N 2 ADSORPTION theory experiment and application OUTLINE



- Slides: 37

N 2 – ADSORPTION theory, experiment and application OUTLINE: - Short review of theory - Experimental methods and machinery at the lab - Case studies

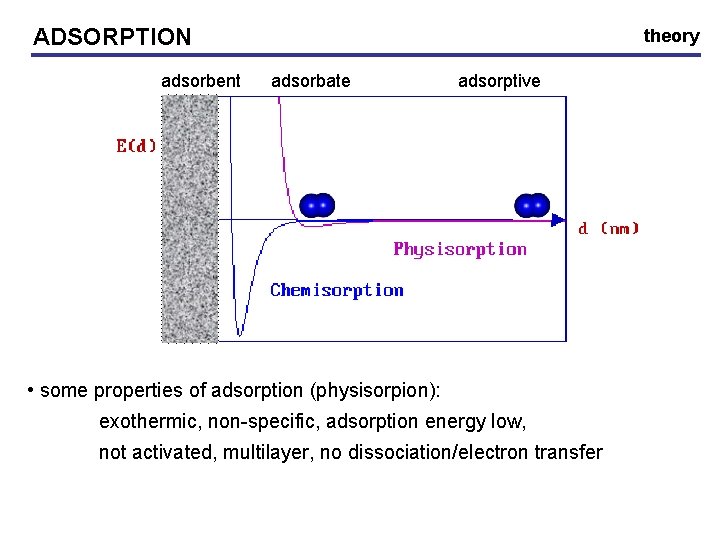
ADSORPTION adsorbent theory adsorbate adsorptive • some properties of adsorption (physisorpion): exothermic, non-specific, adsorption energy low, not activated, multilayer, no dissociation/electron transfer

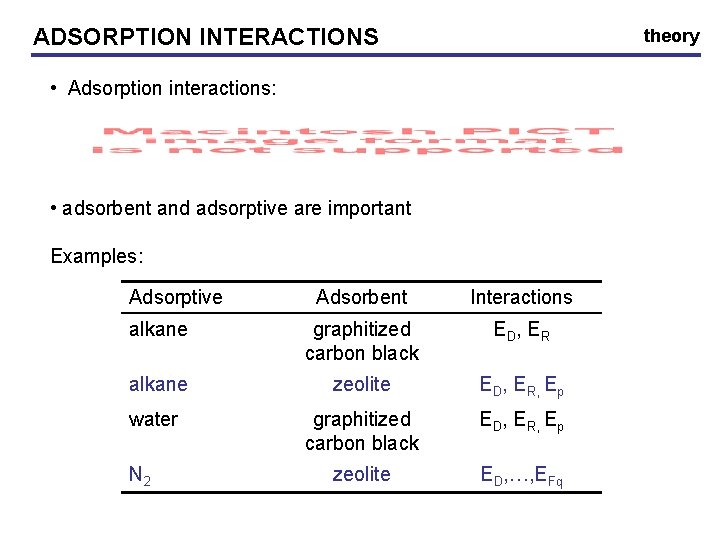
ADSORPTION INTERACTIONS theory • Adsorption interactions: • adsorbent and adsorptive are important Examples: Adsorptive Adsorbent Interactions alkane graphitized carbon black ED, ER alkane zeolite ED, ER, Ep water graphitized carbon black ED, ER, Ep zeolite ED, …, EFq N 2

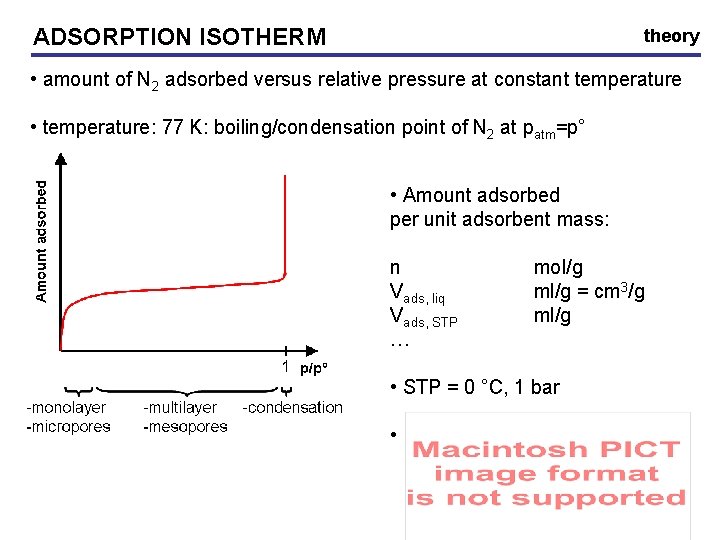
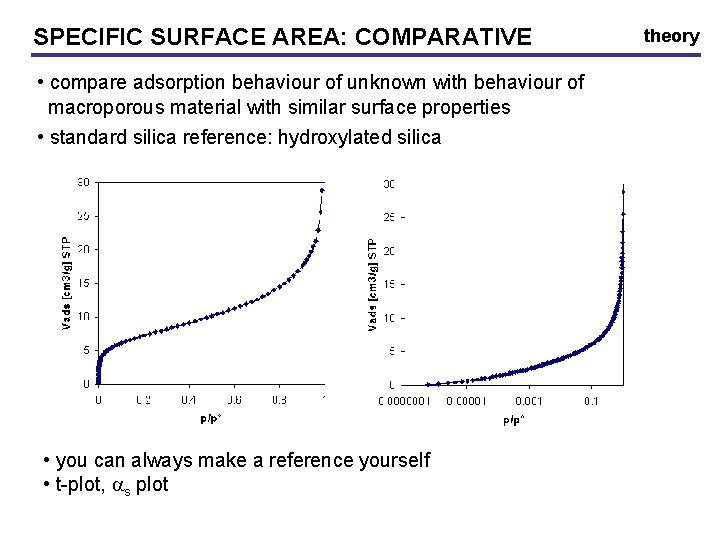
ADSORPTION ISOTHERM theory • amount of N 2 adsorbed versus relative pressure at constant temperature • temperature: 77 K: boiling/condensation point of N 2 at patm=p° • Amount adsorbed per unit adsorbent mass: n Vads, liq Vads, STP … mol/g ml/g = cm 3/g ml/g • STP = 0 °C, 1 bar •

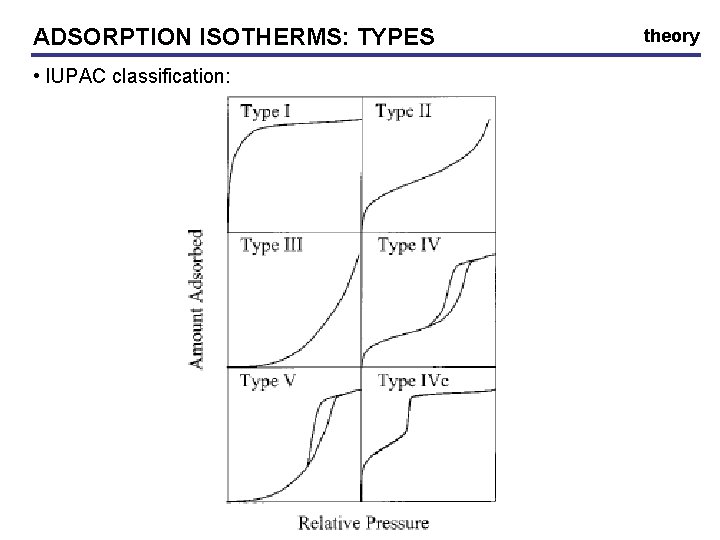
ADSORPTION ISOTHERMS: TYPES • IUPAC classification: theory

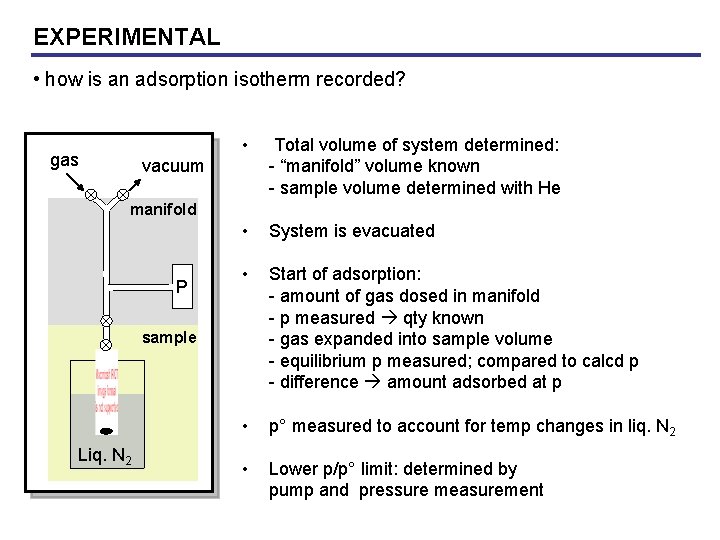
EXPERIMENTAL • how is an adsorption isotherm recorded? gas • Total volume of system determined: - “manifold” volume known - sample volume determined with He • System is evacuated • Start of adsorption: - amount of gas dosed in manifold - p measured qty known - gas expanded into sample volume - equilibrium p measured; compared to calcd p - difference amount adsorbed at p • p° measured to account for temp changes in liq. N 2 • Lower p/p° limit: determined by pump and pressure measurement vacuum manifold P sample Liq. N 2

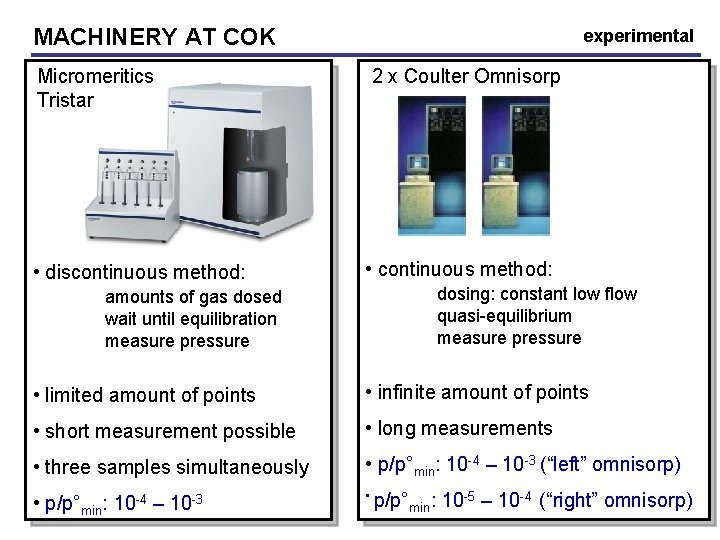
MACHINERY AT COK Micromeritics Tristar • discontinuous method: experimental 2 x Coulter Omnisorp • continuous method: dosing: constant low flow quasi-equilibrium measure pressure amounts of gas dosed wait until equilibration measure pressure • limited amount of points • infinite amount of points • short measurement possible • long measurements • three samples simultaneously • p/p°min: 10 -4 – 10 -3 (“left” omnisorp) • p/p°min: 10 -4 – 10 -3 • p/p° min: 10 -5 – 10 -4 (“right” omnisorp)

SPECIFIC SURFACE AREA theory • 2 frequently used methods: 1. BET 1. Comparative methods: • t-plot or a. S-plot general remark on concept of determination of the specific surface area : - surface roughness – probe molecule size - measured area dependent on size of probe molecule: fractal property

SPECIFIC SURFACE AREA: BET • theory Two steps: 1. Evaluation of the monolayer capacity Vm 2. N 2 cross-sectional area a. N 2: Vm x a. N 2 = S • Not very accurate (20 % error) because: 1. BET model assumptions usually not valid homogeneous surface, no lateral interactions, infinite number of layers possible, n+1: liquefaction energy 2. value of cross-sectional area not determined accurately/ dependent on adsorbent surface (usually: 0. 162 nm 2) 3. Pore size/shape may change interpretation Small cilindrical pores: 10 % underestimation • Mesoporous silica: overestimation

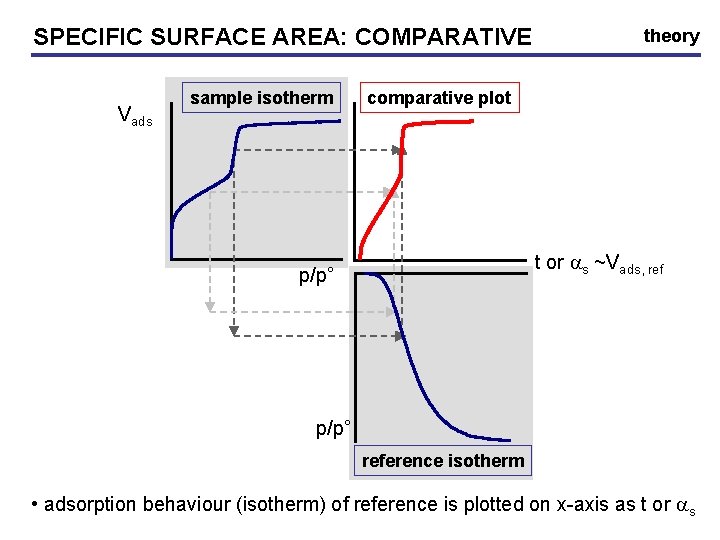
SPECIFIC SURFACE AREA: AREA COMPARATIVE • compare adsorption behaviour of unknown with behaviour of macroporous material with similar surface properties • standard silica reference: hydroxylated silica • you can always make a reference yourself • t-plot, as plot theory

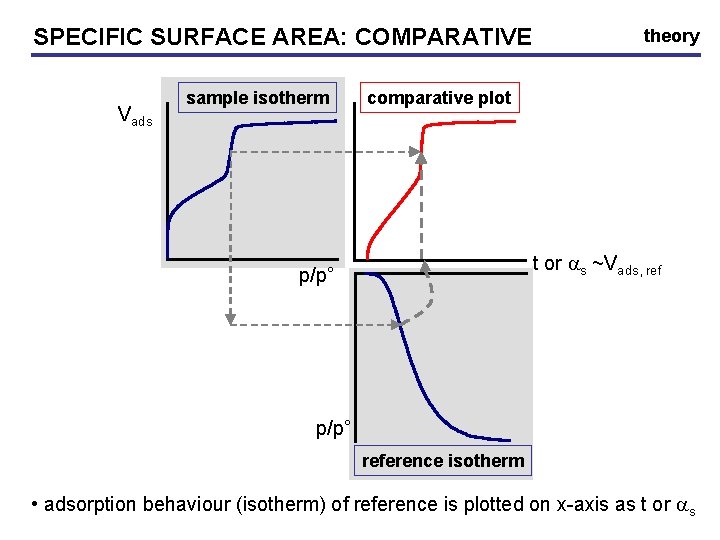
SPECIFIC SURFACE AREA: AREA COMPARATIVE Vads sample isotherm theory comparative plot t or as ~Vads, ref p/p° reference isotherm • adsorption behaviour (isotherm) of reference is plotted on x-axis as t or as

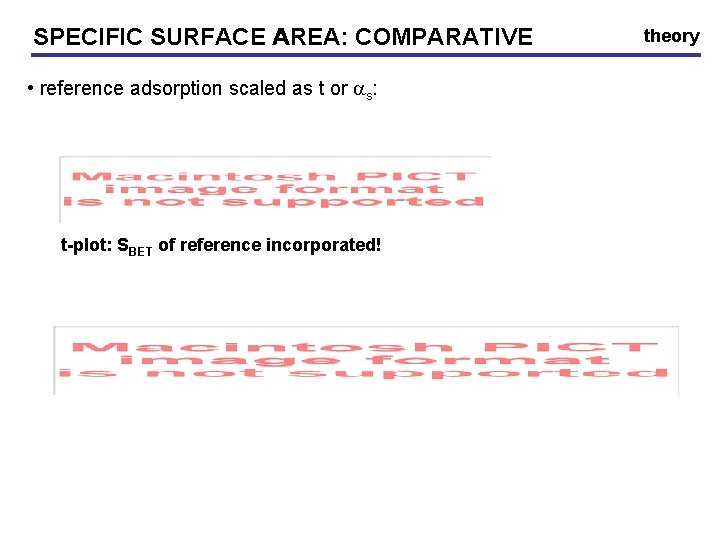
SPECIFIC SURFACE AREA: COMPARATIVE • reference adsorption scaled as t or as: t-plot: SBET of reference incorporated! theory

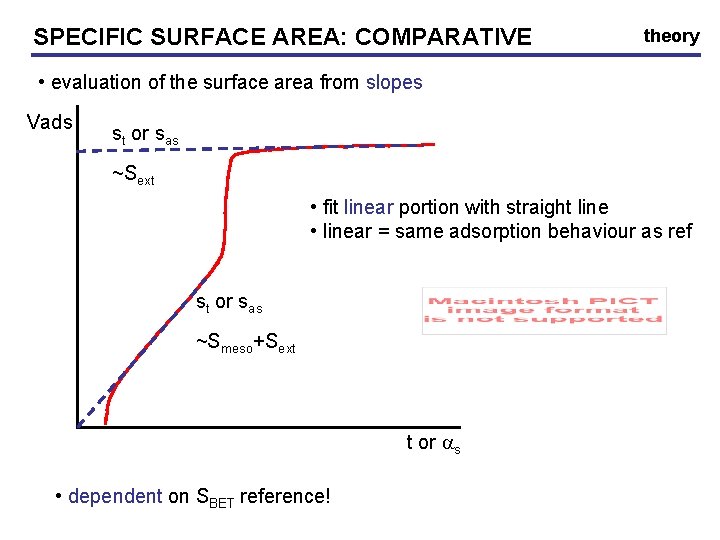
SPECIFIC SURFACE AREA: AREA COMPARATIVE theory • evaluation of the surface area from slopes Vads st or sas ~Sext • fit linear portion with straight line • linear = same adsorption behaviour as ref st or sas ~Smeso+Sext t or as • dependent on SBET reference!

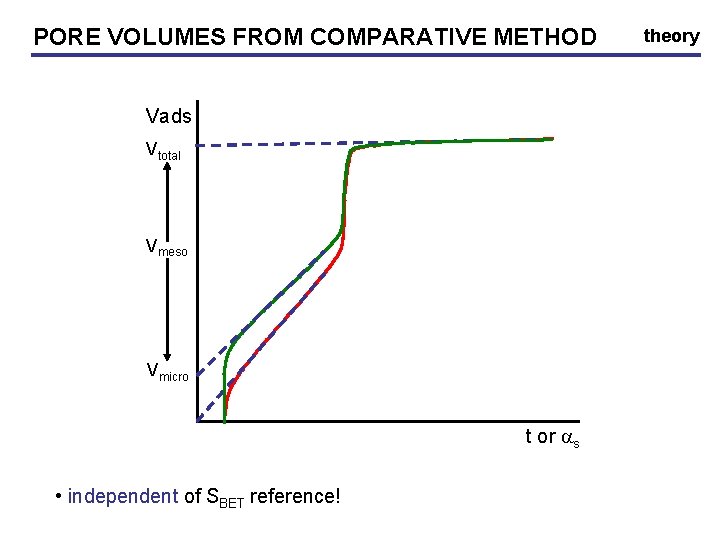
PORE VOLUMES FROM COMPARATIVE METHOD Vads Vtotal Vmeso Vmicro t or as • independent of SBET reference! theory

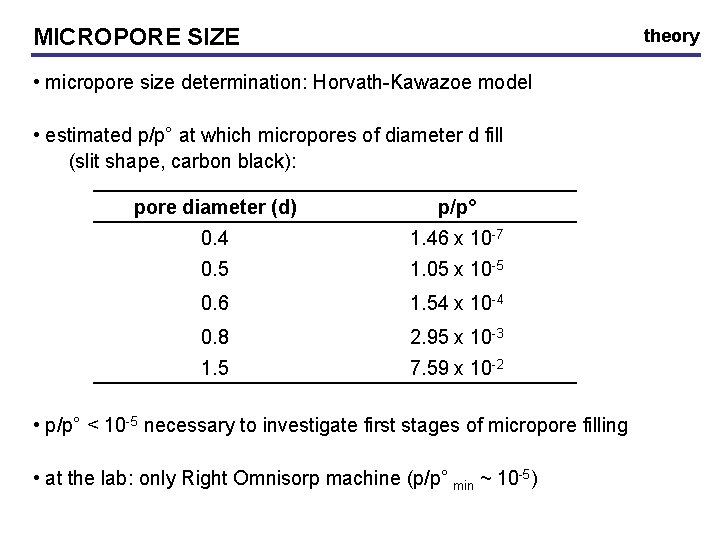
MICROPORE SIZE theory • micropore size determination: Horvath-Kawazoe model • estimated p/p° at which micropores of diameter d fill (slit shape, carbon black): pore diameter (d) p/p° 0. 4 1. 46 x 10 -7 0. 5 1. 05 x 10 -5 0. 6 1. 54 x 10 -4 0. 8 2. 95 x 10 -3 1. 5 7. 59 x 10 -2 • p/p° < 10 -5 necessary to investigate first stages of micropore filling • at the lab: only Right Omnisorp machine (p/p° min ~ 10 -5)

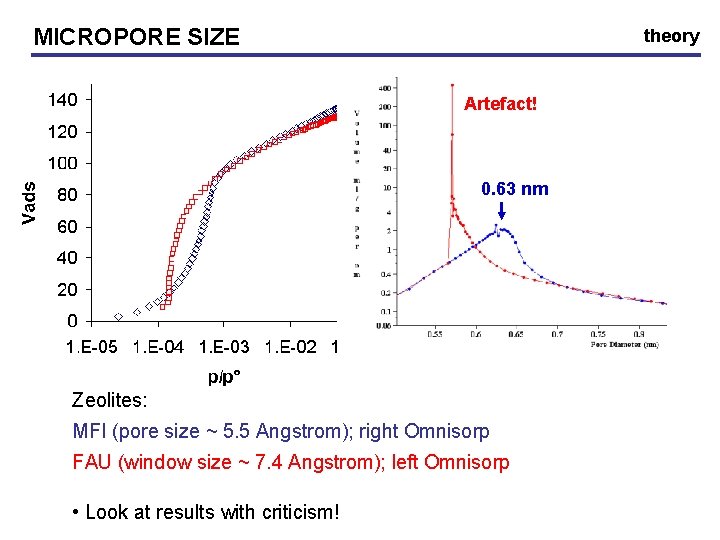
MICROPORE SIZE theory Artefact! 0. 63 nm Zeolites: MFI (pore size ~ 5. 5 Angstrom); right Omnisorp FAU (window size ~ 7. 4 Angstrom); left Omnisorp • Look at results with criticism!

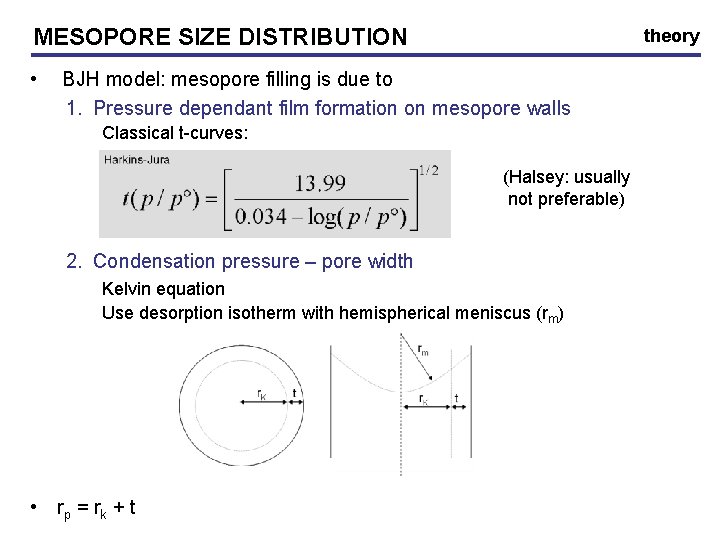
MESOPORE SIZE DISTRIBUTION • theory BJH model: mesopore filling is due to 1. Pressure dependant film formation on mesopore walls Classical t-curves: (Halsey: usually not preferable) 2. Condensation pressure – pore width Kelvin equation Use desorption isotherm with hemispherical meniscus (rm) • rp = rk + t

MESOPORE SIZE DISTRIBUTION BJH model: problems with ordered mesoporous materials 1. Adsorbed layer thickness underestimated (wall curvature) 2. Use of desorption branch: • Instability of condensate at p/p° ~ 0. 4 (4 – 5. 5 nm) during desorption: tensile strength effect Vads • theory 0. 4 • p/p° Desorption branch more prone to material imperfections (network effects)

MESOPORE SIZE DISTRIBUTION theory • improved BJH model: KJS (Kruk – Jaroniec – Sayari) • widely used to determine mesopore size OMM 1. Corrected t-curve based on standard adsorption isotherm 2. Correction for increased film thickness due to mesopore curvature 3. Use of ADsorption isotherm (Kelvin for hemispherical meniscus) • Application between 2 – 10 nm; above 6 nm accuracy decreases • Application for cilindical pores (MCM-41) or cilinder-like pores (MCM-48)

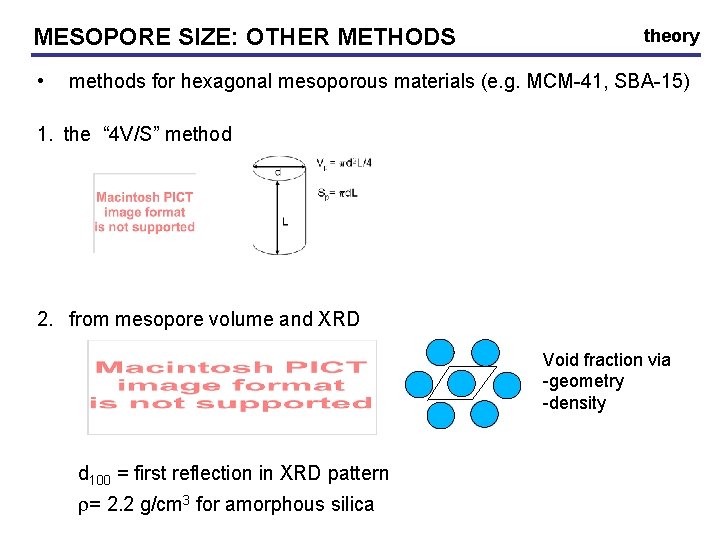
MESOPORE SIZE: OTHER METHODS • theory methods for hexagonal mesoporous materials (e. g. MCM-41, SBA-15) 1. the “ 4 V/S” method 2. from mesopore volume and XRD Void fraction via -geometry -density d 100 = first reflection in XRD pattern r= 2. 2 g/cm 3 for amorphous silica

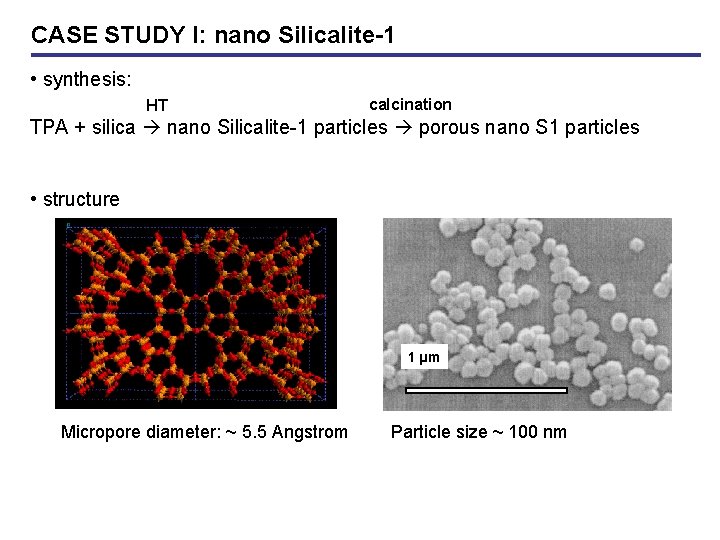
CASE STUDY I: nano Silicalite-1 • synthesis: HT calcination TPA + silica nano Silicalite-1 particles porous nano S 1 particles • structure 1 µm Micropore diameter: ~ 5. 5 Angstrom Particle size ~ 100 nm

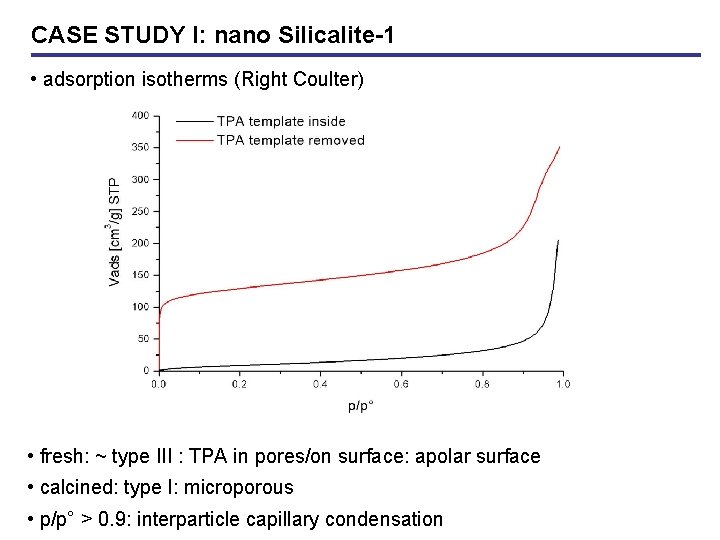
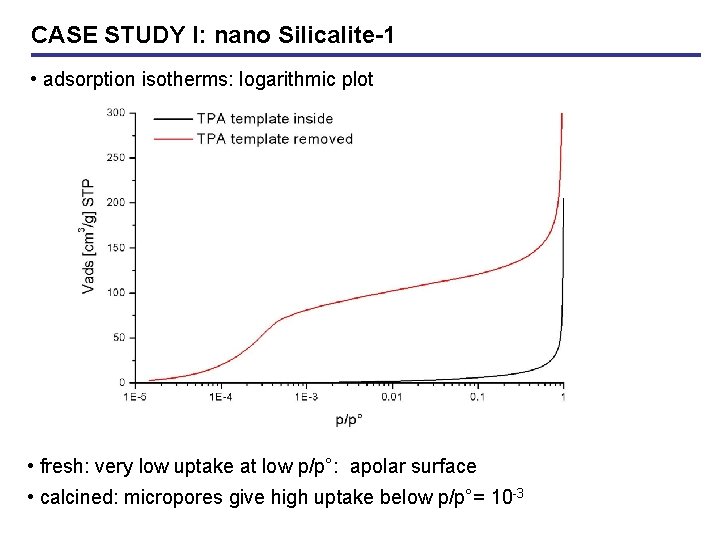
CASE STUDY I: nano Silicalite-1 • adsorption isotherms (Right Coulter) • fresh: ~ type III : TPA in pores/on surface: apolar surface • calcined: type I: microporous • p/p° > 0. 9: interparticle capillary condensation

CASE STUDY I: nano Silicalite-1 • adsorption isotherms: logarithmic plot • fresh: very low uptake at low p/p°: apolar surface • calcined: micropores give high uptake below p/p°= 10 -3

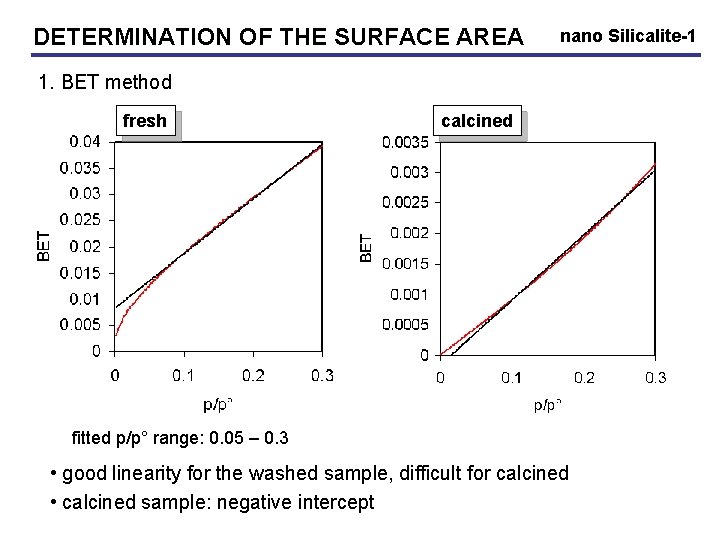
DETERMINATION OF THE SURFACE AREA nano Silicalite-1 1. BET method fresh calcined washed fitted p/p° range: 0. 05 – 0. 3 • good linearity for the washed sample, difficult for calcined • calcined sample: negative intercept

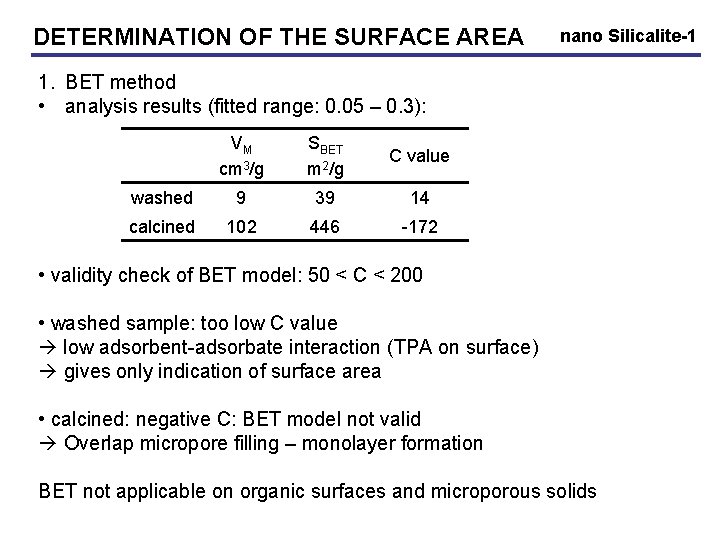
DETERMINATION OF THE SURFACE AREA nano Silicalite-1 1. BET method • analysis results (fitted range: 0. 05 – 0. 3): VM cm 3/g SBET m 2/g C value washed 9 39 14 calcined 102 446 -172 • validity check of BET model: 50 < C < 200 • washed sample: too low C value low adsorbent-adsorbate interaction (TPA on surface) gives only indication of surface area • calcined: negative C: BET model not valid Overlap micropore filling – monolayer formation BET not applicable on organic surfaces and microporous solids

DETERMINATION OF THE SURFACE AREA nano Silicalite-1 2. Comparative method t-plot; SBET, REF = 25 m 2/g fresh • intercept < 0 • no linear regions: adsorption behaviour ≠ reference (organic surface) • t-plot not applicable: bad reference

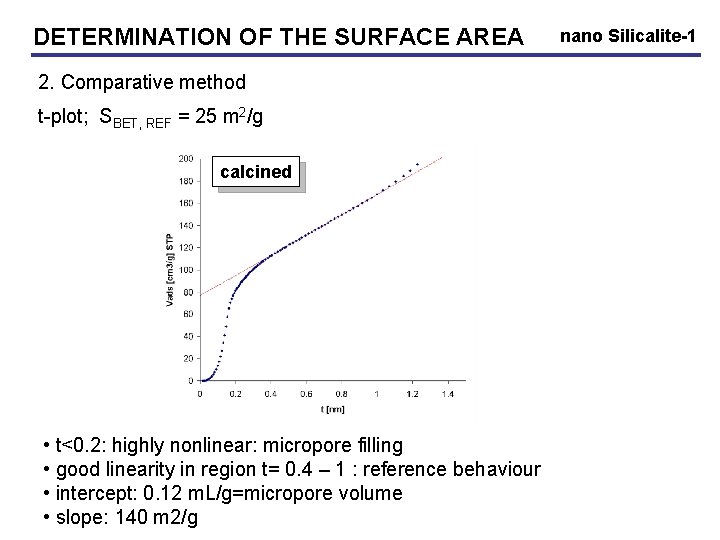
DETERMINATION OF THE SURFACE AREA 2. Comparative method t-plot; SBET, REF = 25 m 2/g calcined • t<0. 2: highly nonlinear: micropore filling • good linearity in region t= 0. 4 – 1 : reference behaviour • intercept: 0. 12 m. L/g=micropore volume • slope: 140 m 2/g nano Silicalite-1

DETERMINATION OF THE SURFACE AREA nano Silicalite-1 Conclusions: • fresh sample: BET better method (40 m 2/g) • calcined sample: comparative method preferable (140 m 2/g) although this method relies indirecly on the BET method, via the BET determination of the reference surface area) • comparative method gives also micropore volume

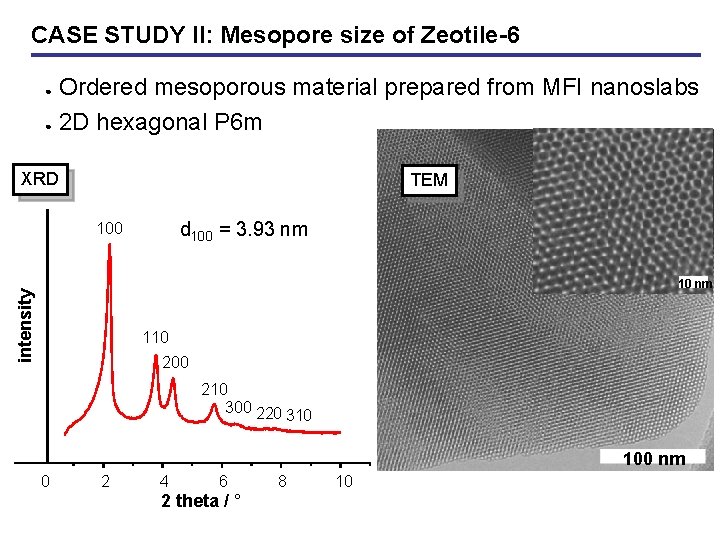
CASE STUDY II: Mesopore size of Zeotile-6 Ordered mesoporous material prepared from MFI nanoslabs ● 2 D hexagonal P 6 m ● XRD TEM d 100 = 3. 93 nm 100 intensity 10 nm 110 200 210 300 220 310 100 nm 0 2 4 6 2 theta / ° 8 10

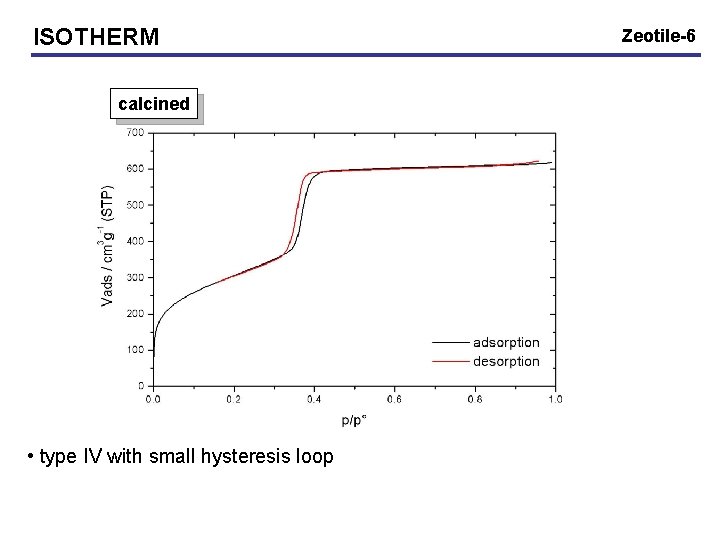
ISOTHERM calcined • type IV with small hysteresis loop Zeotile-6

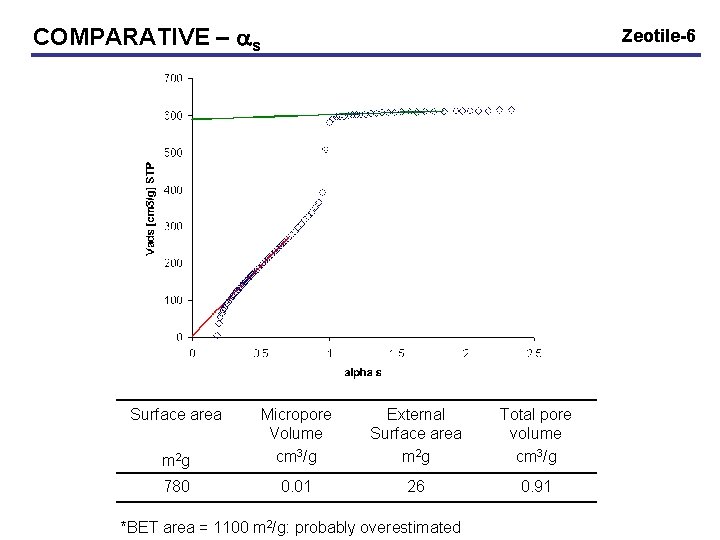
COMPARATIVE – as Surface area Zeotile-6 m 2 g Micropore Volume cm 3/g External Surface area m 2 g Total pore volume cm 3/g 780 0. 01 26 0. 91 *BET area = 1100 m 2/g: probably overestimated

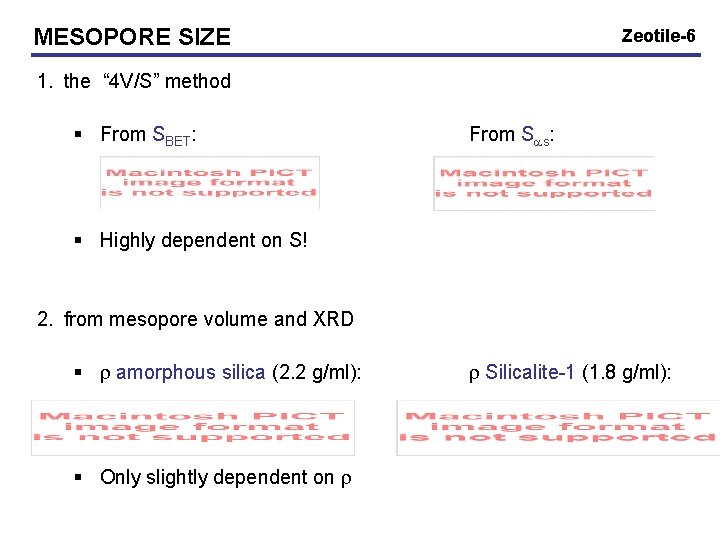
MESOPORE SIZE Zeotile-6 1. the “ 4 V/S” method § From SBET: From Sas: § Highly dependent on S! 2. from mesopore volume and XRD § r amorphous silica (2. 2 g/ml): § Only slightly dependent on r r Silicalite-1 (1. 8 g/ml):

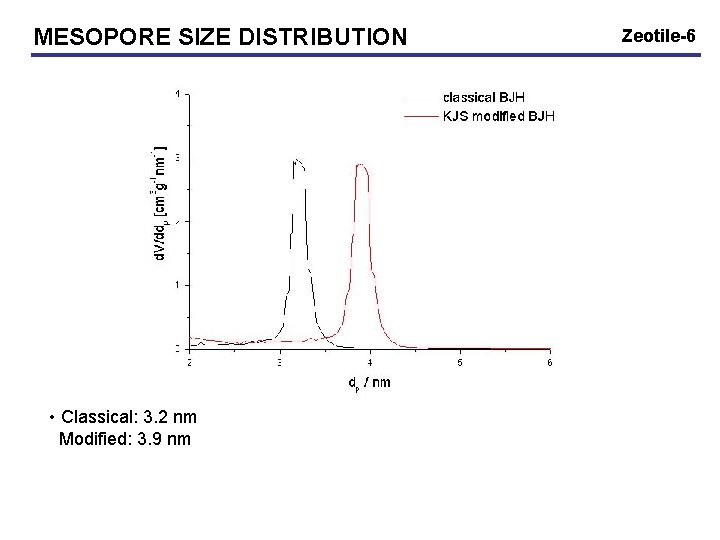
MESOPORE SIZE DISTRIBUTION • Classical: 3. 2 nm Modified: 3. 9 nm Zeotile-6

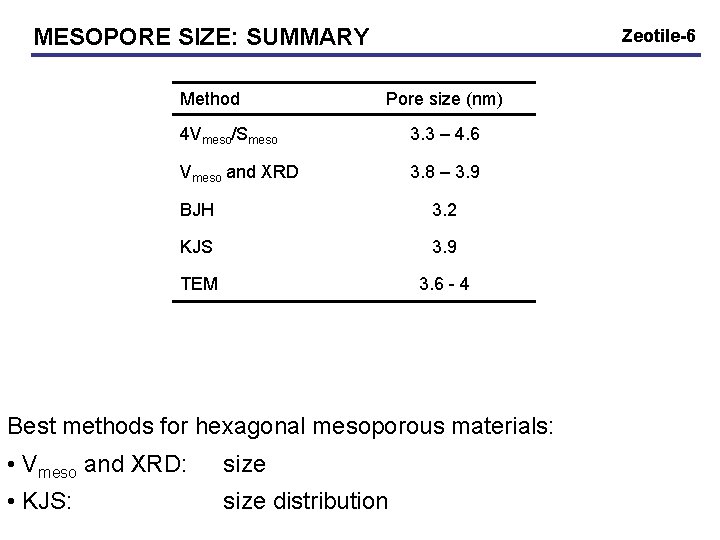
MESOPORE SIZE: SUMMARY Method Zeotile-6 Pore size (nm) 4 Vmeso/Smeso 3. 3 – 4. 6 Vmeso and XRD 3. 8 – 3. 9 BJH 3. 2 KJS 3. 9 TEM 3. 6 - 4 Best methods for hexagonal mesoporous materials: • Vmeso and XRD: size • KJS: size distribution

CONCLUSIONS • surface area : better with comparative method than BET IF good reference • pore volumes: easily determinable with comparative methods • micropore size: limited possibilities in the lab • mesopore size: know that - traditional BJH underestimates pore size of mesoporous silicas, especially in the range of 2 – 6 nm - alternative/improved methods • if detailed analysis needed, verify software settings

SPECIFIC SURFACE AREA: AREA COMPARATIVE Vads sample isotherm theory comparative plot t or as ~Vads, ref p/p° reference isotherm • adsorption behaviour (isotherm) of reference is plotted on x-axis as t or as

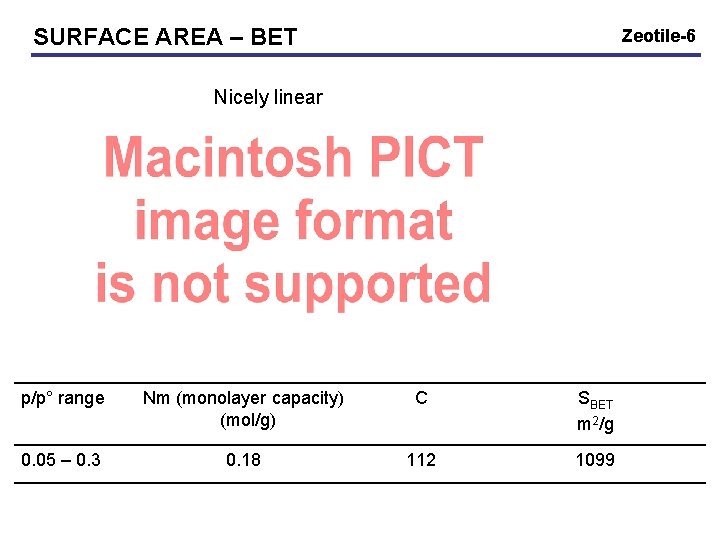
SURFACE AREA – BET Zeotile-6 Nicely linear p/p° range Nm (monolayer capacity) (mol/g) C SBET m 2/g 0. 05 – 0. 3 0. 18 112 1099