Neonatal Neurology 1 Seizures HIE and Cooling CHO



























- Slides: 67

Neonatal Neurology 1: Seizures, HIE, and Cooling CHO NICU Lecture D Durand Revised 10/17/12

Incidence of Seizures • Higher in neonates than any other age group – 3/1000 live births • Most frequent in the first 10 days of life • Most frequent neonatal neurological problem

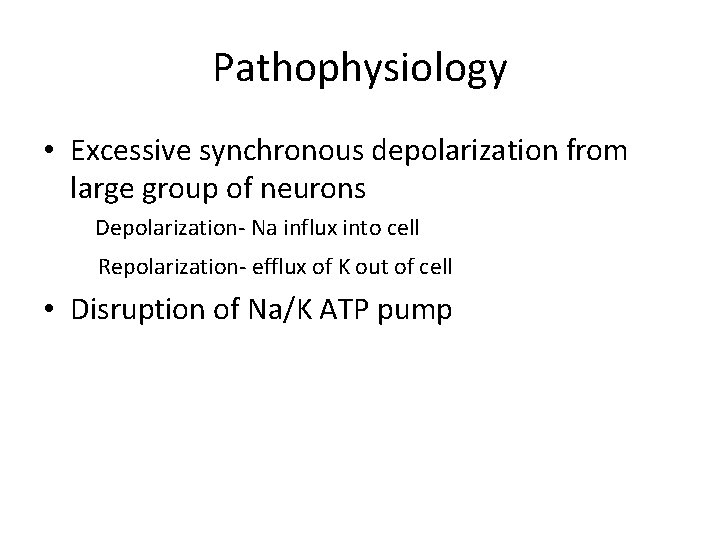
Pathophysiology • Excessive synchronous depolarization from large group of neurons Depolarization- Na influx into cell Repolarization- efflux of K out of cell • Disruption of Na/K ATP pump

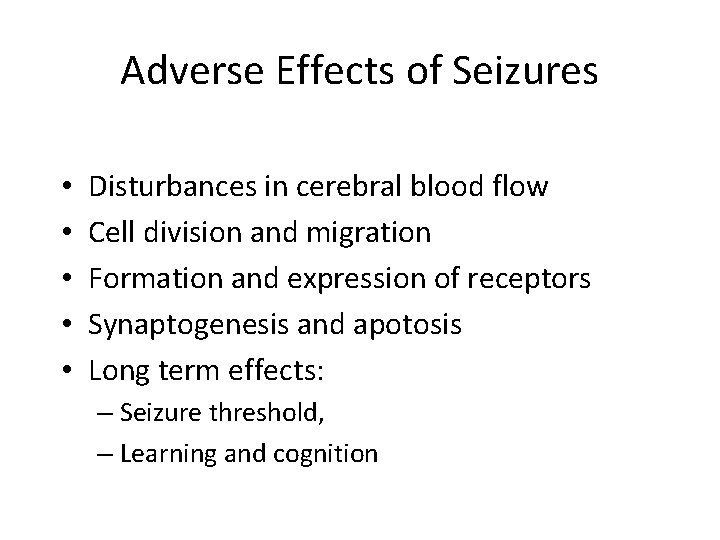
Adverse Effects of Seizures • • • Disturbances in cerebral blood flow Cell division and migration Formation and expression of receptors Synaptogenesis and apotosis Long term effects: – Seizure threshold, – Learning and cognition

Why Are Neonates More Susceptible to Sz? • Increased Excitatory pathways: – Glutamate is the major excitatory neurotransmitter in CNS – Over-expression of glutamate receptors – Neonatal glutamate receptors are more permeable to Ca++, causing increased excitability • Decreased Inhibitory pathways: – GABA is principle inhibitory neurotransmitter – Neonate has decreased binding and expression of receptor

Clonic Seizures • Focal or multifocal, rhythmic movements with slow return movement • May be associated with generalized or focal brain abnormality • Most commonly associated with EEG seizures

Tonic Seizures • Sustained flexion or extension of one extremity or the whole body • Extensive neocortical damage with uninhibited subcortically generated movements • + EEG correlate – Brainstem release?

Nonepileptic Movements • Tremulousness or jitteriness • Stimulous evoked myoclonus from metabolic encephalopathies, CNS malformation • Stopped by gentle passive flexion • Benign sleep myoclonus

Benign Sleep Myoclonus • Onset 1 st week of life • Synchronous jerks of upper and lower extremities during sleep • No EEG correlate • Provoked by benzodiazepines • Ceases upon arousal • Resolves by 2 months • Good prognosis

Causes of Seizures • • Hypoxic ischemic encephalopathy (60%) Arterial and venous stroke (15%) Intracranial hemorrhage (5%) CNS infection (5%) Metabolic disorders (5%) Congenital anomalies (5%) Other (5%)

Diagnose Underlying Etiology • Maternal and labor history – cord gases, placental pathology • Neuroimaging – MRI, US, occasionally CT • R/O infection – CBC, blood cx, LP, viral PCR • Metabolic – Glucose, Electrolytes, Ca++ – NH 3, lactate, pyruvate – Serum AA, Urine OA, very long chain fatty acids

Meningitis/Encephalitis Causes 5% of Seizures • Viral: – TORCH, Enterovirus, Parvovirus – Sz 1 st 72 hrs • Bacterial: – GBS, Listeria, E coli, Strep pneumoniae – Presents at end of 1 st week to 3 months of age • Clues: – Maternal risk factors – Late onset after 1 st week of life

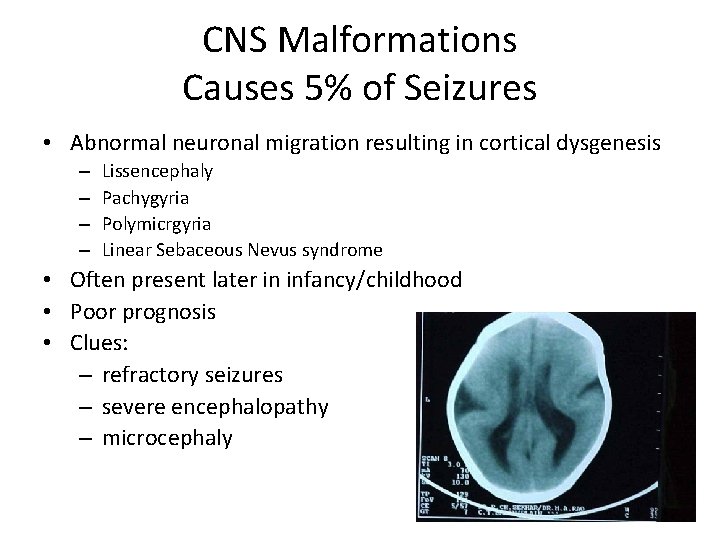
CNS Malformations Causes 5% of Seizures • Abnormal neuronal migration resulting in cortical dysgenesis – – Lissencephaly Pachygyria Polymicrgyria Linear Sebaceous Nevus syndrome • Often present later in infancy/childhood • Poor prognosis • Clues: – refractory seizures – severe encephalopathy – microcephaly

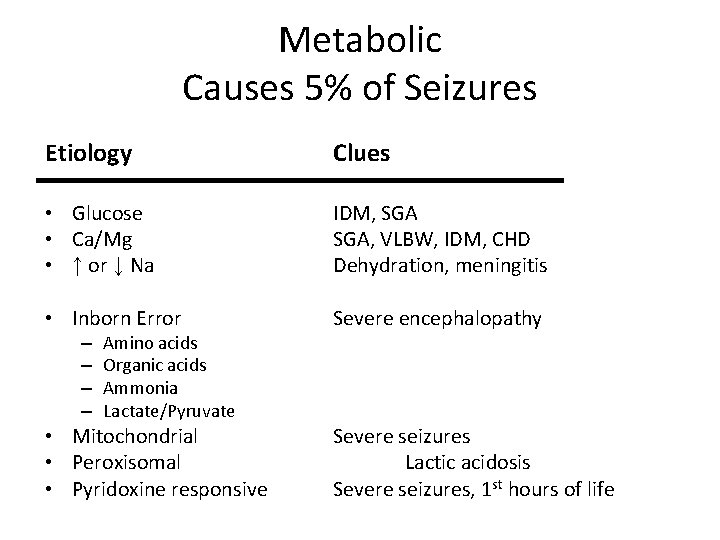
Metabolic Causes 5% of Seizures Etiology Clues • Glucose • Ca/Mg • ↑ or ↓ Na IDM, SGA, VLBW, IDM, CHD Dehydration, meningitis • Inborn Error Severe encephalopathy • Mitochondrial • Peroxisomal • Pyridoxine responsive Severe seizures Lactic acidosis Severe seizures, 1 st hours of life – – Amino acids Organic acids Ammonia Lactate/Pyruvate

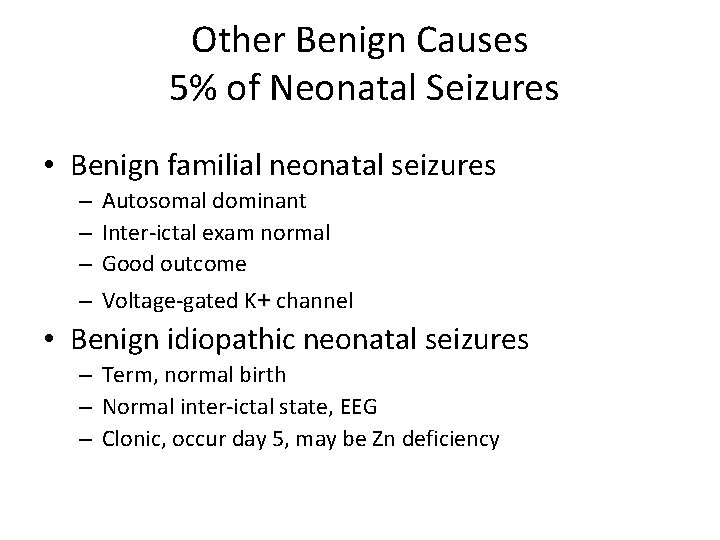
Other Benign Causes 5% of Neonatal Seizures • Benign familial neonatal seizures – – Autosomal dominant Inter-ictal exam normal Good outcome Voltage-gated K+ channel • Benign idiopathic neonatal seizures – Term, normal birth – Normal inter-ictal state, EEG – Clonic, occur day 5, may be Zn deficiency

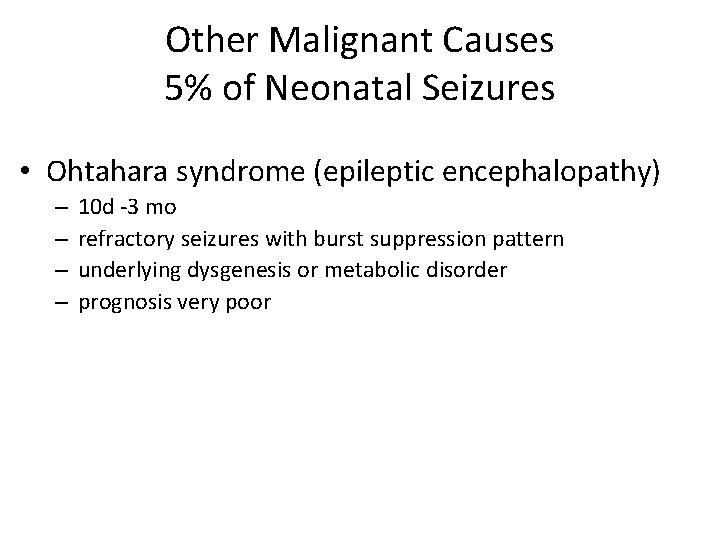
Other Malignant Causes 5% of Neonatal Seizures • Ohtahara syndrome (epileptic encephalopathy) – – 10 d -3 mo refractory seizures with burst suppression pattern underlying dysgenesis or metabolic disorder prognosis very poor

Neurologic Exam • Alertness – Lethargy, irritability • Spontaneous motor activity – Smooth, symmetric • Tone and muscle strength – Normal increased flexor tone – Muscle bulk • Cranial nerves – Corneal reflexes, facial symmetry, suck, swallow • Reflexes – DTR, clonus • Sensory – Response to touch

Neuroimaging • Ultrasound – IVH in premature infants • MRI – Developmental anomalies – Diagnostic and prognostic info for term infants with HIE • CT scan – Hemorrhagic lesions in term infants – Portable, radiation risk depends on urgency

Clinical Seizures Without EEG Correlate • May represent uninhibited brainstem reflexes • Discharges from deep cerebral structures and brainstem may not reach the cortical surface • Or may be normal movement – Myoclonic jerks

EEG Seizures Without Clinical Correlate • Occurs after treatment with anticonvulsants • Approximately 50% of EEG seizures not controlled by phenobarbital • Remember when using paralytic agents

Treatment • More difficult to suppress than in older children • Treatment is worthwhile because seizures: – May cause hemodynamic or respiratory compromise – Disrupt cerebral autoregulation – May result in cerebral energy failure and further injury

Treatment Options • Phenobarbitol • Dilantin • Kepra (? ) • Benzodiazepams

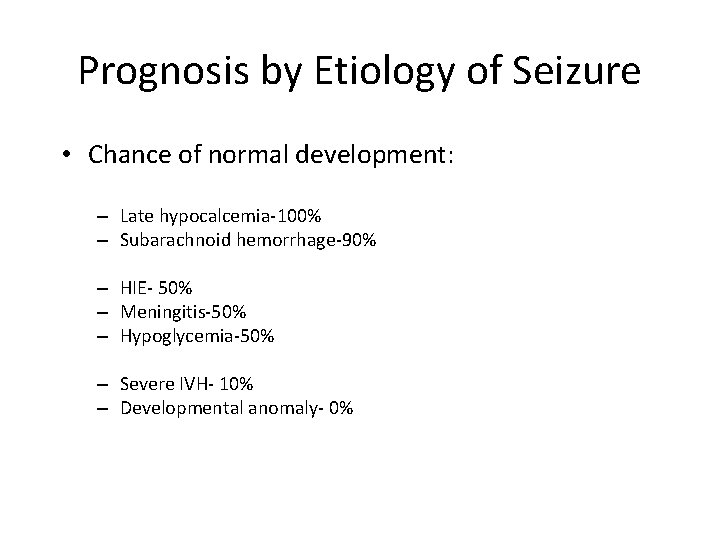
Prognosis by Etiology of Seizure • Chance of normal development: – Late hypocalcemia-100% – Subarachnoid hemorrhage-90% 50% normal outcome – HIE- 50% – Meningitis-50% – Hypoglycemia-50% – Severe IVH- 10% – Developmental anomaly- 0% Almost all are normal

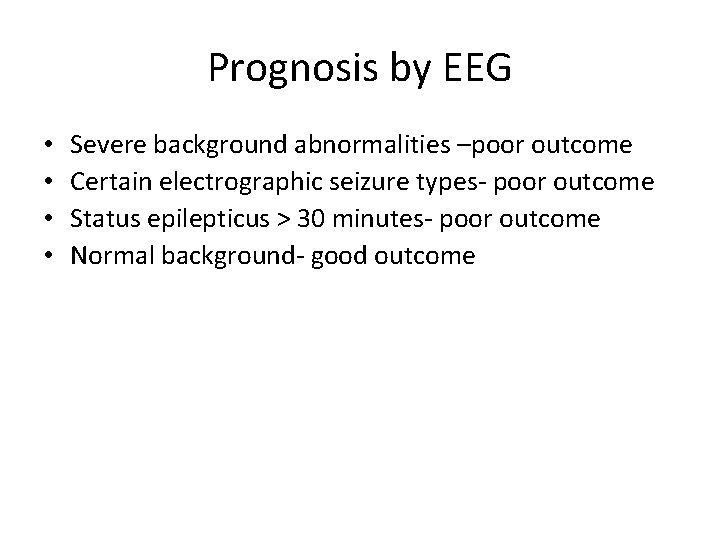
Prognosis by EEG • • Severe background abnormalities –poor outcome Certain electrographic seizure types- poor outcome Status epilepticus > 30 minutes- poor outcome Normal background- good outcome

Hypoxemic Ischemic Encephalopathy & Asphyxia

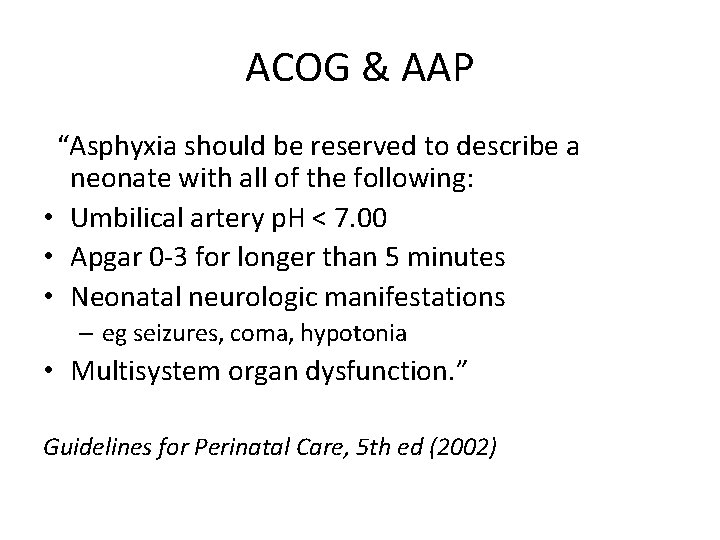
ACOG & AAP “Asphyxia should be reserved to describe a neonate with all of the following: • Umbilical artery p. H < 7. 00 • Apgar 0 -3 for longer than 5 minutes • Neonatal neurologic manifestations – eg seizures, coma, hypotonia • Multisystem organ dysfunction. ” Guidelines for Perinatal Care, 5 th ed (2002)

Apgar Score • Developed in 1953 • Designed to help with decisions about clinical care, not prognosis • Poor correlation with long-term outcome – 1 minute Apgar does not correlate with outcome – 5 min Apgar poorly correlated with outcome

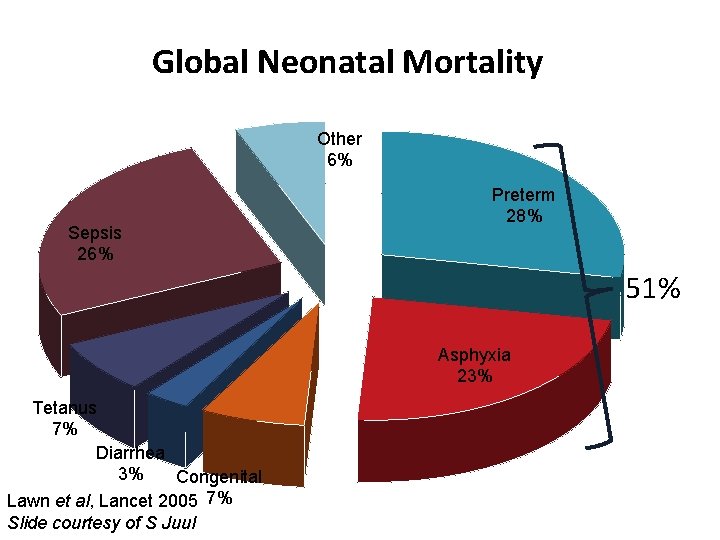
Global Neonatal Mortality Other 6% Sepsis 26% Preterm 28% 51% Asphyxia 23% Tetanus 7% Diarrhea 3% Congenital Lawn et al, Lancet 2005 7% Slide courtesy of S Juul

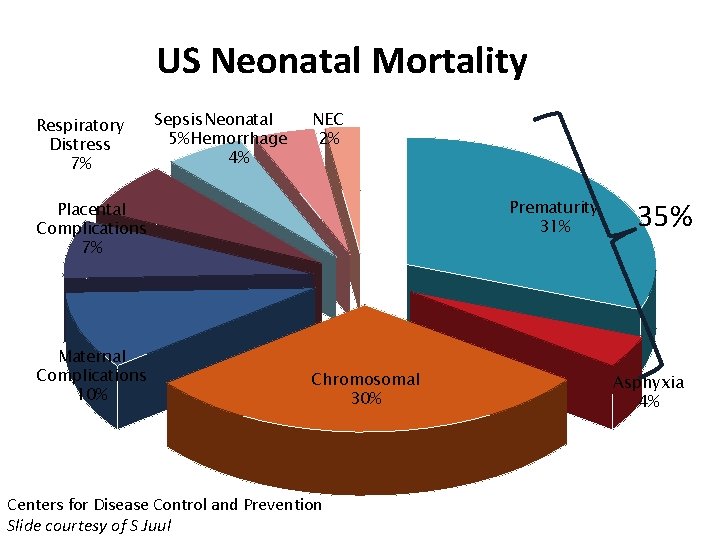
US Neonatal Mortality Respiratory Distress 7% Sepsis Neonatal 5% Hemorrhage 4% NEC 2% Prematurity 31% Placental Complications 7% Maternal Complications 10% Chromosomal 30% Centers for Disease Control and Prevention Slide courtesy of S Juul 35% Asphyxia 4%

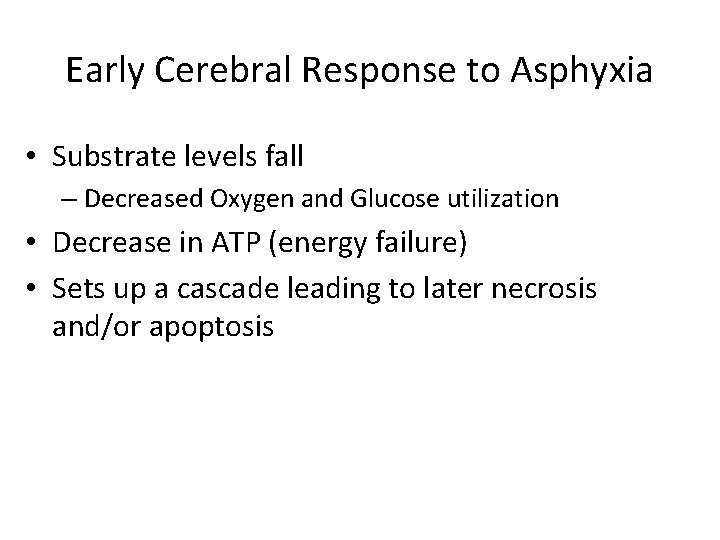
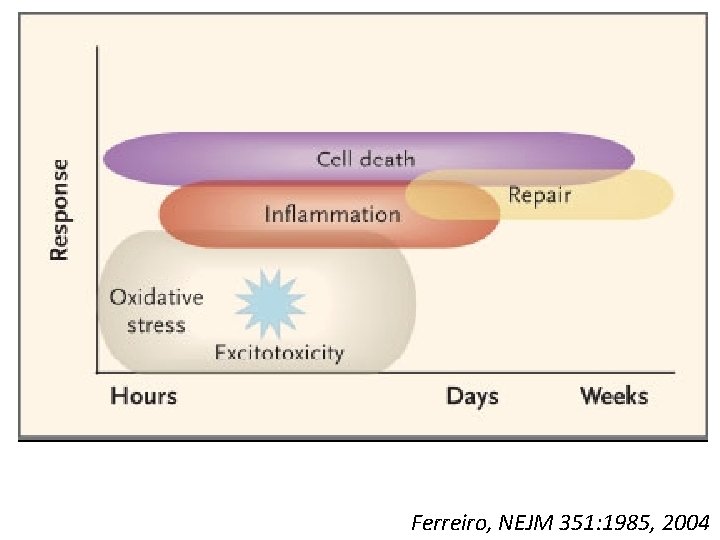
Early Cerebral Response to Asphyxia • Substrate levels fall – Decreased Oxygen and Glucose utilization • Decrease in ATP (energy failure) • Sets up a cascade leading to later necrosis and/or apoptosis

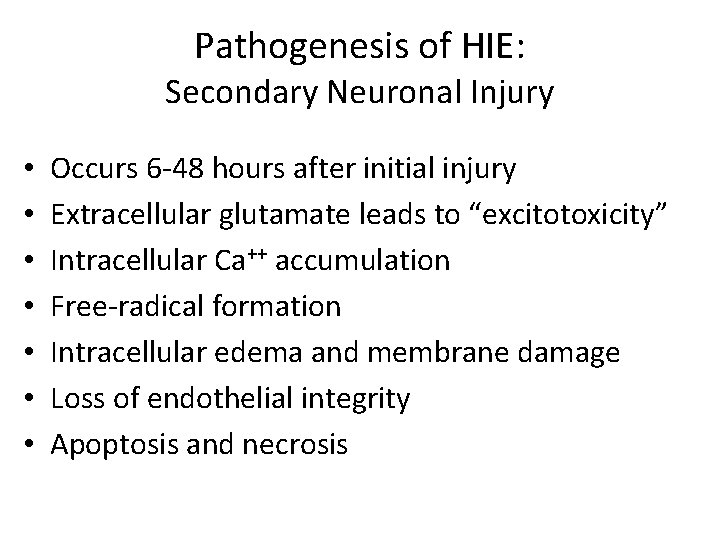
Pathogenesis of HIE: Secondary Neuronal Injury • • Occurs 6 -48 hours after initial injury Extracellular glutamate leads to “excitotoxicity” Intracellular Ca++ accumulation Free-radical formation Intracellular edema and membrane damage Loss of endothelial integrity Apoptosis and necrosis

Ferreiro, NEJM 351: 1985, 2004

Standard Therapies for Neonatal HIE • Basic support: – Maintain blood pressure in normal range – Maintain blood gases in normal range – Maintain electrolytes in normal range – Maintain blood glucose in normal range • Treat obvious seizures with anticonvulsants

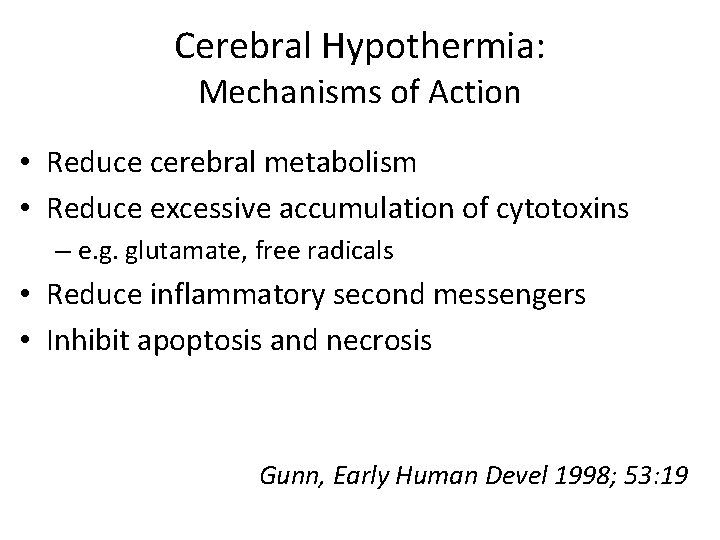
Cerebral Hypothermia: Mechanisms of Action • Reduce cerebral metabolism • Reduce excessive accumulation of cytotoxins – e. g. glutamate, free radicals • Reduce inflammatory second messengers • Inhibit apoptosis and necrosis Gunn, Early Human Devel 1998; 53: 19

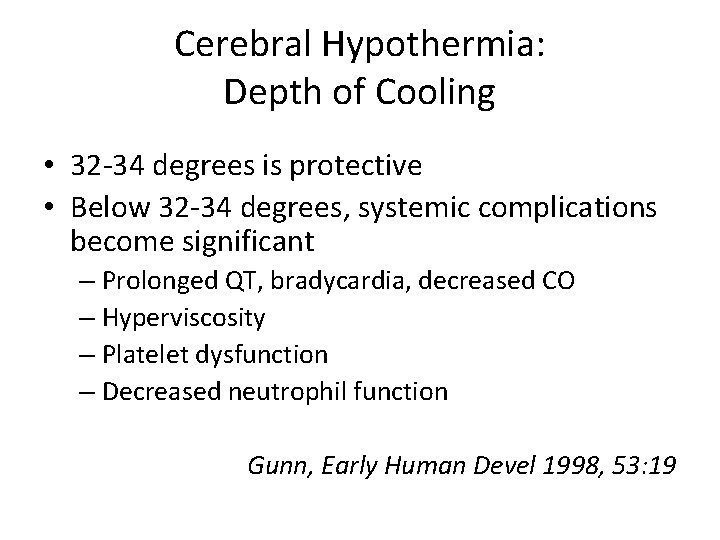
Cerebral Hypothermia: Depth of Cooling • 32 -34 degrees is protective • Below 32 -34 degrees, systemic complications become significant – Prolonged QT, bradycardia, decreased CO – Hyperviscosity – Platelet dysfunction – Decreased neutrophil function Gunn, Early Human Devel 1998, 53: 19

Cerebral Hypothermia: Animal Model • Near-term fetal sheep • Cerebral ischemia x 30 min • 5. 5 hrs later, randomized to cooling or sham cooling • Rx’d 72 hrs • Cooled animals did better – Improved EEG, decreased neuronal loss Gunn, Pediatrics 1998; 102: 1098

Cerebral Hypothermia: Animal Model • Animals did better if cooling started earlier – 90 min after cerebral ischemia • No protection seen after 8. 5 hours

Cool Cap Study “Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial” Gluckman et al, Lancet, 2005

Inclusion Criteria: History • Gestation > 36 wks • One of the following: – Apgar score < 5 at 10 minutes – Continued need for resuscitation at 10 min of age – Umbilical cord or arterial p. H < 7. 0 within 60 min of birth – Umbilical cord or arterial Base Deficit > 16 within 60 min of birth

Inclusion Criteria: Physical Exam • Moderate to severe encephalopathy • Altered state of consciousness and at least one of the following: – Lethargy, stupor, coma – Hypotonia – Abnormal reflexes – Absent or weak suck – Seizures

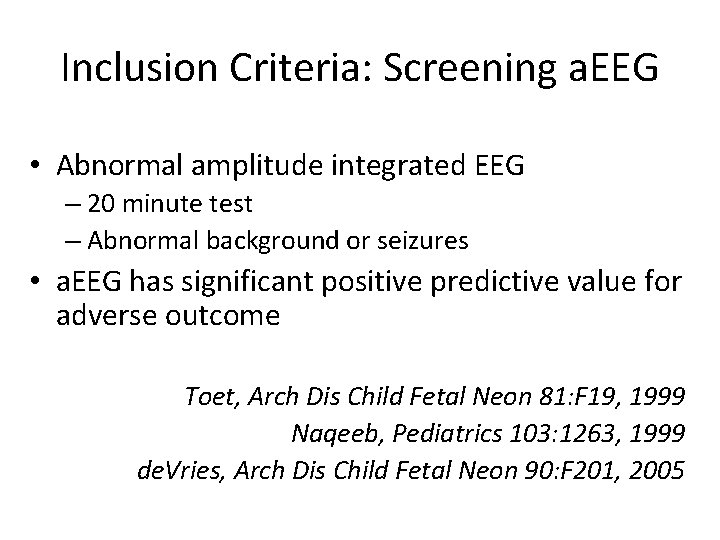
Inclusion Criteria: Screening a. EEG • Abnormal amplitude integrated EEG – 20 minute test – Abnormal background or seizures • a. EEG has significant positive predictive value for adverse outcome Toet, Arch Dis Child Fetal Neon 81: F 19, 1999 Naqeeb, Pediatrics 103: 1263, 1999 de. Vries, Arch Dis Child Fetal Neon 90: F 201, 2005

Cerebral Function Monitor (CFM) • • 3 lead amplitude integrated EEG (a. EEG) Easy to apply 20 minute tracing Easy to interpret

Cerebral Function Monitor

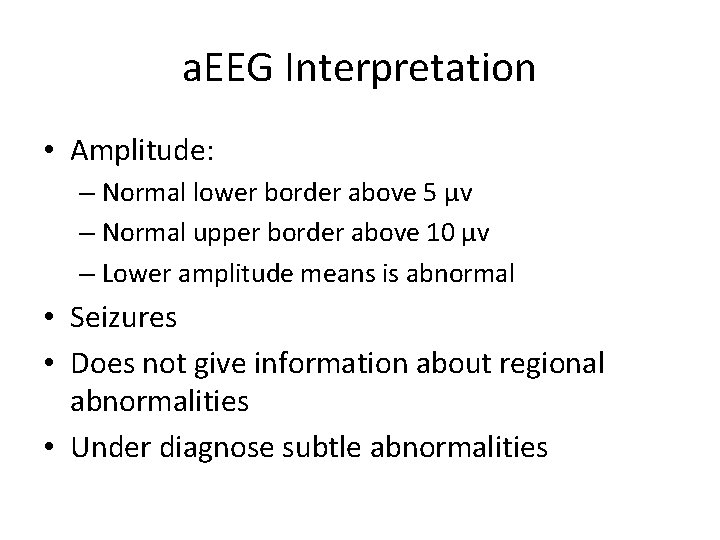
a. EEG Interpretation • Amplitude: – Normal lower border above 5 µv – Normal upper border above 10 µv – Lower amplitude means is abnormal • Seizures • Does not give information about regional abnormalities • Under diagnose subtle abnormalities

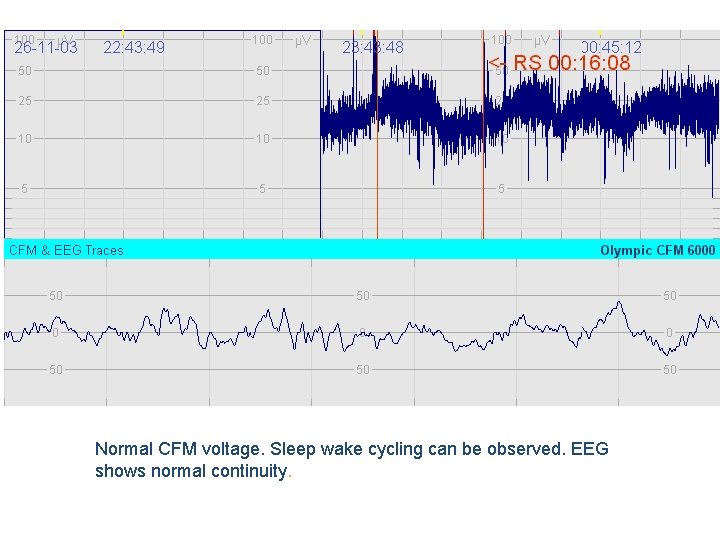
Normal CFM voltage. Sleep wake cycling can be observed. EEG shows normal continuity.

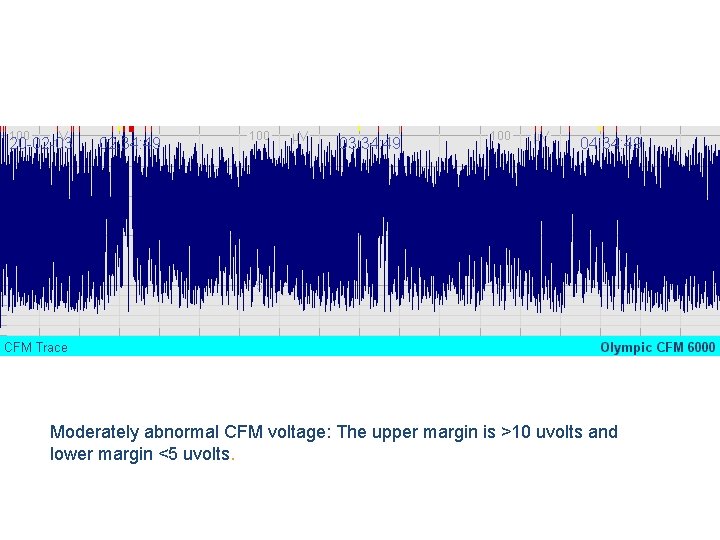
Moderately abnormal CFM voltage: The upper margin is >10 uvolts and lower margin <5 uvolts.

Cerebral Cooling Technique • Goal: Maximal cerebral cooling with only mild core cooling • Cool water (approx 10 degrees) circulates through cap • Radiant warmer adjusted to maintain rectal temp 34 -35 degrees • Both cooling and re-warming done gradually – 0. 5 degree/hour


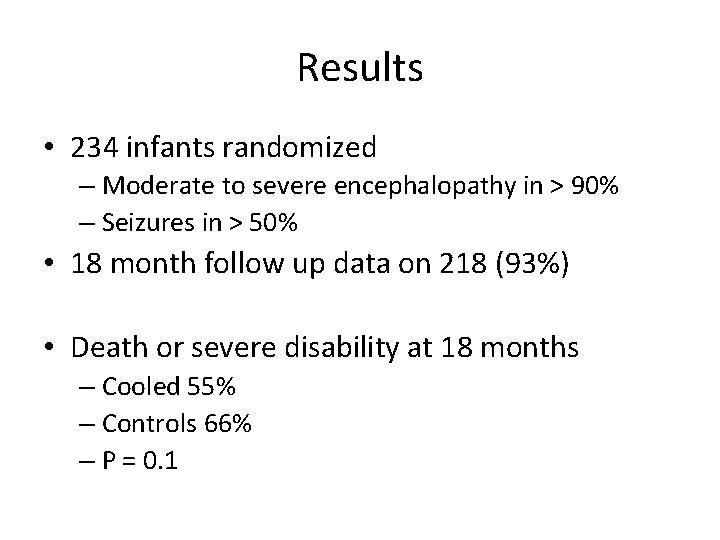
Results • 234 infants randomized – Moderate to severe encephalopathy in > 90% – Seizures in > 50% • 18 month follow up data on 218 (93%) • Death or severe disability at 18 months – Cooled 55% – Controls 66% – P = 0. 1

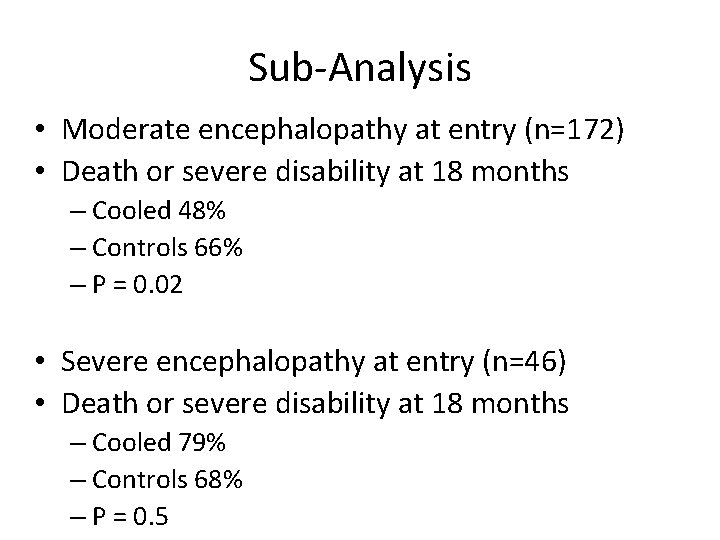
Sub-Analysis • Moderate encephalopathy at entry (n=172) • Death or severe disability at 18 months – Cooled 48% – Controls 66% – P = 0. 02 • Severe encephalopathy at entry (n=46) • Death or severe disability at 18 months – Cooled 79% – Controls 68% – P = 0. 5

Whole Body Cooling NICHD Network • • Cooling blankets Esophageal temperature 33. 5 o. C No radiant heat 72 hrs cooling, 6 hrs re-warming Shankaran, N Engl J Med 2005, 353: 1574


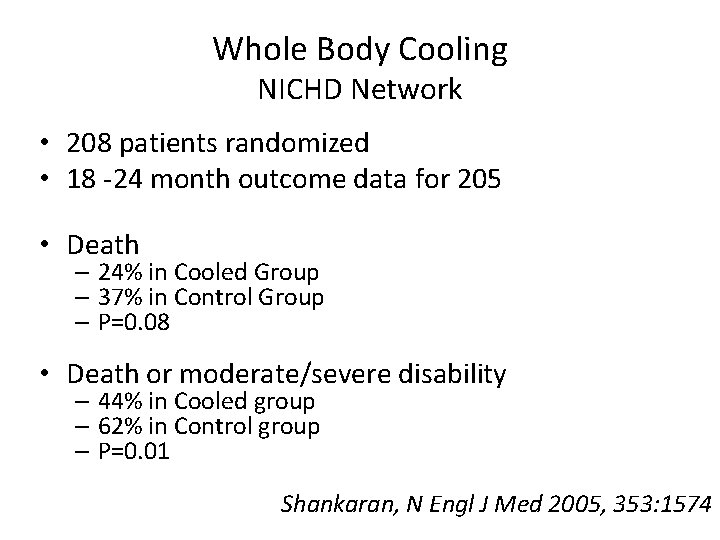
Whole Body Cooling NICHD Network • 208 patients randomized • 18 -24 month outcome data for 205 • Death – 24% in Cooled Group – 37% in Control Group – P=0. 08 • Death or moderate/severe disability – 44% in Cooled group – 62% in Control group – P=0. 01 Shankaran, N Engl J Med 2005, 353: 1574

Current US Consensus • Cooling improves outcome from HIE – No longer experimental • Selective cerebral cooling and whole body cooling are (probably) similarly effective • Cooling should (probably) be offered to patients with either moderate or severe HIE • Cooling should only be done as part of an organized program

Cooling is Not Easy! • Careful servo of cooling device to keep temperature in narrow range • With over-cooling: – Pulmonary hypertension – Bradycardia • With re-warming: – Vasodilation – Hypotension

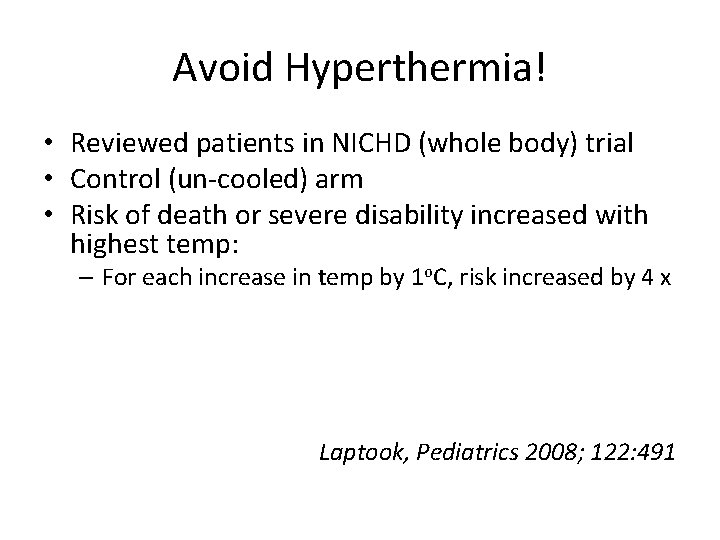
Avoid Hyperthermia! • Reviewed patients in NICHD (whole body) trial • Control (un-cooled) arm • Risk of death or severe disability increased with highest temp: – For each increase in temp by 1 o. C, risk increased by 4 x Laptook, Pediatrics 2008; 122: 491

Passive Cooling • • • Begin at delivery hospital Do not turn on radiant warmer Measure rectal temperature every 15 minutes Maintain rectal temperature 33 -35 °C Watch for complications of cooling: – Pulmonary hypertension – Systemic hypotension

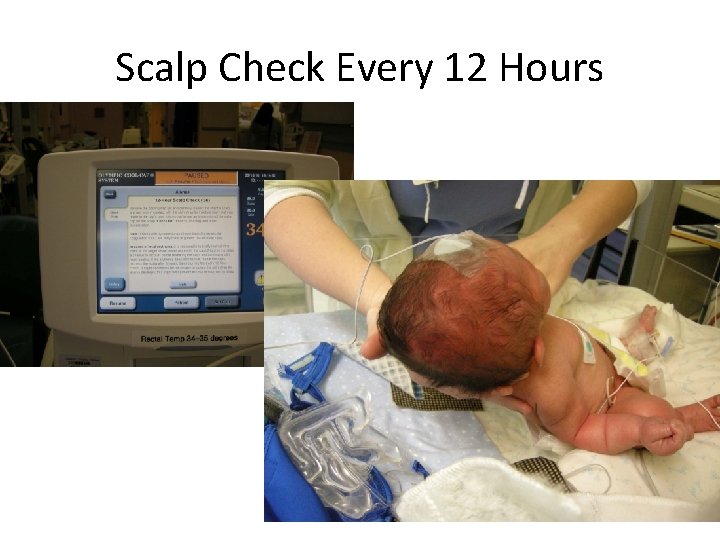
Scalp Check Every 12 Hours

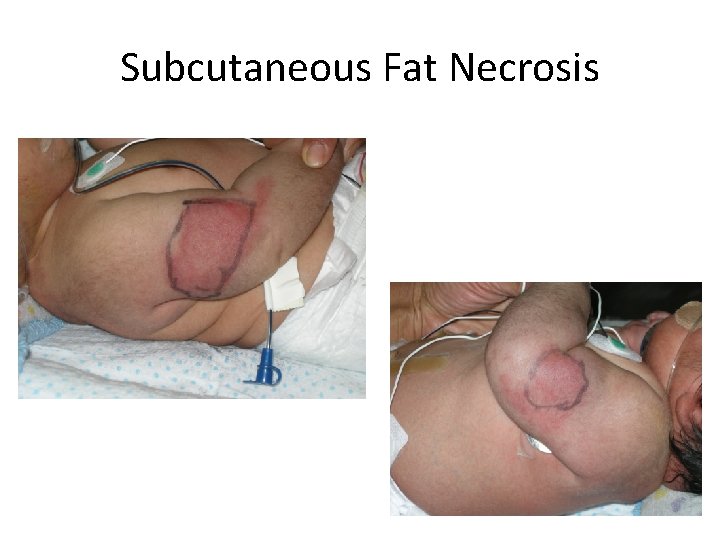
Subcutaneous Fat Necrosis

Passive Cooling • • • Begin at delivery hospital Do not turn on radiant warmer Measure rectal temperature every 15 minutes Maintain rectal temperature 34 -35 °C Watch for complications of cooling: – Pulmonary hypertension – Systemic hypotension

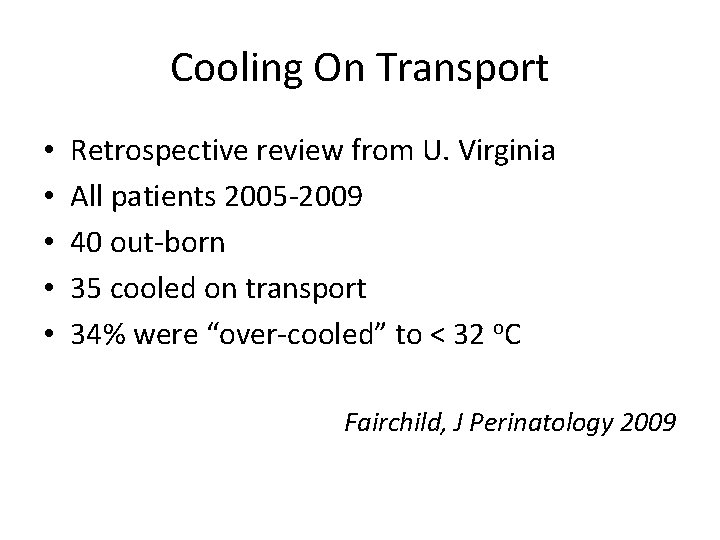
Cooling On Transport • • • Retrospective review from U. Virginia All patients 2005 -2009 40 out-born 35 cooled on transport 34% were “over-cooled” to < 32 o. C Fairchild, J Perinatology 2009

Other Neuroprotective Rx? • Mg++ – Small trial of post-natal Rx in term HIE Bhat, Pediatrics 2009; 123: e 764 • Allopurinol – Animal data encouraging – Most data focused on prenatal administration to mother • Xenon – Animal data very encouraging – Given by inhalation to intubated mother – Expensive! • Stem Cells • Erythropoietin (Epo)

Erythropoietin – Not Just for RBCs • Erythropoietin Receptor (Epo. R) is expressed by multiple cell types • Somatic – – Erythrocytes Endothelial cells Myocardiocytes Enterocytes • Nervous System – – – Neurons Oligodendrocytes Schwann cells Astrocytes Microglia

Endogenous Erythropoeitin in Brain • Epo is required for normal brain development • Epo and Epo. R are expressed in the human fetal brain by 7 weeks gestation • Epo. R is expressed in mouse neural tube neuroepithelium, and in neural crest and mesenchyme-derived cells • Epo expression follows by 0. 5 – 1 d • Epo production in brain is increased by hypoxia

Erythropoietin Safety • Extensively studied in preterm infants for RBC production and neuroprotection – More than 30 clinical trials – More than 2500 babies – No significant safety issues – Still some concerns about ROP in preemies • Widely used in preterm and term infants to prevent or treat anemia

Erythropoietin Neuroprotection Animal Studies • Erythropoietin is neuroprotective: – Benefit in short-term and long-term studies – Protects both white matter and gray matter • Multiple mechanisms: – – – – Decreases glutamate toxicity Decreases oxidative injury Decreases NO mediated injury Decreases inflammation Decreases apoptosis Promotes cell growth Promotes angiogenesis

Erythropoietin for HIE Early Clinical Trials • 167 term patients with mod-severe HIE randomized to Erythropoietin vs Placebo (without cooling) • Erythropoietin patients did better Zhu, Pediatrics 2009; 124: e 218 • Non-randomized study, showed term infants with HIE who received Erythropoietin had improved neurologic outcome at 6 months Elmahdy, Pediatrics 2010; 25: e 1135 • NEAT Trial (Neonatal Erythropoietin in Asphyxiated Term Infants) – Dose escalation and safety trial
 Freepik
Freepik Cho cho
Cho cho Freepik
Freepik Cursos de inicial 3 años
Cursos de inicial 3 años Cho cho chon chonp
Cho cho chon chonp Hagfish slime macromolecule
Hagfish slime macromolecule Tremors and seizures
Tremors and seizures Basic mechanisms underlying seizures and epilepsy
Basic mechanisms underlying seizures and epilepsy Non epileptic seizure
Non epileptic seizure Pediatric seizures
Pediatric seizures Lorna myers pnes
Lorna myers pnes Psychomotor seizures
Psychomotor seizures Psychomotor seizures
Psychomotor seizures Icp monitoring
Icp monitoring Spina bifida occulta
Spina bifida occulta Seizures
Seizures Zomisamide
Zomisamide Tonic vs clonic
Tonic vs clonic Epilepsy
Epilepsy Benign febrile convulsion
Benign febrile convulsion Simple partial seizures vs complex
Simple partial seizures vs complex Simple partial seizures vs complex
Simple partial seizures vs complex Levene staging
Levene staging Open hie
Open hie Open hie
Open hie Simone hie
Simone hie Roshini jayasankar
Roshini jayasankar Open hie
Open hie Apgar
Apgar Sarnat staging
Sarnat staging Hie transistor
Hie transistor Camden hie
Camden hie Terminology services
Terminology services Open hie
Open hie Intranet kfmc
Intranet kfmc Akaims
Akaims Albert camus tujec
Albert camus tujec The walton centre for neurology and neurosurgery
The walton centre for neurology and neurosurgery Yorkshire neonatal network
Yorkshire neonatal network Neurology strength scale
Neurology strength scale Vanderbilt neurology residents
Vanderbilt neurology residents Mayzent fdo
Mayzent fdo Mary bridge neurology
Mary bridge neurology Duplex ultrasound vs doppler
Duplex ultrasound vs doppler West coast neurology
West coast neurology Surgery shelf percentiles
Surgery shelf percentiles Rrerl
Rrerl Nlff neuro
Nlff neuro Nex exam neurology
Nex exam neurology Department of neurology
Department of neurology Dr forbes
Dr forbes Dr nin bajaj
Dr nin bajaj Joseph berger md neurology
Joseph berger md neurology Contrallateral
Contrallateral Nlff neuro
Nlff neuro Umass memorial medical center pharmacy residency
Umass memorial medical center pharmacy residency Midwest neurology
Midwest neurology Neurology
Neurology Miqtu
Miqtu Neurology near loomis
Neurology near loomis Lahey clinic anesthesiology residency
Lahey clinic anesthesiology residency Nociceptive sensation
Nociceptive sensation Uf neurology residents
Uf neurology residents Midwest neurology
Midwest neurology Erlanger neurology
Erlanger neurology Cerebral palsy iap
Cerebral palsy iap Vasculopatia lenticulo estriada
Vasculopatia lenticulo estriada Circulação neonatal
Circulação neonatal