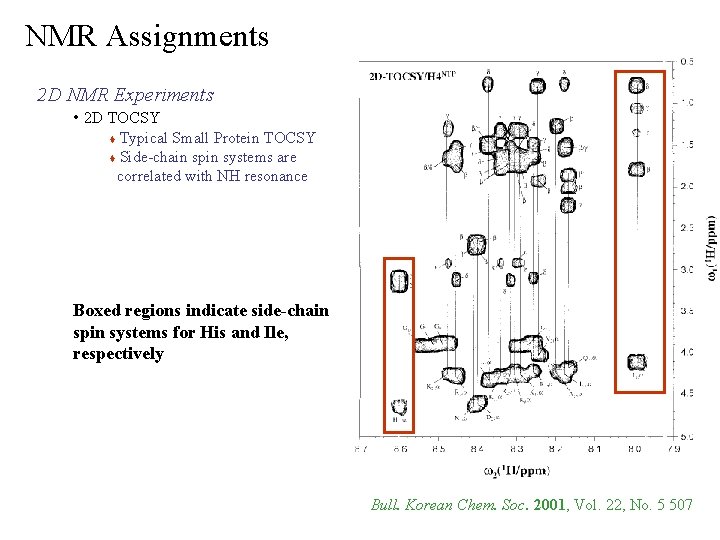
NMR Assignments 2 D NMR Experiments • 2 D TOCSY Typical Small Protein TOCSY Side-chain spin systems are correlated with NH resonance Boxed regions indicate side-chain spin systems for His and Ile, respectively Bull. Korean Chem. Soc. 2001, Vol. 22, No. 5 507

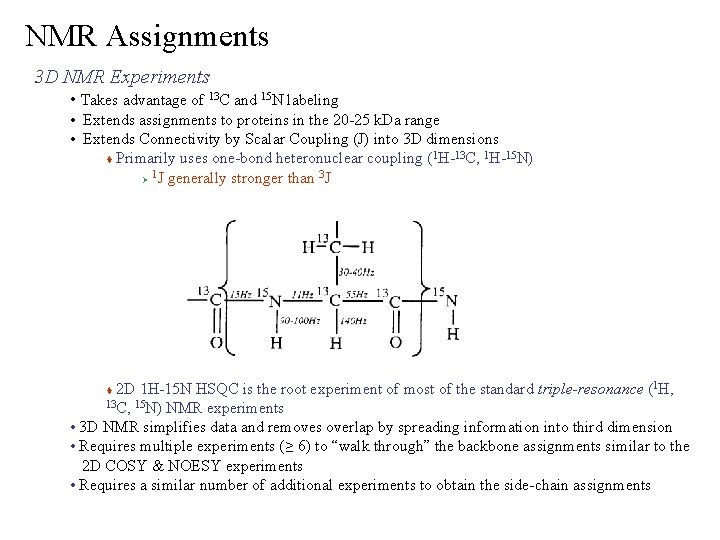
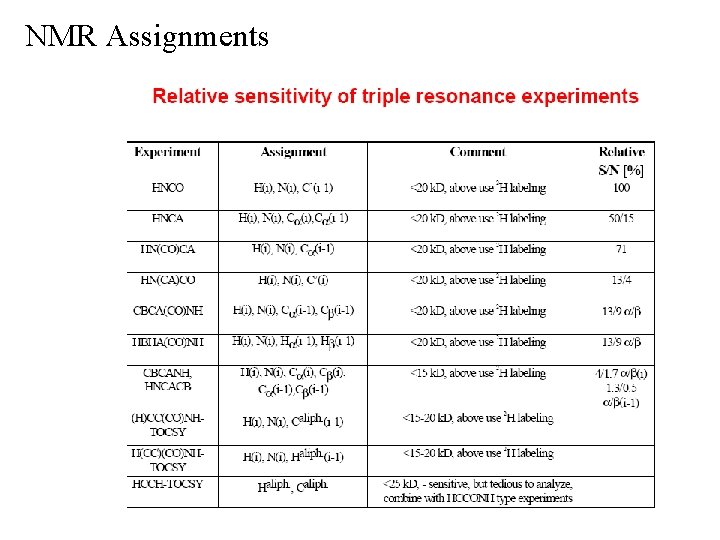
NMR Assignments 3 D NMR Experiments • Takes advantage of 13 C and 15 N labeling • Extends assignments to proteins in the 20 -25 k. Da range • Extends Connectivity by Scalar Coupling (J) into 3 D dimensions 1 13 1 15 Primarily uses one-bond heteronuclear coupling ( H- C, H- N) 1 3 Ø J generally stronger than J 2 D 1 H-15 N HSQC is the root experiment of most of the standard triple-resonance (1 H, 13 C, 15 N) NMR experiments • 3 D NMR simplifies data and removes overlap by spreading information into third dimension • Requires multiple experiments (≥ 6) to “walk through” the backbone assignments similar to the 2 D COSY & NOESY experiments • Requires a similar number of additional experiments to obtain the side-chain assignments

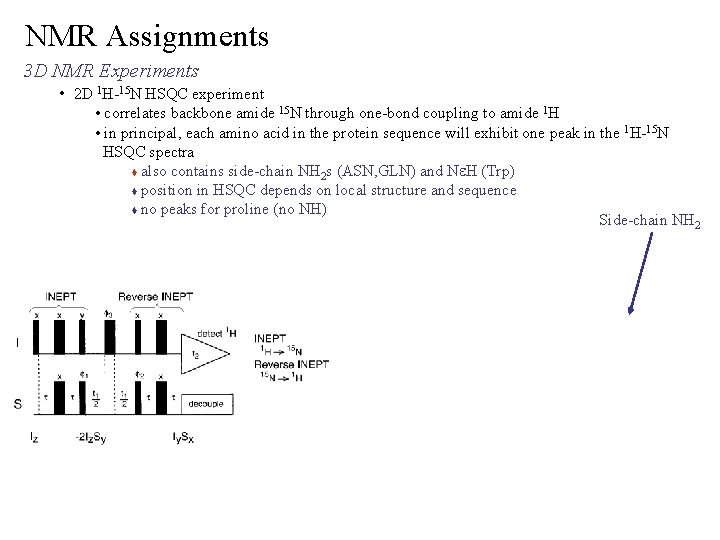
NMR Assignments 3 D NMR Experiments • 2 D 1 H-15 N HSQC experiment • correlates backbone amide 15 N through one-bond coupling to amide 1 H • in principal, each amino acid in the protein sequence will exhibit one peak in the 1 H-15 N HSQC spectra also contains side-chain NH 2 s (ASN, GLN) and Ne. H (Trp) position in HSQC depends on local structure and sequence no peaks for proline (no NH) Side-chain NH 2

NMR Assignments 3 D NMR Experiments • Consider a 3 D experiment as a collection of 2 D experiments z-dimension is the 15 N chemical shift • 1 H-15 N HSQC spectra is modulated to include correlation through coupling to a another backbone atom • All the 3 D triple resonance experiments are then related by the common 1 H, 15 N chemical shifts of the HSQC spectra • The backbone assignments are then obtained by piecing together all the “jigsaw” puzzles pieces from the various NMR experiments to reassemble the backbone

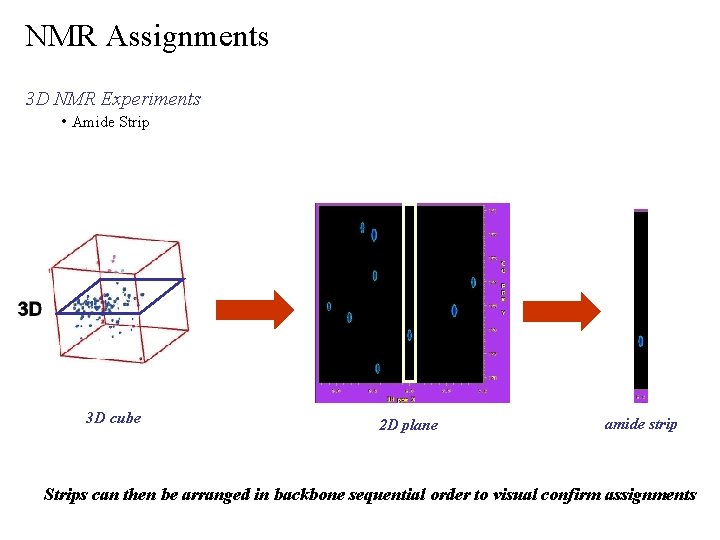
NMR Assignments 3 D NMR Experiments • Amide Strip 3 D cube 2 D plane amide strip Strips can then be arranged in backbone sequential order to visual confirm assignments

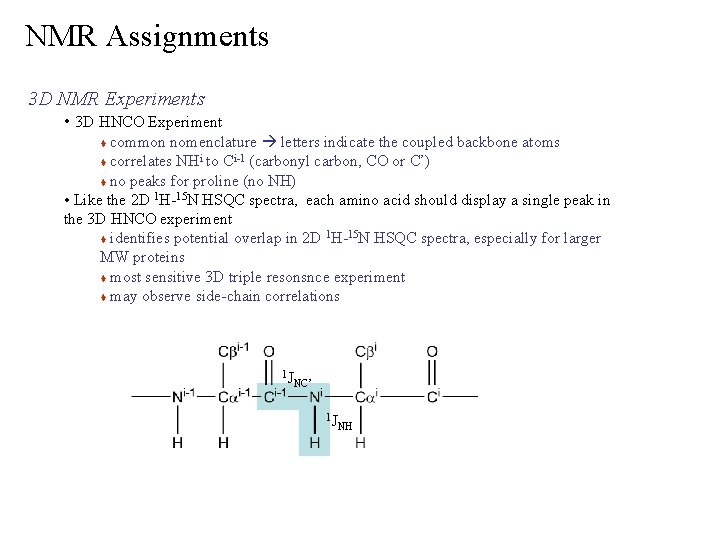
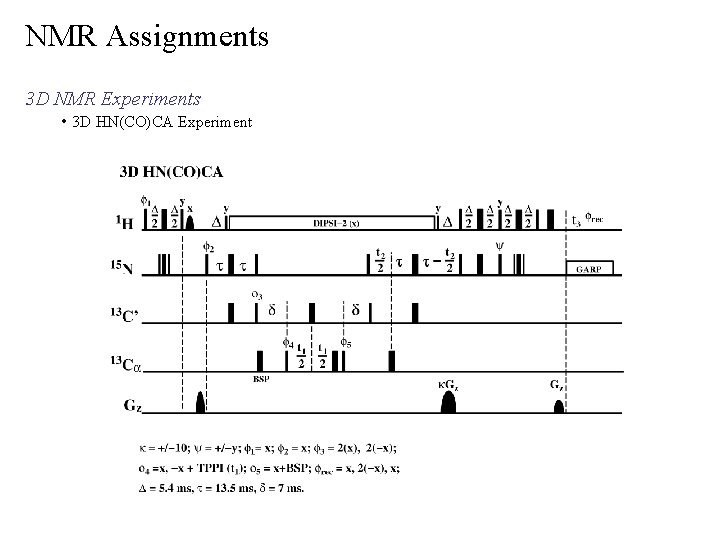
NMR Assignments 3 D NMR Experiments • 3 D HNCO Experiment common nomenclature letters indicate the coupled backbone atoms i i-1 (carbonyl carbon, CO or C’) correlates NH to C no peaks for proline (no NH) • Like the 2 D 1 H-15 N HSQC spectra, each amino acid should display a single peak in the 3 D HNCO experiment 1 15 identifies potential overlap in 2 D H- N HSQC spectra, especially for larger MW proteins most sensitive 3 D triple resonsnce experiment may observe side-chain correlations 1 J NC’ 1 J NH

NMR Assignments 3 D NMR Experiments • 3 D HNCO Experiment

NMR Assignments 3 D NMR Experiments • 3 D HNCO Experiment One expanded plane or slice from a 3 D HNCO experiment, where the 15 N chemical shift is 118. 21 ppm A total of 128 planes, with a digital resolution of 0. 28 ppm per plane for the entire experiment. slice through 3 D cube

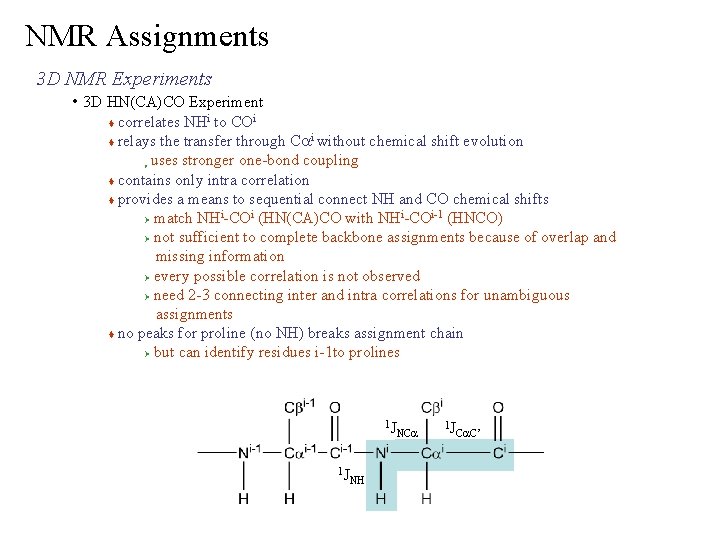
NMR Assignments 3 D NMR Experiments • 3 D HN(CA)CO Experiment correlates NHi to COi i relays the transfer through Ca without chemical shift evolution uses stronger one-bond coupling contains only intra correlation provides a means to sequential connect NH and CO chemical shifts i i-1 (HNCO) Ø match NH -CO (HN(CA)CO with NH -CO Ø not sufficient to complete backbone assignments because of overlap and missing information Ø every possible correlation is not observed Ø need 2 -3 connecting inter and intra correlations for unambiguous assignments no peaks for proline (no NH) breaks assignment chain Ø but can identify residues i-1 to prolines Ø 1 J 1 J NH NCa 1 J Ca. C’

NMR Assignments 3 D NMR Experiments • 3 D HN(CA)CO Experiment

NMR Assignments 3 D NMR Experiments • 3 D HN(CA)CO Experiment Connects HNi-COi with HNi-COi-1 HNCO and HN(CA)CO pair for one residues NH Amide “Strips” from the 3 D HNCO and HN(CA)CO experiments arranged in sequential order Journal of Biomolecular NMR, 9 (1997) 11– 24

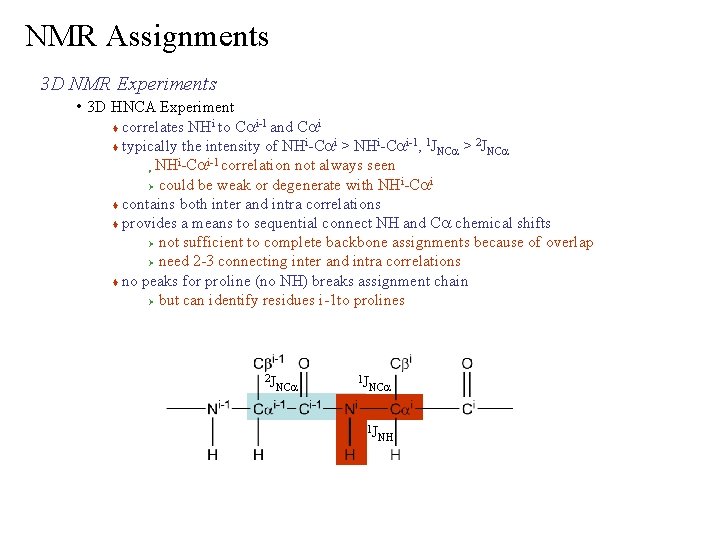
NMR Assignments 3 D NMR Experiments • 3 D HNCA Experiment correlates NHi to Cai-1 and Cai i i-1 1 2 typically the intensity of NH -Ca > NH -Ca , JNCa > JNCa NHi-Cai-1 correlation not always seen i i Ø could be weak or degenerate with NH -Ca contains both inter and intra correlations provides a means to sequential connect NH and Ca chemical shifts Ø not sufficient to complete backbone assignments because of overlap Ø need 2 -3 connecting inter and intra correlations no peaks for proline (no NH) breaks assignment chain Ø but can identify residues i-1 to prolines Ø 2 J NCa 1 J NH

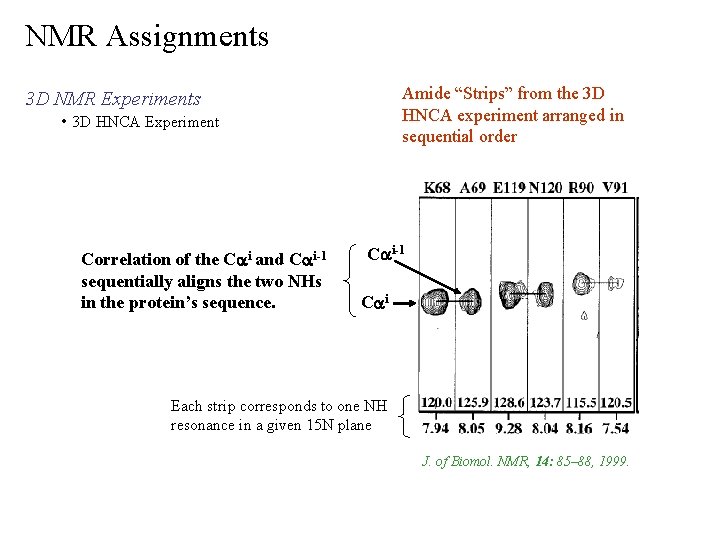
NMR Assignments 3 D NMR Experiments • 3 D HNCA Experiment

NMR Assignments Amide “Strips” from the 3 D HNCA experiment arranged in sequential order 3 D NMR Experiments • 3 D HNCA Experiment Correlation of the Cai and Cai-1 sequentially aligns the two NHs in the protein’s sequence. Cai-1 Cai Each strip corresponds to one NH resonance in a given 15 N plane J. of Biomol. NMR, 14: 85– 88, 1999.

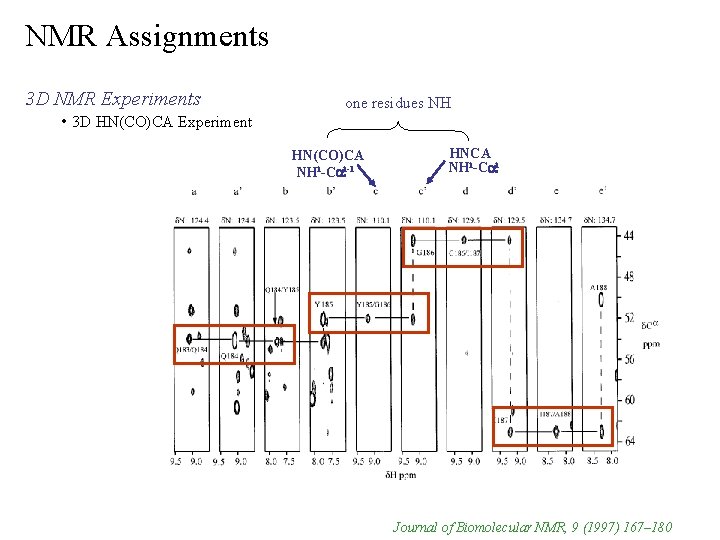
NMR Assignments 3 D NMR Experiments • 3 D HN(CO)CA Experiment correlates NHi to Cai-1 1 relays through JNC’ without chemical shift evolution NHi-Cai-1 correlation is more sensitive than HNCA experiment i i-1 assignments Ø unambiguous NH -Ca Ø avoids possible overlap in HNCA experiment companion experiment to HNCA provides a means to sequential connect NH and Ca chemical shifts i i-1 (HN(CO)CA) Ø NH -Ca (HNCA) matches with NH -Ca Ønot sufficient to complete backbone assignments because of overlap Ø need 2 -3 connecting inter and intra correlations no peaks for proline (no NH) breaks assignment chain Ø but can identify residues i-1 to prolines Ø 1 J C’Ca 1 J NC’ 1 J NH

NMR Assignments 3 D NMR Experiments • 3 D HN(CO)CA Experiment

NMR Assignments 3 D NMR Experiments • 3 D HN(CO)CA Experiment one residues NH HN(CO)CA NHi-Cai-1 HNCA NHi-Cai Journal of Biomolecular NMR, 9 (1997) 167– 180

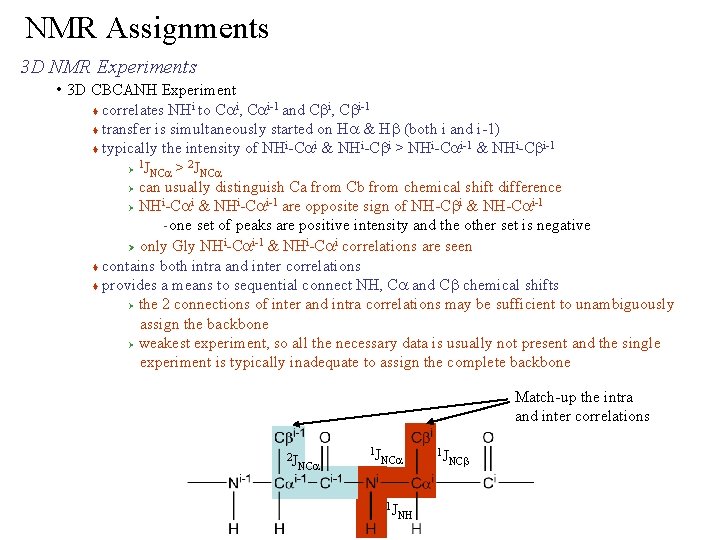
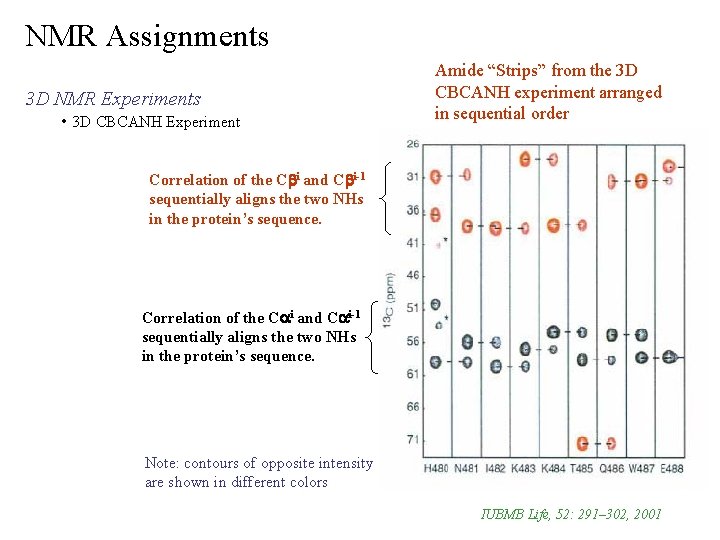
NMR Assignments 3 D NMR Experiments • 3 D CBCANH Experiment correlates NHi to Cai, Cai-1 and Cbi, Cbi-1 transfer is simultaneously started on Ha & Hb (both i and i-1) i i i-1 & NHi-Cbi-1 typically the intensity of NH -Ca & NH -Cb > NH -Ca 1 2 Ø JNCa > JNCa Ø can usually distinguish Ca from Cb from chemical shift difference i i-1 are opposite sign of NH-Cbi & NH-Cai-1 Ø NH -Ca & NH -Ca one set of peaks are positive intensity and the other set is negative i i-1 & NHi-Cai correlations are seen Ø only Gly NH -Ca contains both intra and inter correlations provides a means to sequential connect NH, Ca and Cb chemical shifts Ø the 2 connections of inter and intra correlations may be sufficient to unambiguously assign the backbone Ø weakest experiment, so all the necessary data is usually not present and the single experiment is typically inadequate to assign the complete backbone – Match-up the intra and inter correlations 2 J NCa 1 J NH 1 J NCb

NMR Assignments 3 D NMR Experiments • 3 D CBCANH Experiment

NMR Assignments 3 D NMR Experiments • 3 D CBCANH Experiment Amide “Strips” from the 3 D CBCANH experiment arranged in sequential order Correlation of the Cbi and Cbi-1 sequentially aligns the two NHs in the protein’s sequence. Correlation of the Cai and Cai-1 sequentially aligns the two NHs in the protein’s sequence. Note: contours of opposite intensity are shown in different colors IUBMB Life, 52: 291– 302, 2001

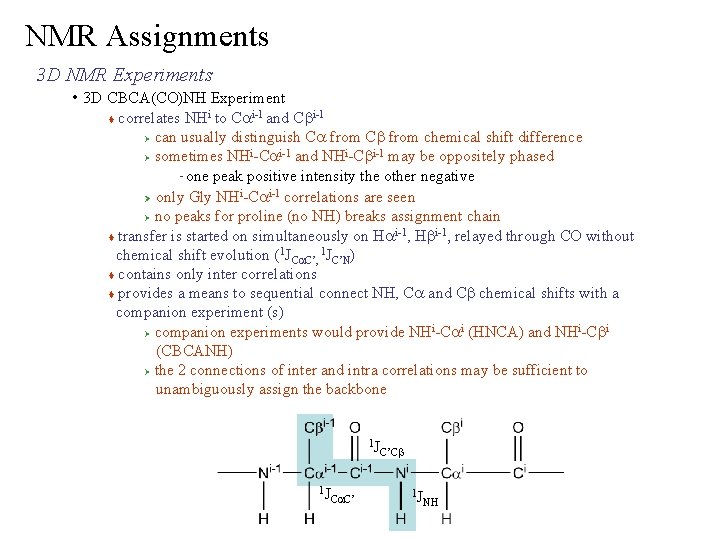
NMR Assignments 3 D NMR Experiments • 3 D CBCA(CO)NH Experiment correlates NHi to Cai-1 and Cbi-1 Ø can usually distinguish Ca from Cb from chemical shift difference i i-1 and NHi-Cbi-1 may be oppositely phased Ø sometimes NH -Ca one peak positive intensity the other negative i i-1 correlations are seen Ø only Gly NH -Ca Ø no peaks for proline (no NH) breaks assignment chain i-1 transfer is started on simultaneously on Ha , Hb , relayed through CO without chemical shift evolution (1 JCa. C’, 1 JC’N) contains only inter correlations provides a means to sequential connect NH, Ca and Cb chemical shifts with a companion experiment (s) i i Ø companion experiments would provide NH -Ca (HNCA) and NH -Cb (CBCANH) Ø the 2 connections of inter and intra correlations may be sufficient to unambiguously assign the backbone – 1 J 1 J Ca. C’ C’Cb 1 J NH

NMR Assignments 3 D NMR Experiments • 3 D CBCA(CO)NH Experiment

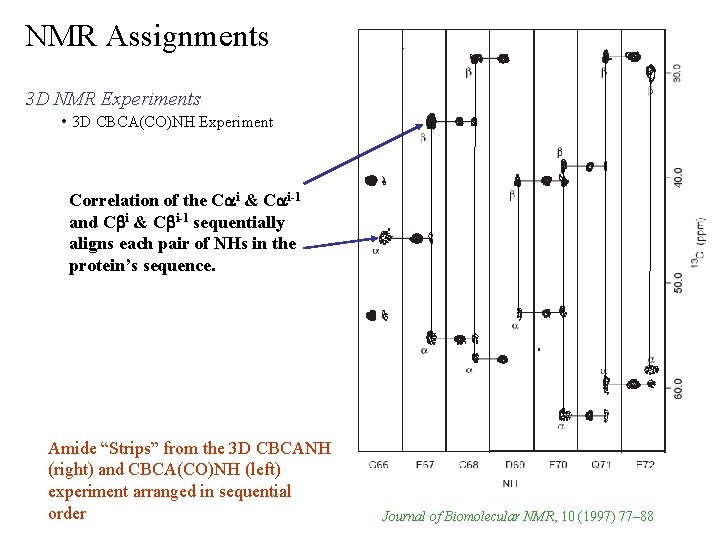
NMR Assignments 3 D NMR Experiments • 3 D CBCA(CO)NH Experiment Correlation of the Cai & Cai-1 and Cbi & Cbi-1 sequentially aligns each pair of NHs in the protein’s sequence. Amide “Strips” from the 3 D CBCANH (right) and CBCA(CO)NH (left) experiment arranged in sequential order Journal of Biomolecular NMR, 10 (1997) 77– 88

NMR Assignments

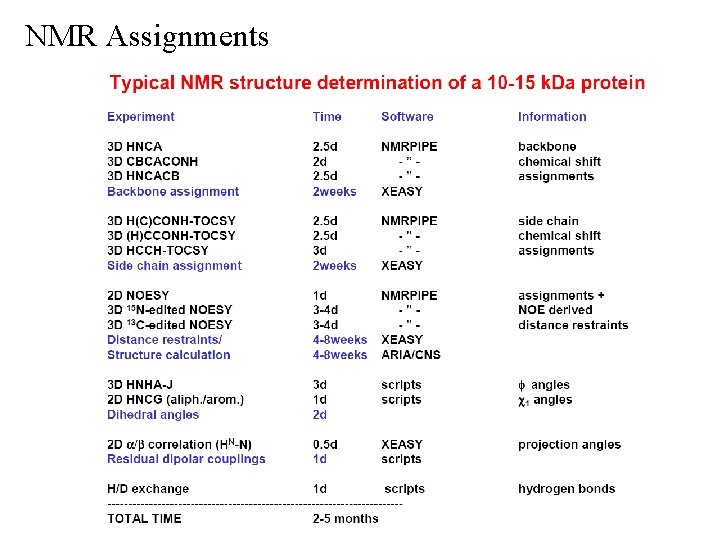
NMR Assignments 3 D NMR Experiments • Typically collect 1024 x 64 x 40 complex points in each dimension • Typical digital resolution is 0. 02 ppm (1 H) x 0. 15 ppm (13 C) x 0. 28 ppm (15 N) resolution is better in some experiments that require smaller sweep-width. need to allow for significant error when comparing chemical shift values from different NMR experiments conservative use twice digital resolution • Typical experiment time is 2. 5 days

NMR Assignments

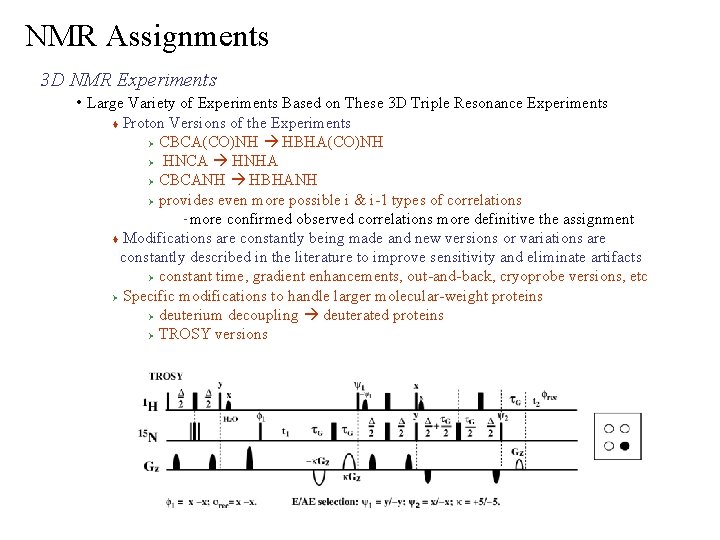
NMR Assignments 3 D NMR Experiments • Large Variety of Experiments Based on These 3 D Triple Resonance Experiments Proton Versions of the Experiments Ø CBCA(CO)NH HBHA(CO)NH Ø HNCA HNHA Ø CBCANH HBHANH Ø provides even more possible i & i-1 types of correlations more confirmed observed correlations more definitive the assignment Modifications are constantly being made and new versions or variations are constantly described in the literature to improve sensitivity and eliminate artifacts Ø constant time, gradient enhancements, out-and-back, cryoprobe versions, etc Ø Specific modifications to handle larger molecular-weight proteins Ø deuterium decoupling deuterated proteins Ø TROSY versions –

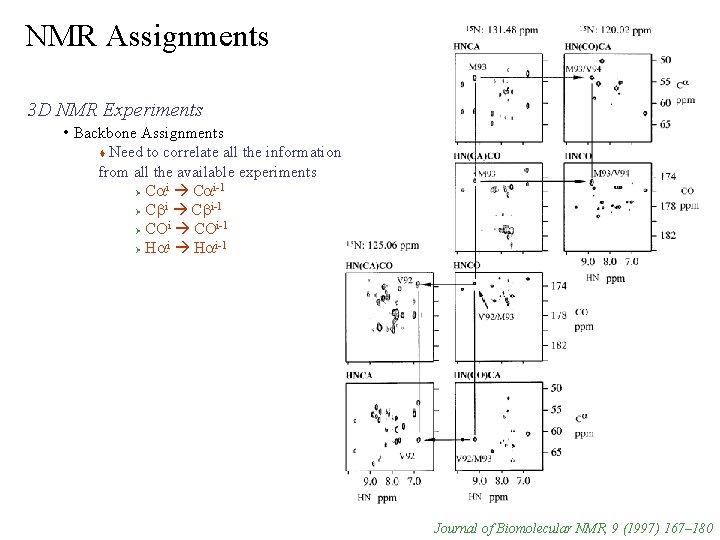
NMR Assignments 3 D NMR Experiments • Backbone Assignments Need to correlate all the information from all the available experiments i i-1 Ø Ca i i-1 Ø Cb i i-1 Ø CO i i-1 Ø Ha Journal of Biomolecular NMR, 9 (1997) 167– 180

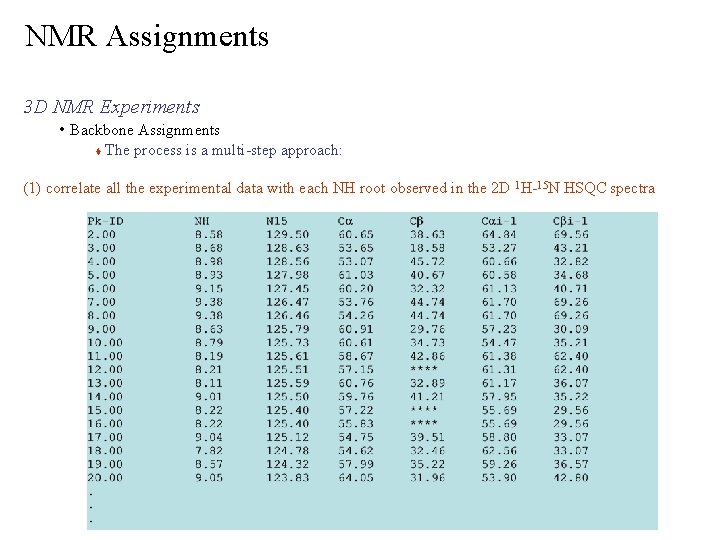
NMR Assignments 3 D NMR Experiments • Backbone Assignments The process is a multi-step approach: (1) correlate all the experimental data with each NH root observed in the 2 D 1 H-15 N HSQC spectra

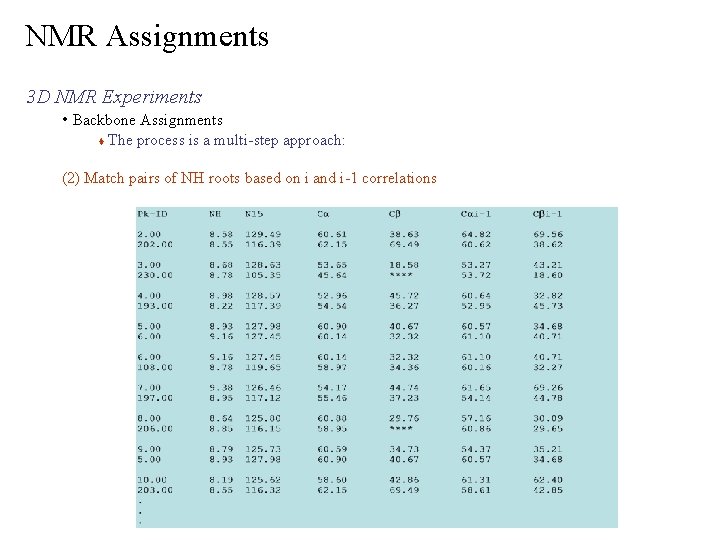
NMR Assignments 3 D NMR Experiments • Backbone Assignments The process is a multi-step approach: (2) Match pairs of NH roots based on i and i-1 correlations

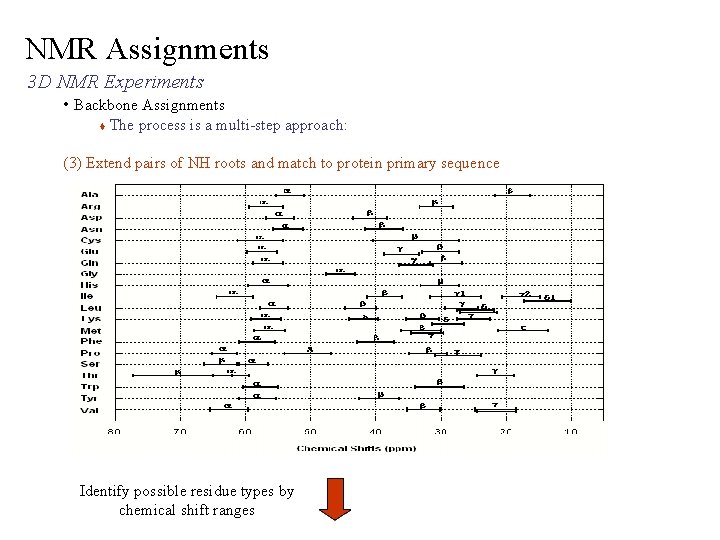
NMR Assignments 3 D NMR Experiments • Backbone Assignments The process is a multi-step approach: (3) Extend pairs of NH roots and match to protein primary sequence Identify overlapping spin-system pairs connect spinsystem pairs

NMR Assignments 3 D NMR Experiments • Backbone Assignments The process is a multi-step approach: (3) Extend pairs of NH roots and match to protein primary sequence Identify possible residue types by chemical shift ranges

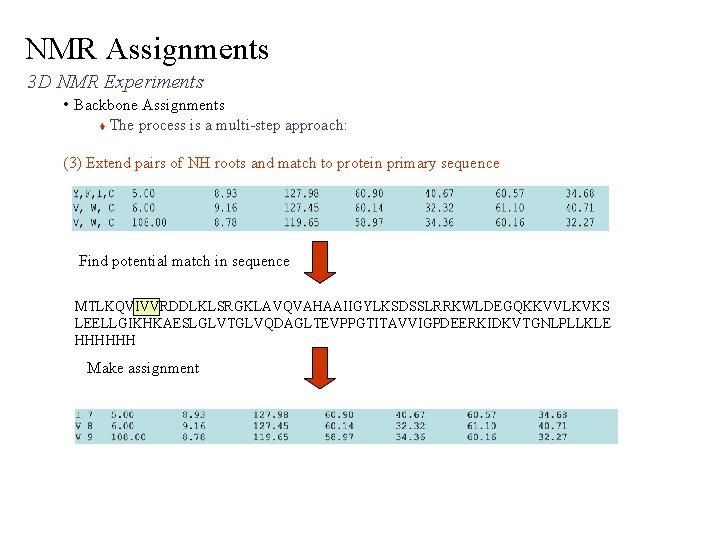
NMR Assignments 3 D NMR Experiments • Backbone Assignments The process is a multi-step approach: (3) Extend pairs of NH roots and match to protein primary sequence Find potential match in sequence MTLKQVIVVRDDLKLSRGKLAVQVAHAAIIGYLKSDSSLRRKWLDEGQKKVVLKVKS LEELLGIKHKAESLGLVTGLVQDAGLTEVPPGTITAVVIGPDEERKIDKVTGNLPLLKLE HHHHHH Make assignment

NMR Assignments 3 D NMR Experiments • Side-chain Assignments Help confirm the backbone assignment Similar in principal to 2 D assignment approach Ø Correlate entire spin-system with NH backbone Ø Use TOCSY to observe entire spin-system Ø CC(CO)NH & HCC(CO)NH – Relay magnetization from NH through side-chain carbon or hydrogen chemical shifts – Start simultaneously on all side-chain hydrogens – Also, overlap with Ca and Cb chemical shifts from other triple-resonance experiments to confirm side-chain assignments

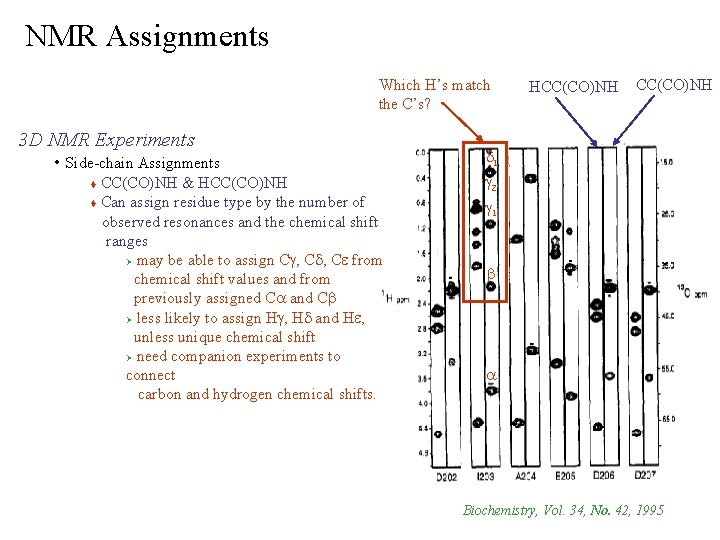
NMR Assignments Which H’s match the C’s? 3 D NMR Experiments • Side-chain Assignments CC(CO)NH & HCC(CO)NH Can assign residue type by the number of observed resonances and the chemical shift ranges Ø may be able to assign Cg, Cd, Ce from chemical shift values and from previously assigned Ca and Cb Ø less likely to assign Hg, Hd and He, unless unique chemical shift Ø need companion experiments to connect carbon and hydrogen chemical shifts. HCC(CO)NH d 1 g 2 g 1 b a Biochemistry, Vol. 34, No. 42, 1995

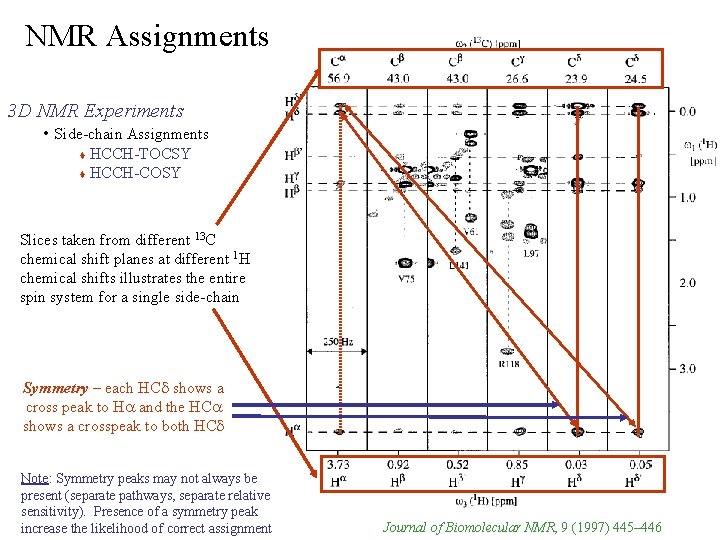
NMR Assignments 3 D NMR Experiments • Side-chain Assignments HCCH-TOCSY & HCCH-COSY 1 13 C via coupling Ø relays magnetization from side-chain and backbone H & constants Ø Experiments have symmetry – 1 Ha-13 Ca diagonal shows cross peak to 1 Hb AND – 1 Hb-13 Cb diagonal shows cross peak to 1 Ha Ø does not correlate to backbone NH no direct connection with other tripleresonance experiments – sample can be collected in D 2 O

NMR Assignments 3 D NMR Experiments • Side-chain Assignments HCCH-TOCSY HCCH-COSY Slices taken from different 13 C chemical shift planes at different 1 H chemical shifts illustrates the entire spin system for a single side-chain Symmetry – each HCd shows a cross peak to Ha and the HCa shows a crosspeak to both HCd Note: Symmetry peaks may not always be present (separate pathways, separate relative sensitivity). Presence of a symmetry peak increase the likelihood of correct assignment Journal of Biomolecular NMR, 9 (1997) 445– 446

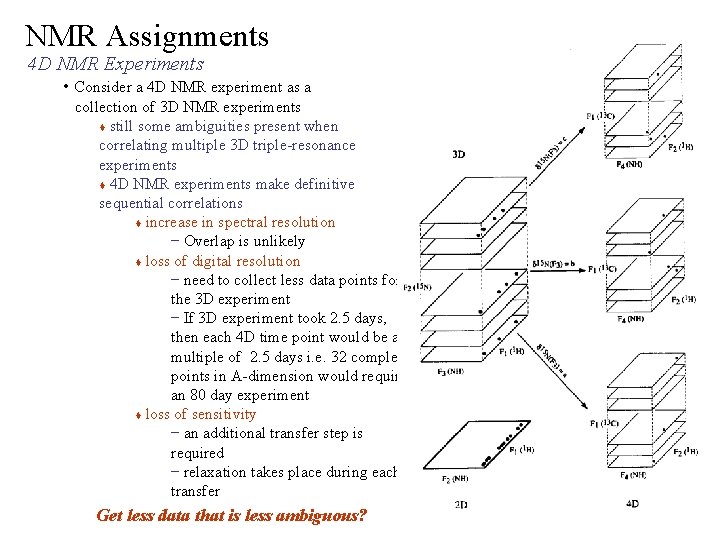
NMR Assignments 4 D NMR Experiments • Consider a 4 D NMR experiment as a collection of 3 D NMR experiments still some ambiguities present when correlating multiple 3 D triple-resonance experiments 4 D NMR experiments make definitive sequential correlations increase in spectral resolution – Overlap is unlikely loss of digital resolution – need to collect less data points for the 3 D experiment – If 3 D experiment took 2. 5 days, then each 4 D time point would be a multiple of 2. 5 days i. e. 32 complex points in A-dimension would require an 80 day experiment loss of sensitivity – an additional transfer step is required – relaxation takes place during each transfer Get less data that is less ambiguous?

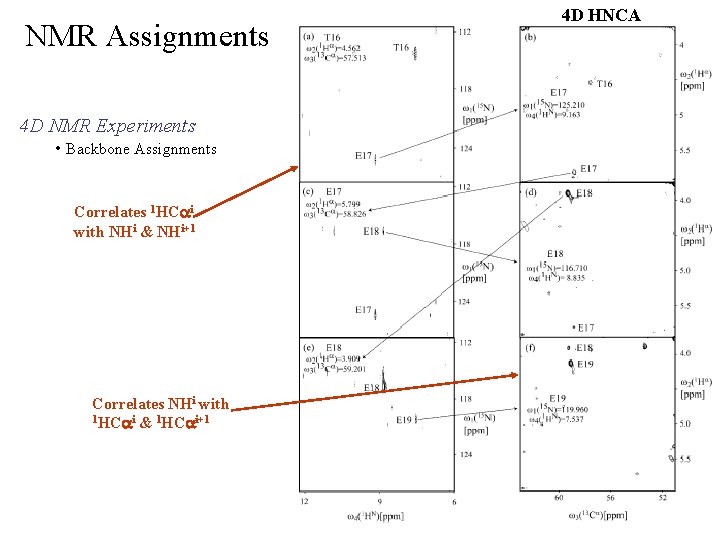
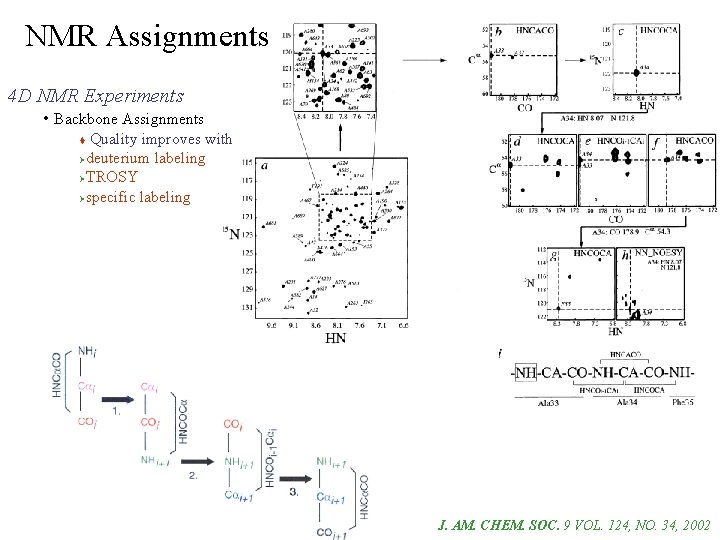
NMR Assignments 4 D NMR Experiments • Backbone Assignments Correlates 1 HCai with NHi & NHi+1 Correlates NHi with 1 HCai & 1 HCai+1 4 D HNCA

NMR Assignments 4 D NMR Experiments • Backbone Assignments Quality improves with Ødeuterium labeling ØTROSY Øspecific labeling J. AM. CHEM. SOC. 9 VOL. 124, NO. 34, 2002