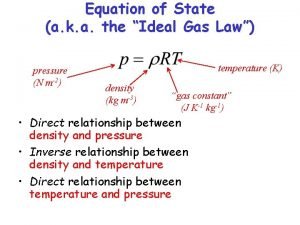
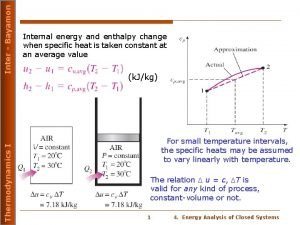
Thermodynamic of Adsorption 1 Thermodynamics of Adsorption Equao




![[adsorbate]° = 1 mol/L 9 [adsorbate]° = 1 mol/L 9](https://slidetodoc.com/presentation_image_h2/e3c1d0780c4ae838ede16fdba15dc384/image-9.jpg)

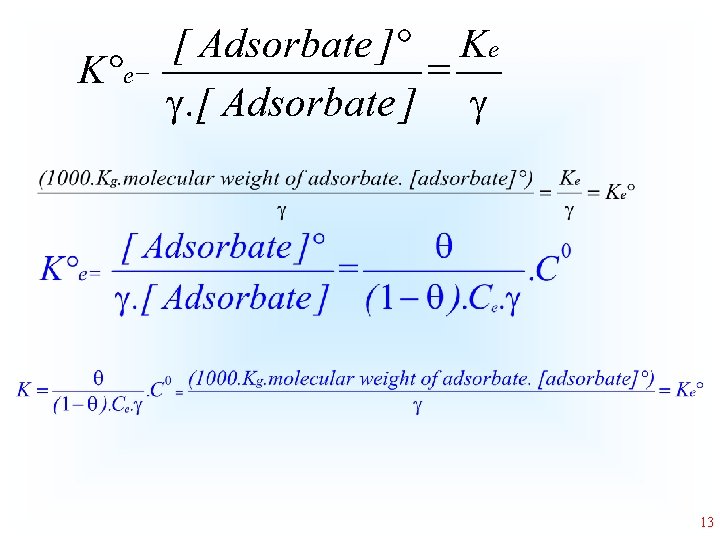













- Slides: 64

Thermodynamic of Adsorption 1

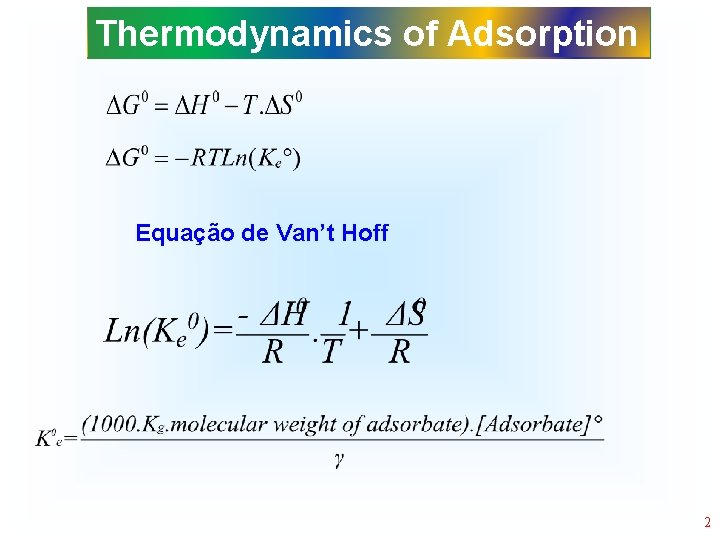
Thermodynamics of Adsorption Equação de Van’t Hoff 2

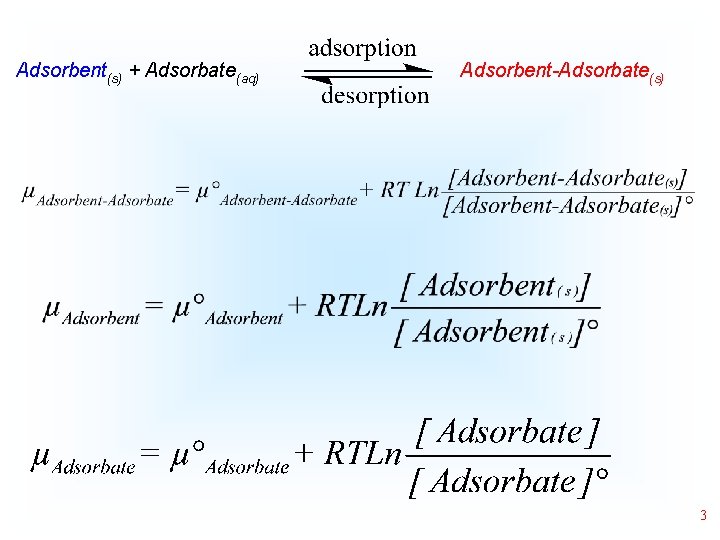
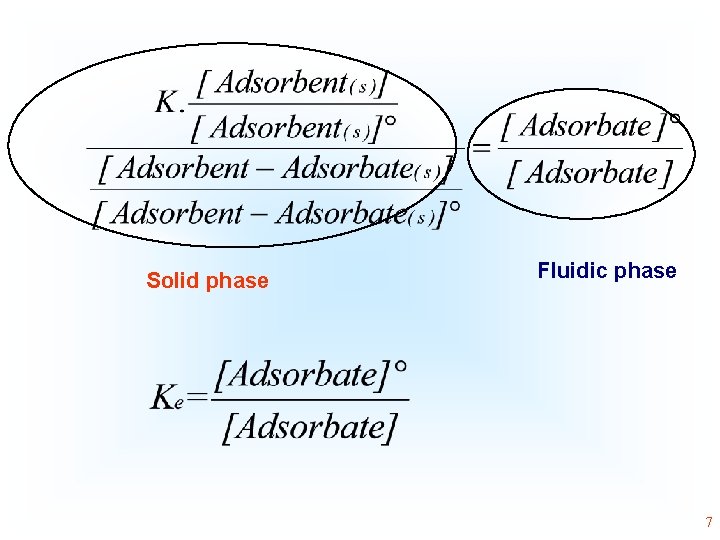
Adsorbent(s) + Adsorbate(aq) Adsorbent-Adsorbate(s) 3

Adsorbent(s) + Adsorbate(aq) Adsorbent-Adsorbate(s) Gad= µAdsobent-Adsorbate- (µAdsorbent + µAdsorbate) • 4

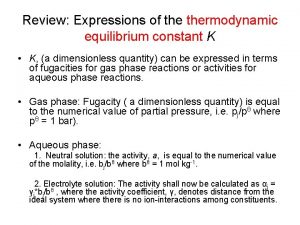
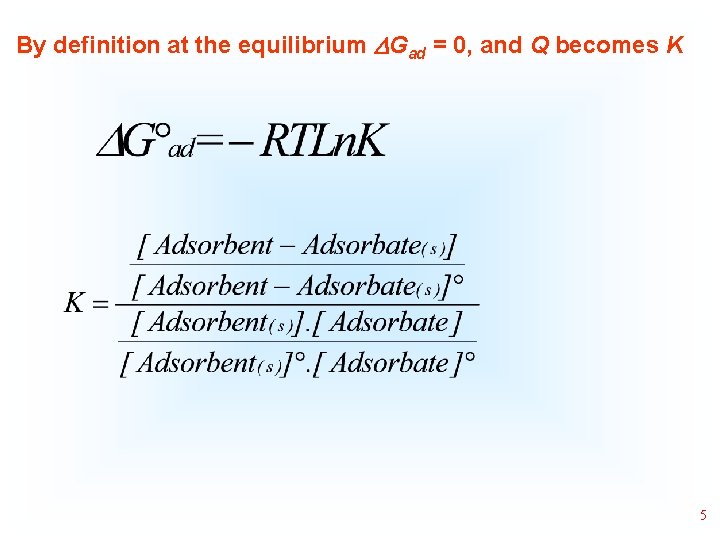
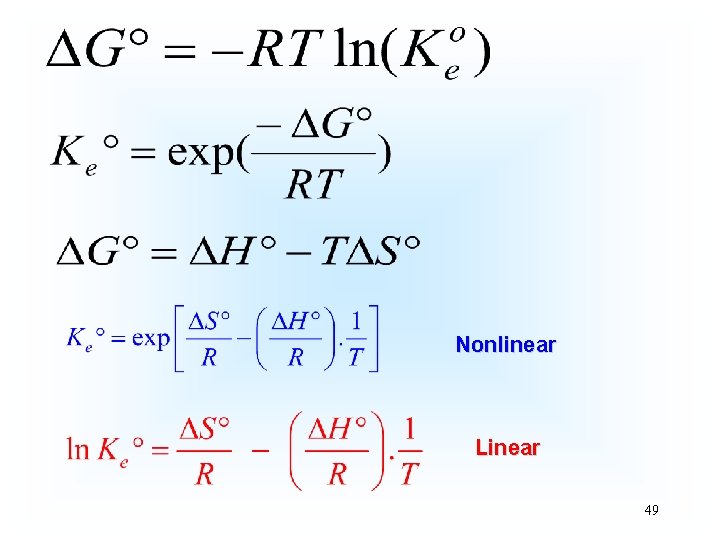
By definition at the equilibrium Gad = 0, and Q becomes K 5

The adsorption is a heterogeneous equilibrium where the adsorbate is present in a fluidic phase (aqueous solution, or gas) and the adsorbent will be present in a solid phase. Usually, the concentration of the solid phase [Adsorbent] and [Adsorbent-Adsorbate] is considered constant, because the number of moles of the solid phase divided by the volume of the solid phase is practically constant, because the solid phases did not alter their volume during the process of the adsorption 6

Solid phase Fluidic phase 7

The adsorption of an adsorbate by a solid adsorbent is far away of an ideal solution, the equilibrium constant should be expressed regarding activity, instead of concentration • Where [Adsorbate] is the molar concentration of the adsorbate (mol L-1); and is the coefficient 8
![adsorbate 1 molL 9 [adsorbate]° = 1 mol/L 9](https://slidetodoc.com/presentation_image_h2/e3c1d0780c4ae838ede16fdba15dc384/image-9.jpg)
[adsorbate]° = 1 mol/L 9

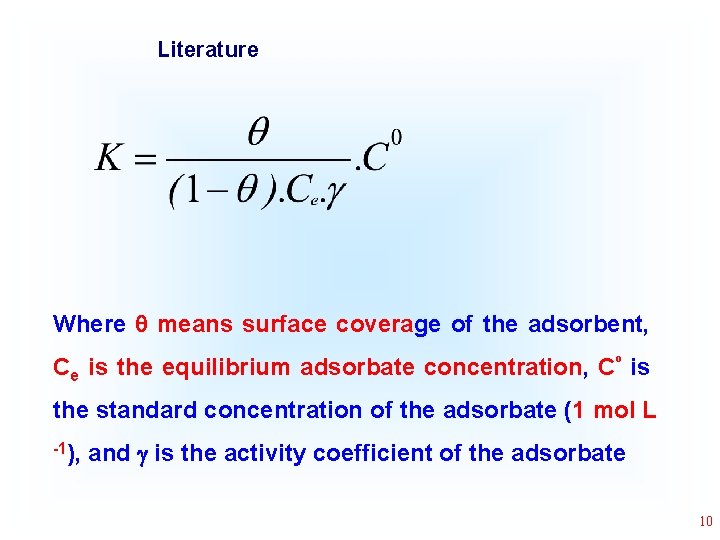
Literature Where means surface coverage of the adsorbent, Ce is the equilibrium adsorbate concentration, Cº is the standard concentration of the adsorbate (1 mol L -1), and is the activity coefficient of the adsorbate 10

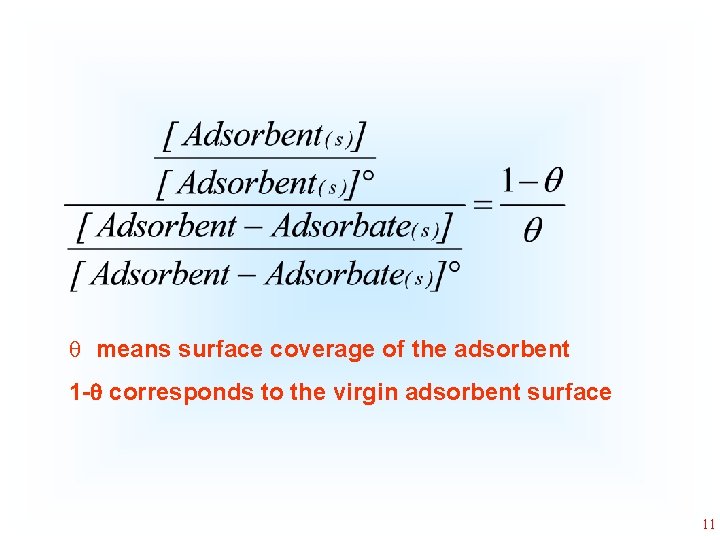
q means surface coverage of the adsorbent 1 - corresponds to the virgin adsorbent surface 11

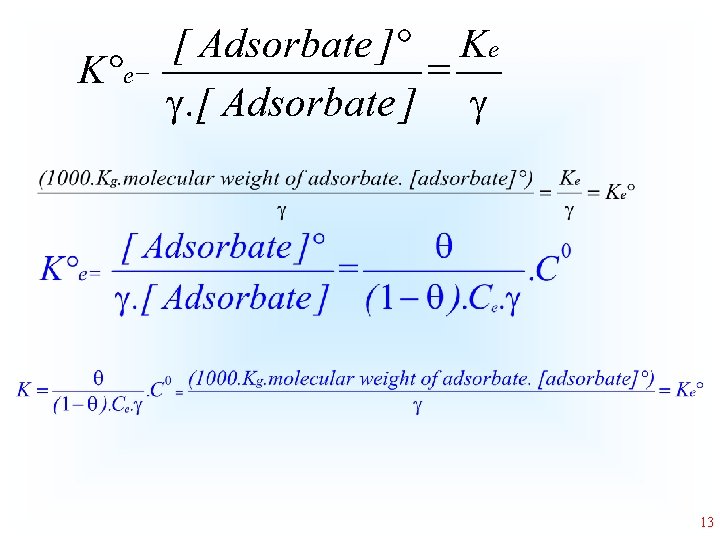
13

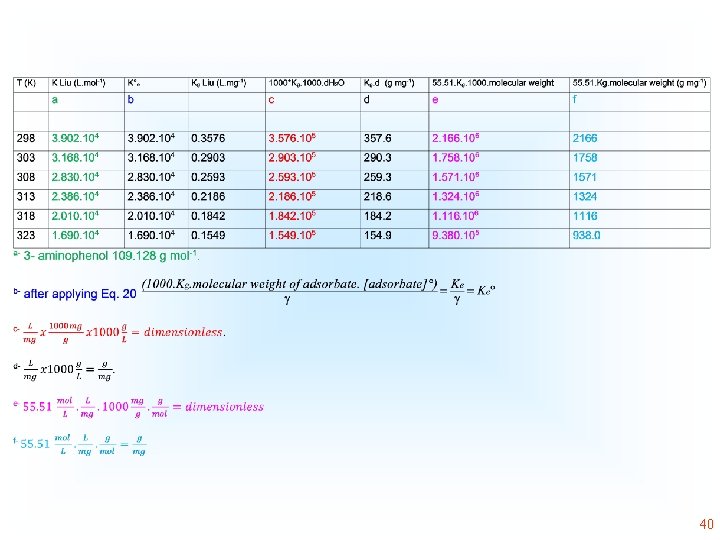
K°e is thermodynamic equilibrium constant that is dimensionless. It is calculated by converting the units of Kg (the best isotherm model fitted, such as K of the Liu equilibrium constant, or K of the Sips isotherm model or KL, the Langmuir equilibrium constant), that are given initially in L mg-1 into L mol-1. This conversion is obtained by the multiplication of the value of K (L mg-1) by 1000, to convert L mg-1 into L g-1 and subsequently making the multiplication of this result by the molecular weight of the adsorbate (g mol-1) multiplied by the unitary standard concentration of the adsorbate (1 mol L-1) and making the division by the activity coefficient (dimensionless). For this calculation, it is considered that the adsorbate solution is very diluted to consider that the activity coefficient is unitary. The parameter K°e becomes dimensionless after making these calculations. 14

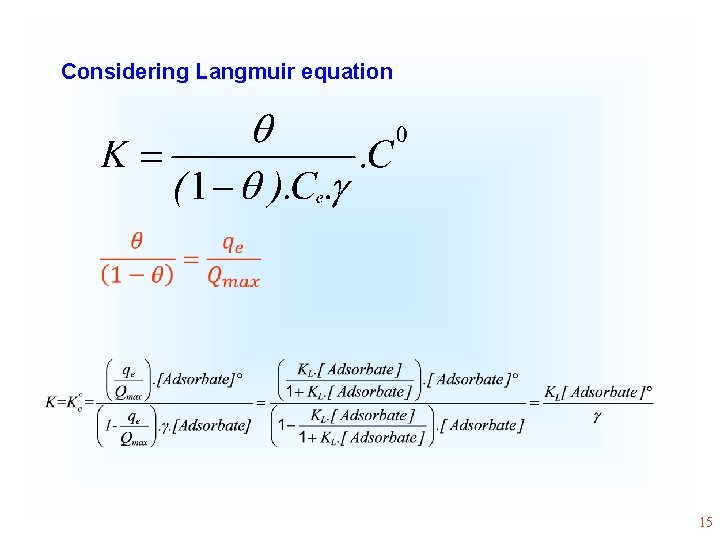
Considering Langmuir equation • 15

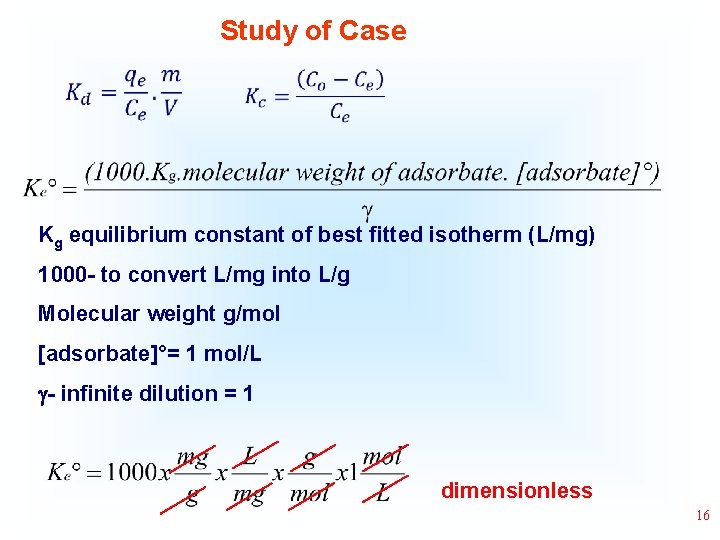
Study of Case • • Kg equilibrium constant of best fitted isotherm (L/mg) 1000 - to convert L/mg into L/g Molecular weight g/mol [adsorbate]°= 1 mol/L - infinite dilution = 1 dimensionless 16

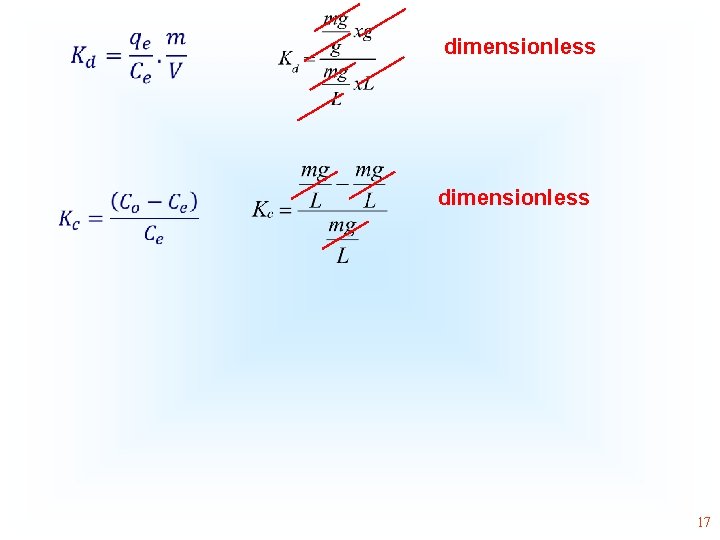
• • dimensionless 17

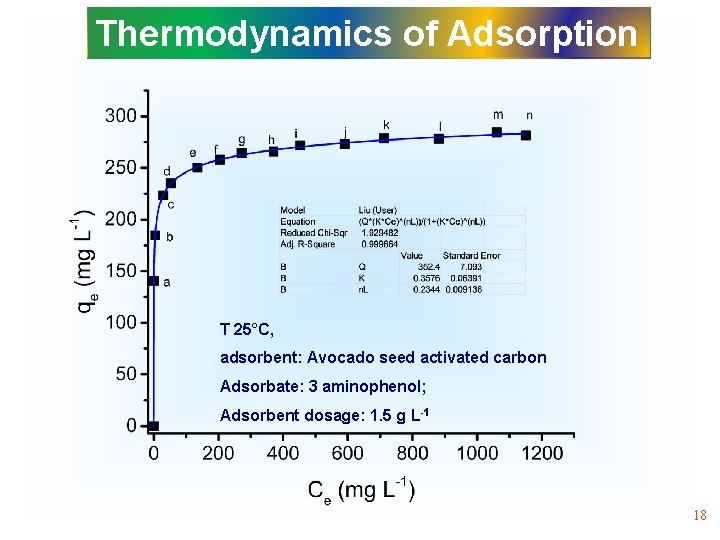
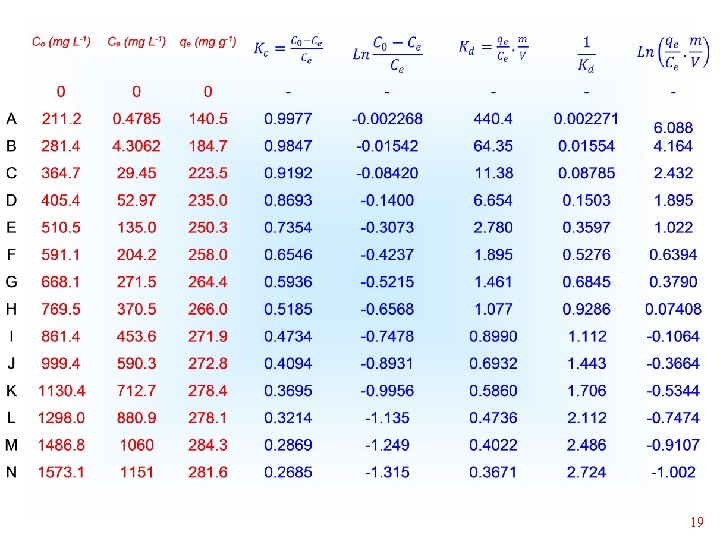
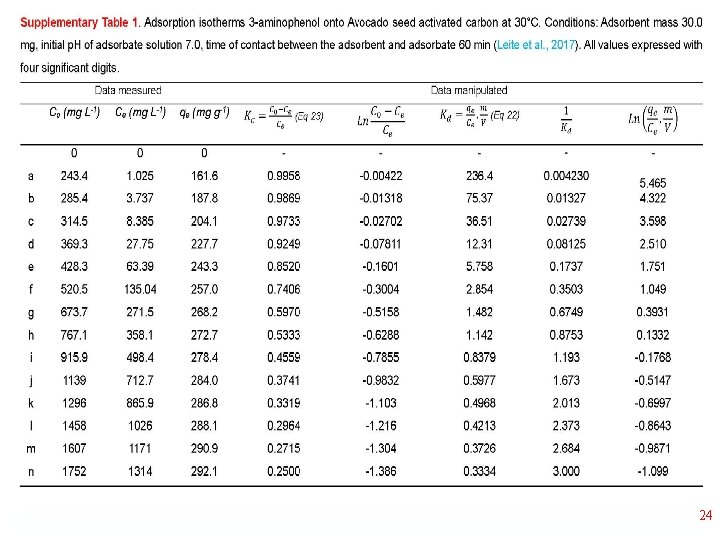
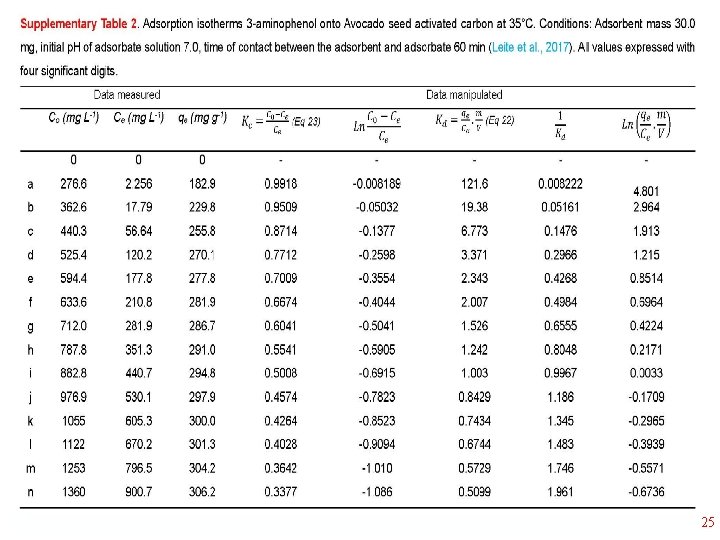
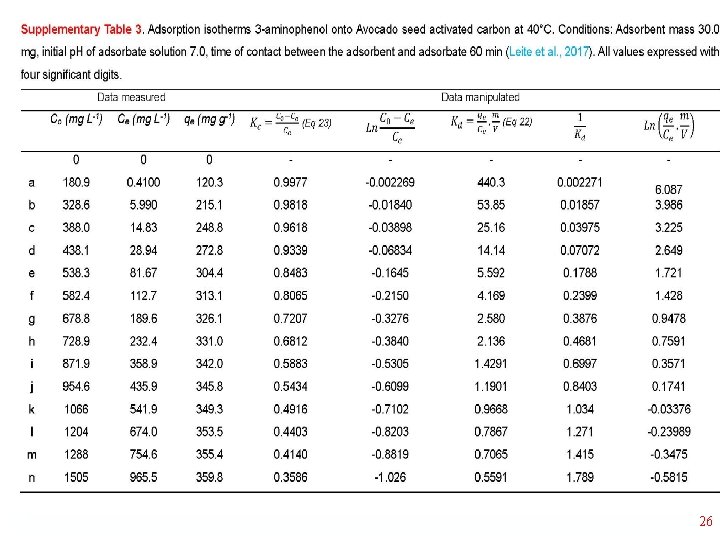
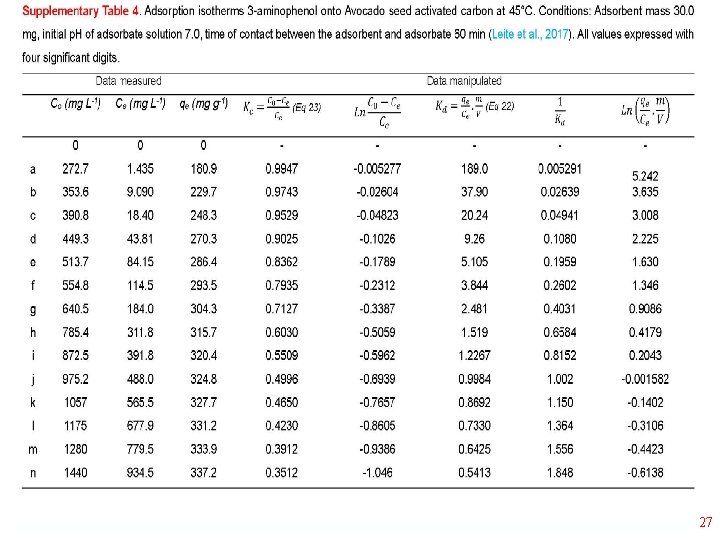
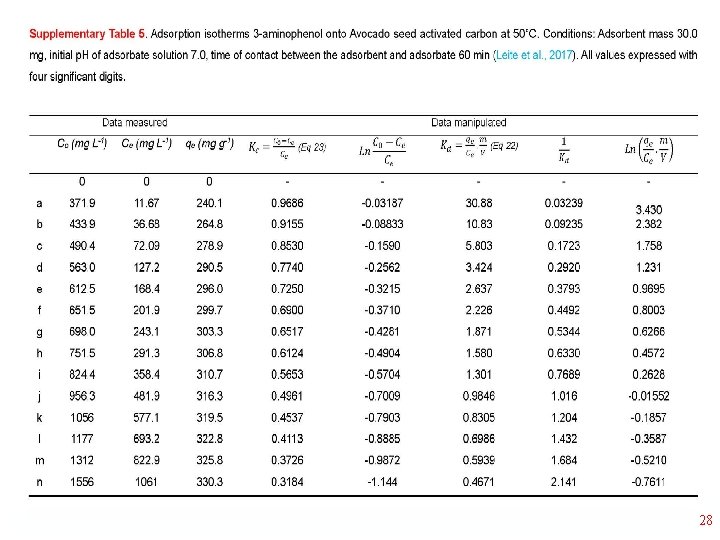
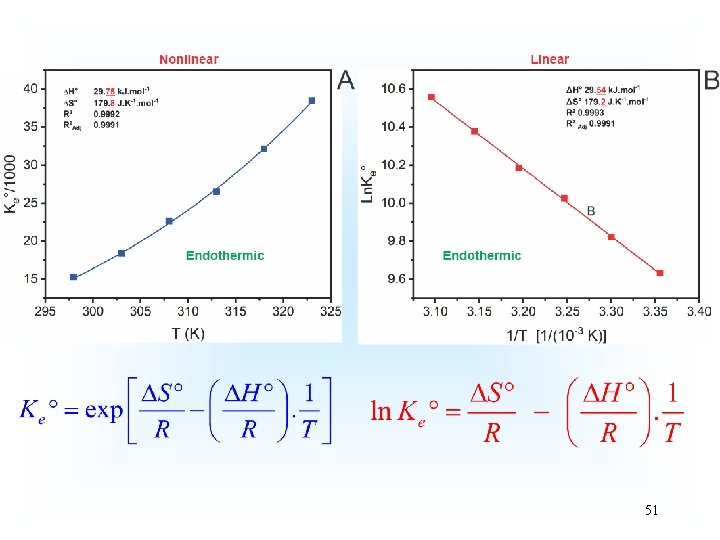
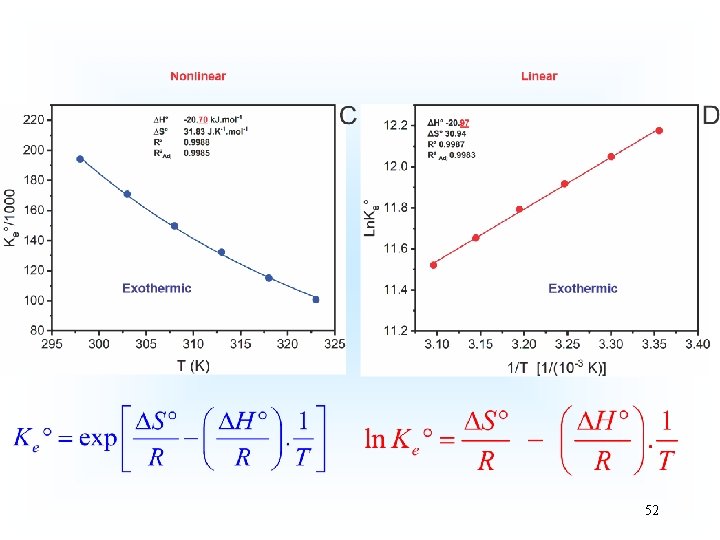
Thermodynamics of Adsorption T 25°C, adsorbent: Avocado seed activated carbon Adsorbate: 3 aminophenol; Adsorbent dosage: 1. 5 g L-1 18

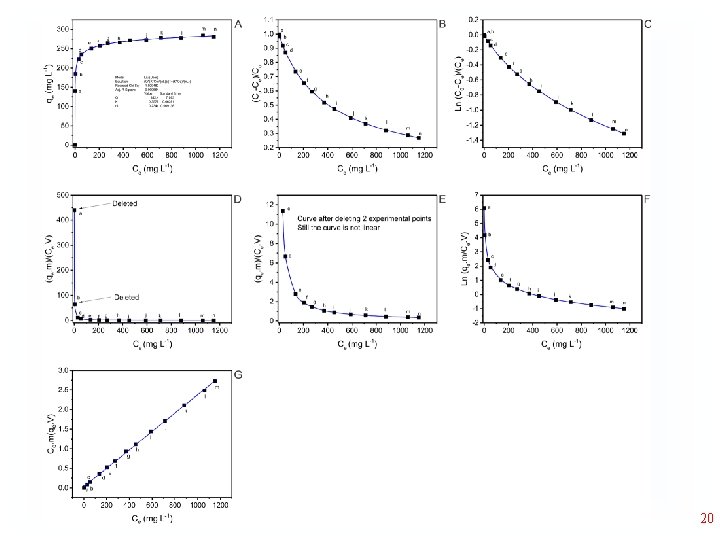
19

20

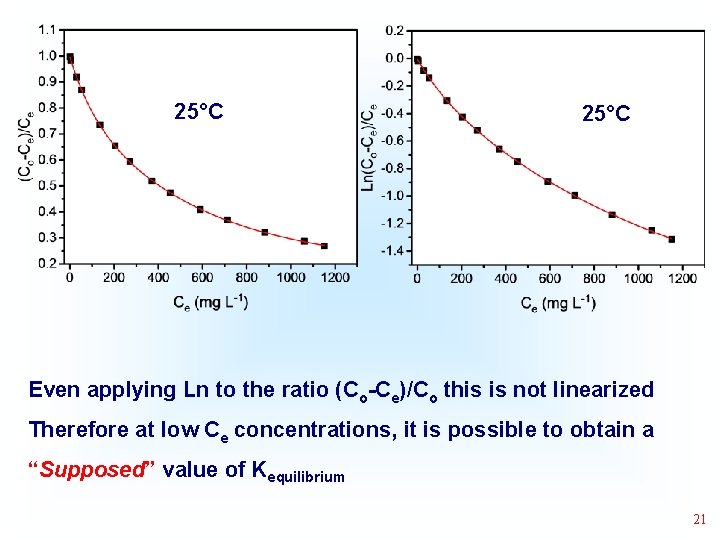
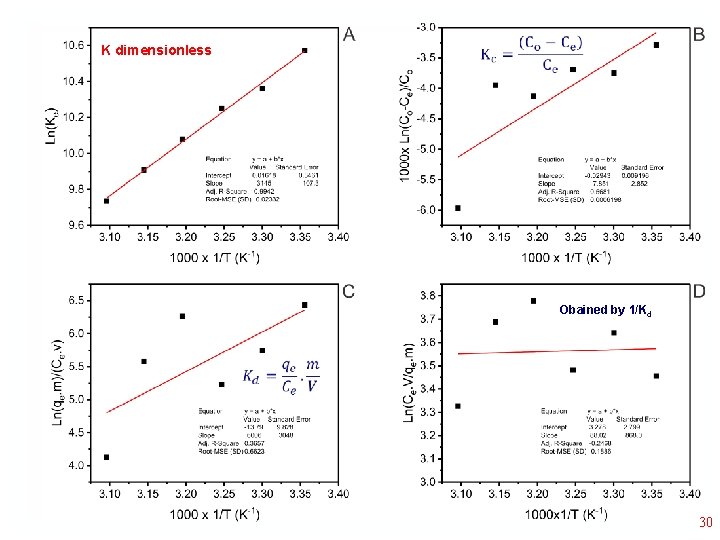
25°C Even applying Ln to the ratio (Co-Ce)/Co this is not linearized Therefore at low Ce concentrations, it is possible to obtain a “Supposed” value of Kequilibrium 21

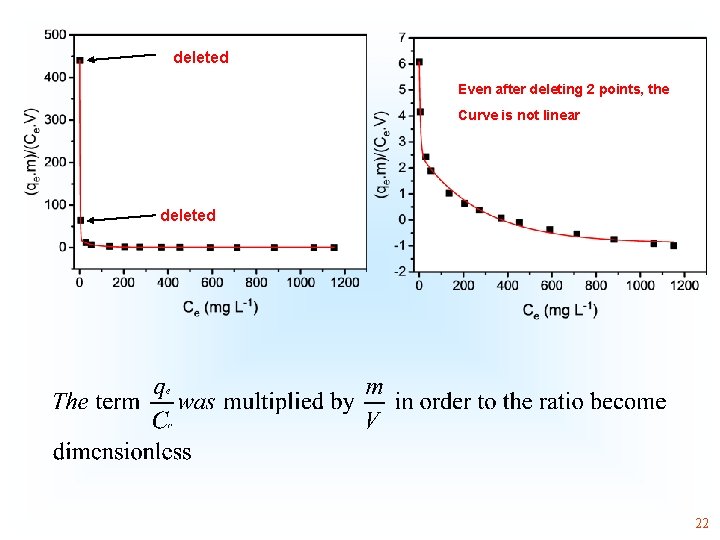
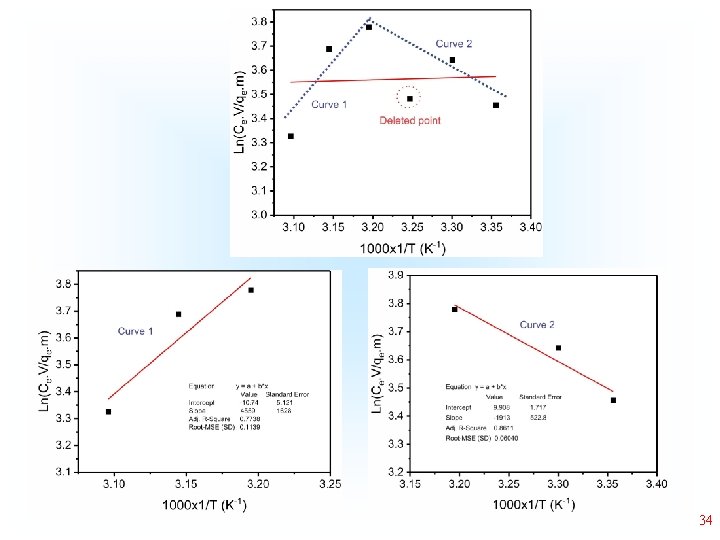
deleted Even after deleting 2 points, the Curve is not linear deleted 22

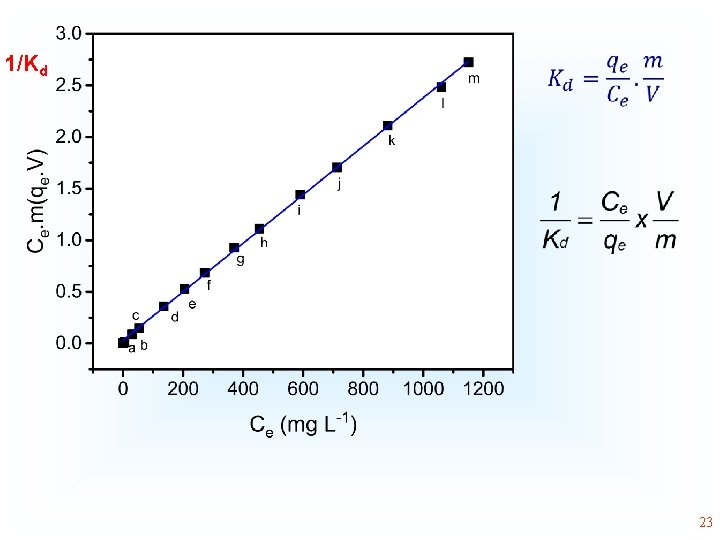
1/Kd • 23

24

25

26

27

28

29

• K dimensionless Obained by 1/Kd • 30

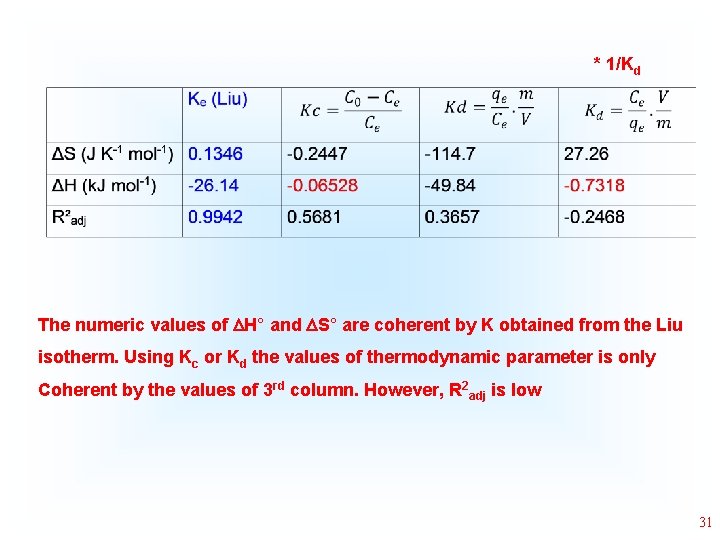
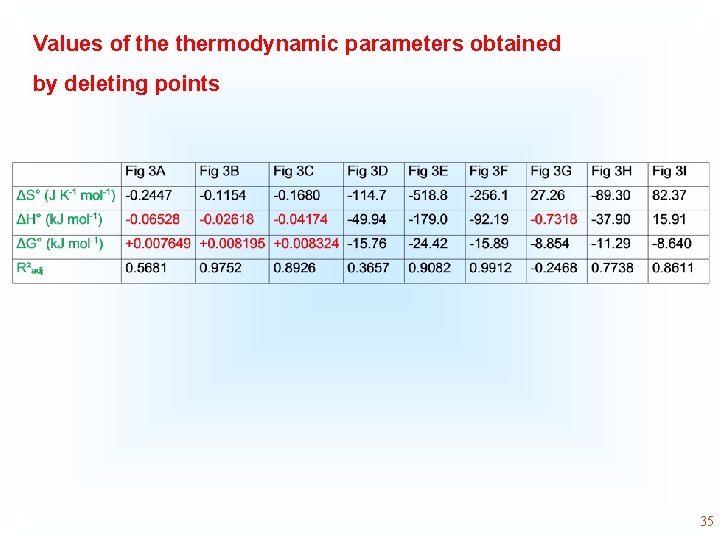
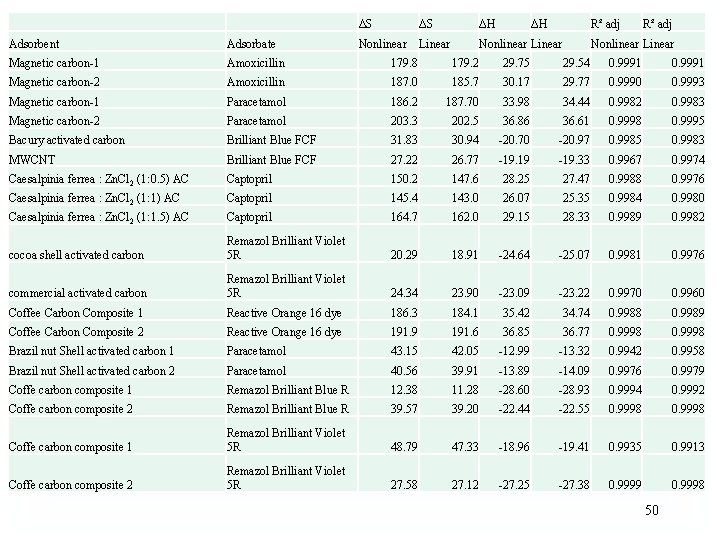
* 1/Kd The numeric values of H° and S° are coherent by K obtained from the Liu isotherm. Using Kc or Kd the values of thermodynamic parameter is only Coherent by the values of 3 rd column. However, R 2 adj is low 31

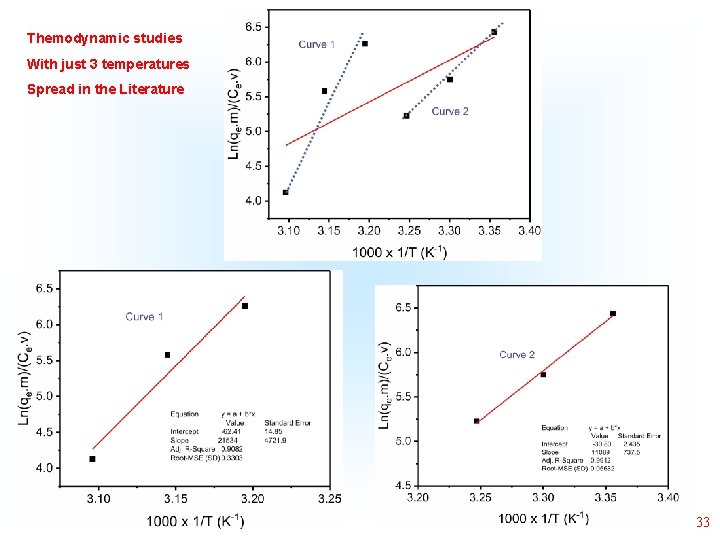
Themodynamic studies With just 3 temperatures Spread in the Literature 32

Themodynamic studies With just 3 temperatures Spread in the Literature 33

34

Values of thermodynamic parameters obtained by deleting points 35

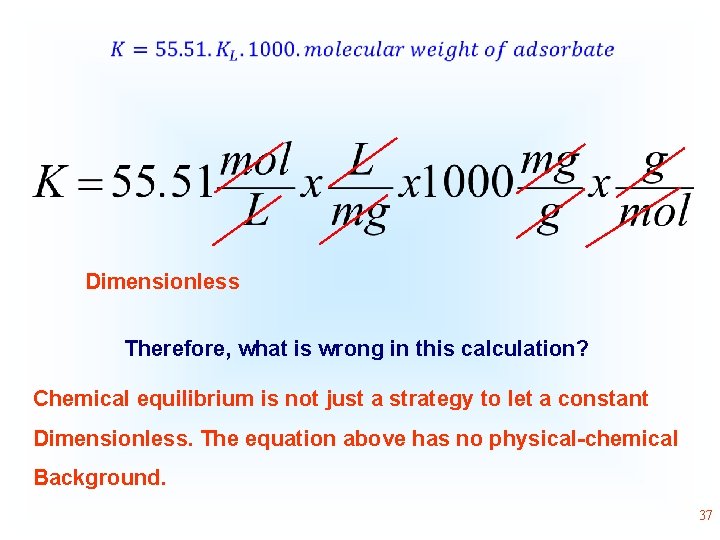
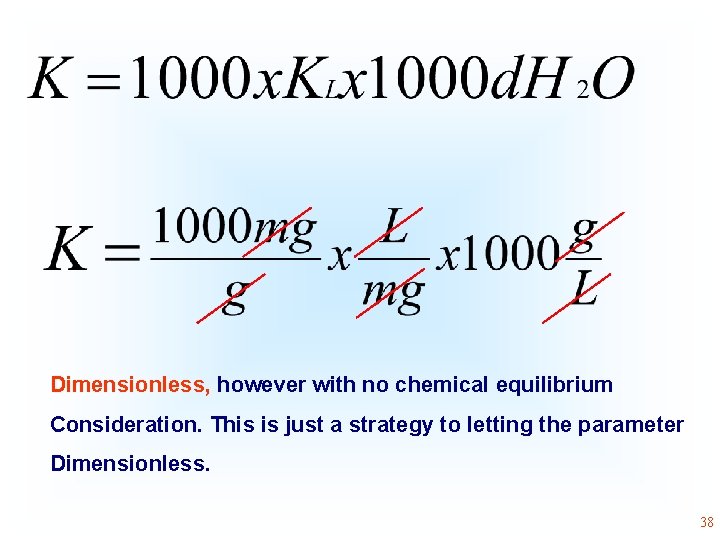
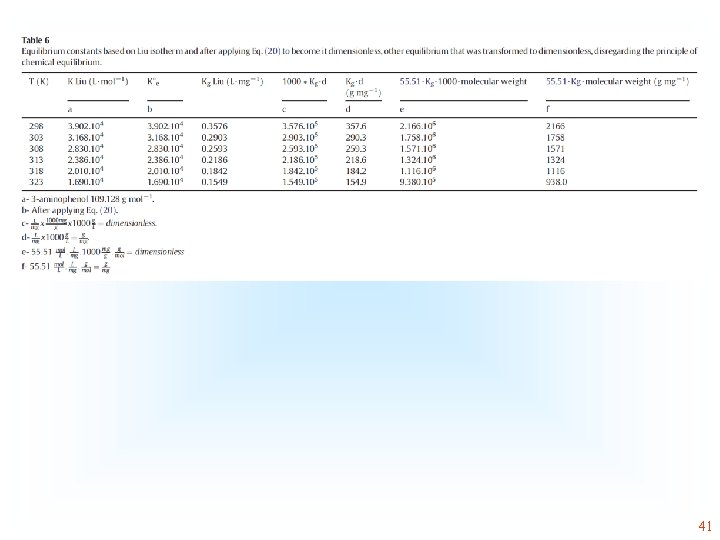
Different strategies for becoming dimensionless, without considering concept of chemical equilibrium constant the physical-chemical Although some authors obtain the equilibrium constant from isotherms, they do not know how to proceed to become it dimensionless by Eq 20. Some authors multiply the value of Langmuir equilibrium constant, as (L mg-1) by 1000 and then by the molality of water. Considering the amount of mol of water present in 1 kg and considering the molecular weight of water 18. 015 g mol-1, 1 kg of water will present 55. 51 mol of water. Therefore, the molal concentration of water in an aqueous solution will be 55. 51 mol kg-1 and considering that 1 kg of water will occupy a volume of 1 L, the molal concentration of water in aqueous solution will be very close to 55. 51 mol L-1 (molar concentration of water). Transforming the Langmuir, Liu or Sips equilibrium constant (L mg-1) into L mol-1 and then multiplying it by 55. 51 mol L-1, the K becomes dimensionless. 36

• Dimensionless Therefore, what is wrong in this calculation? Chemical equilibrium is not just a strategy to let a constant Dimensionless. The equation above has no physical-chemical Background. 37

Dimensionless, however with no chemical equilibrium Consideration. This is just a strategy to letting the parameter Dimensionless. 38

Some authors, commit mistakes in this conversion, therefore The following equations are also presented in the literature Unit is g/mg 39

40

41

42

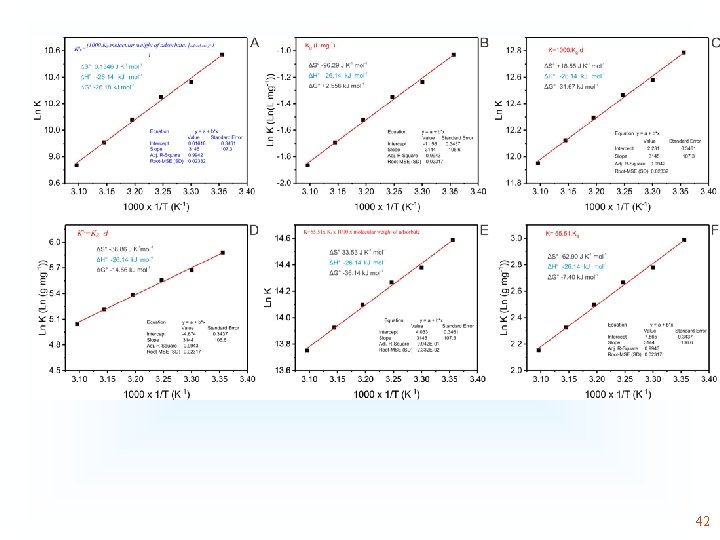
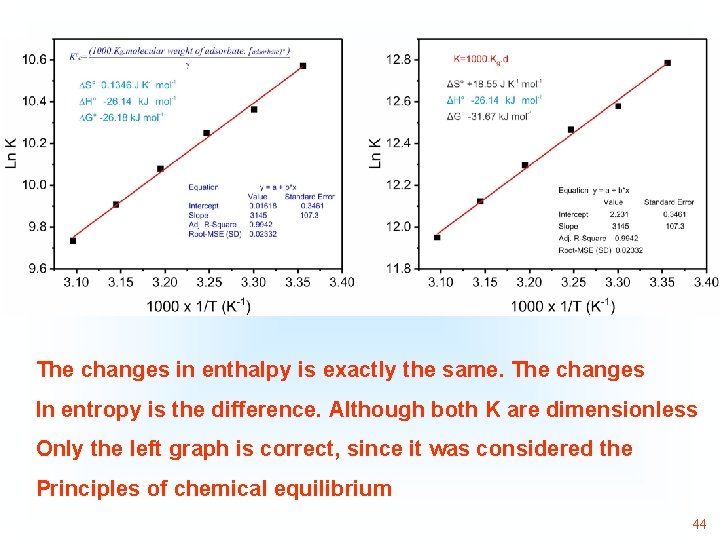
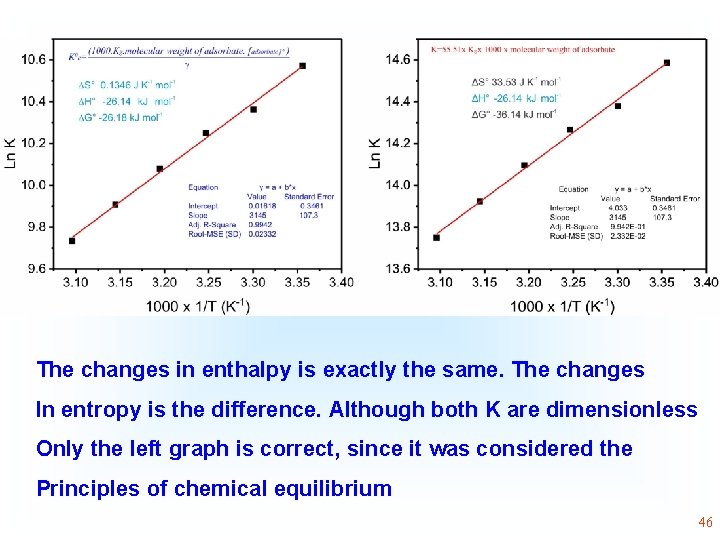
Although there is an error of concept, since Kg of the model Was used in L/mg without converting it to dimensionless, The changes in enthalpy are exactly the same 43

The changes in enthalpy is exactly the same. The changes In entropy is the difference. Although both K are dimensionless Only the left graph is correct, since it was considered the Principles of chemical equilibrium 44

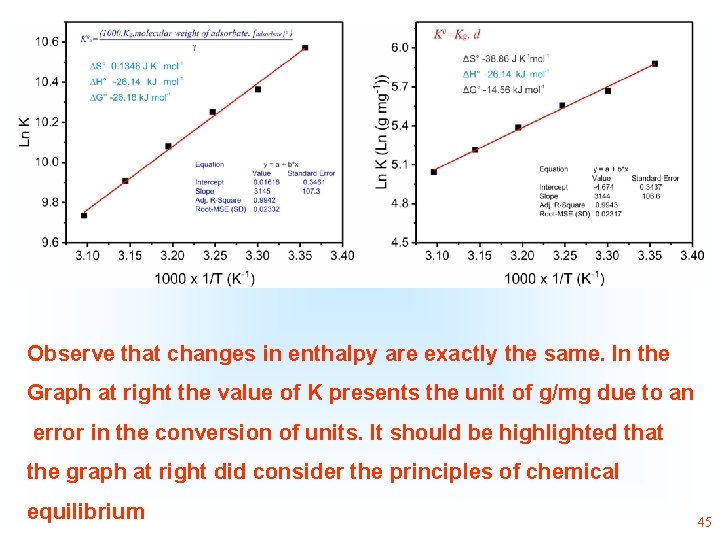
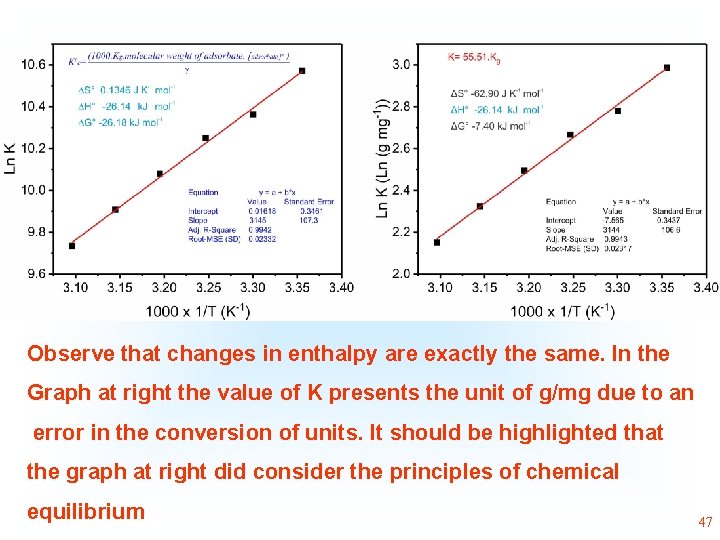
Observe that changes in enthalpy are exactly the same. In the Graph at right the value of K presents the unit of g/mg due to an error in the conversion of units. It should be highlighted that the graph at right did consider the principles of chemical equilibrium 45

The changes in enthalpy is exactly the same. The changes In entropy is the difference. Although both K are dimensionless Only the left graph is correct, since it was considered the Principles of chemical equilibrium 46

Observe that changes in enthalpy are exactly the same. In the Graph at right the value of K presents the unit of g/mg due to an error in the conversion of units. It should be highlighted that the graph at right did consider the principles of chemical equilibrium 47

The Thermodynamic equilibrium constant should be Dimensionless, however it is necessary to follow the Principles of chemical equilibrium. It is not enough to use The equilibrium constant obtained by the isotherms of Langmuir, Sips, Liu (L/mg) and make an wrong strategy of Multiply this value by the molar concentration of water or Density of water, that the value of K will be correct. The worst thing is, that although this error occurs and it is Spread in the literature, the value of H° is correct, although The values of S° and G° will be wrong. 48

Nonlinear Linear 49

ΔS ΔS ΔH ΔH R² adj Nonlinear Linear R² adj Adsorbent Adsorbate Nonlinear Linear Magnetic carbon-1 Amoxicillin 179. 8 179. 2 29. 75 29. 54 0. 9991 Magnetic carbon-2 Amoxicillin 187. 0 185. 7 30. 17 29. 77 0. 9990 0. 9993 Magnetic carbon-1 Paracetamol 186. 2 187. 70 33. 98 34. 44 0. 9982 0. 9983 Magnetic carbon-2 Paracetamol 203. 3 202. 5 36. 86 36. 61 0. 9998 0. 9995 Bacury activated carbon Brilliant Blue FCF 31. 83 30. 94 -20. 70 -20. 97 0. 9985 0. 9983 MWCNT Brilliant Blue FCF 27. 22 26. 77 -19. 19 -19. 33 0. 9967 0. 9974 Caesalpinia ferrea : Zn. Cl 2 (1: 0. 5) AC Captopril 150. 2 147. 6 28. 25 27. 47 0. 9988 0. 9976 Caesalpinia ferrea : Zn. Cl 2 (1: 1) AC Captopril 145. 4 143. 0 26. 07 25. 35 0. 9984 0. 9980 Caesalpinia ferrea : Zn. Cl 2 (1: 1. 5) AC Captopril 164. 7 162. 0 29. 15 28. 33 0. 9989 0. 9982 cocoa shell activated carbon Remazol Brilliant Violet 5 R 20. 29 18. 91 -24. 64 -25. 07 0. 9981 0. 9976 commercial activated carbon Remazol Brilliant Violet 5 R 24. 34 23. 90 -23. 09 -23. 22 0. 9970 0. 9960 Coffee Carbon Composite 1 Reactive Orange 16 dye 186. 3 184. 1 35. 42 34. 74 0. 9988 0. 9989 Coffee Carbon Composite 2 Reactive Orange 16 dye 191. 9 191. 6 36. 85 36. 77 0. 9998 Brazil nut Shell activated carbon 1 Paracetamol 43. 15 42. 05 -12. 99 -13. 32 0. 9942 0. 9958 Brazil nut Shell activated carbon 2 Paracetamol 40. 56 39. 91 -13. 89 -14. 09 0. 9976 0. 9979 Coffe carbon composite 1 Remazol Brilliant Blue R 12. 38 11. 28 -28. 60 -28. 93 0. 9994 0. 9992 Coffe carbon composite 2 Remazol Brilliant Blue R 39. 57 39. 20 -22. 44 -22. 55 0. 9998 Coffe carbon composite 1 Remazol Brilliant Violet 5 R 48. 79 47. 33 -18. 96 -19. 41 0. 9935 0. 9913 Coffe carbon composite 2 Remazol Brilliant Violet 5 R 27. 58 27. 12 -27. 25 -27. 38 0. 9999 0. 9998 50

51

52

53

54

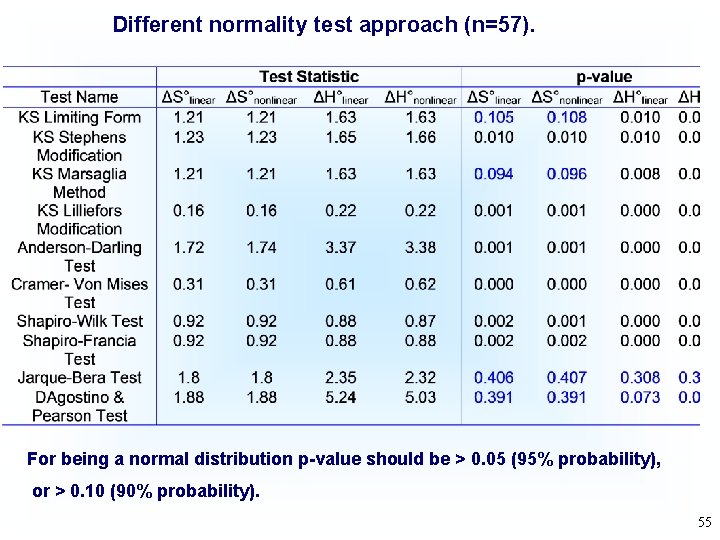
Different normality test approach (n=57). For being a normal distribution p-value should be > 0. 05 (95% probability), or > 0. 10 (90% probability). 55

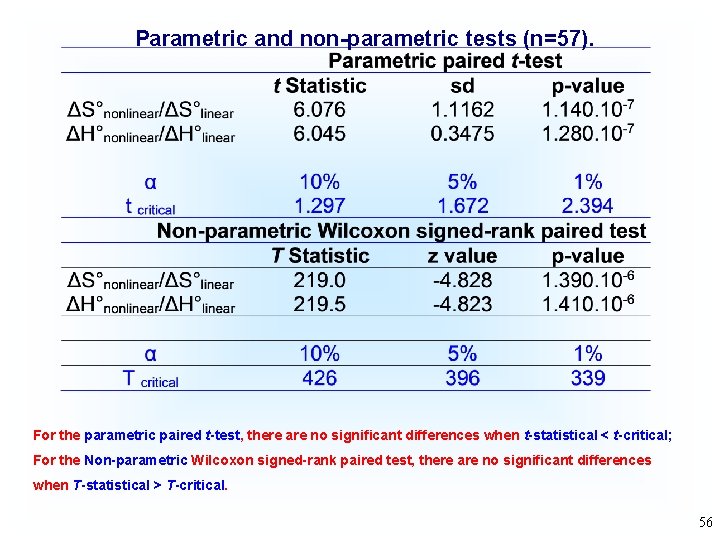
Parametric and non-parametric tests (n=57). For the parametric paired t-test, there are no significant differences when t-statistical < t-critical; For the Non-parametric Wilcoxon signed-rank paired test, there are no significant differences when T-statistical > T-critical. 56

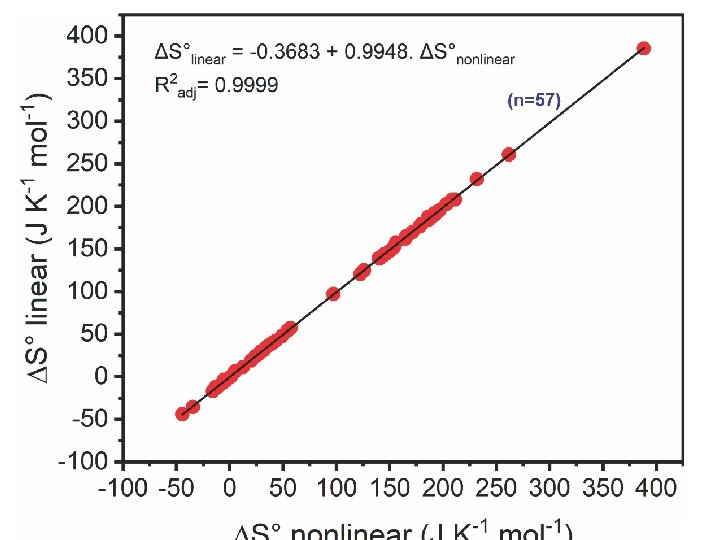
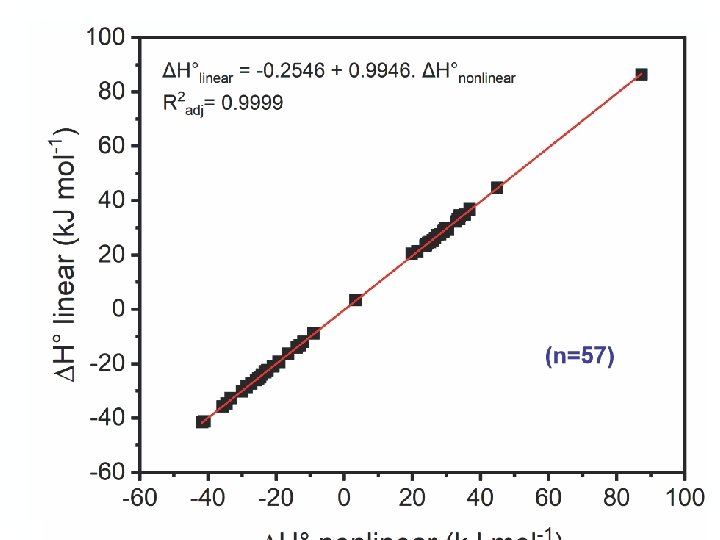
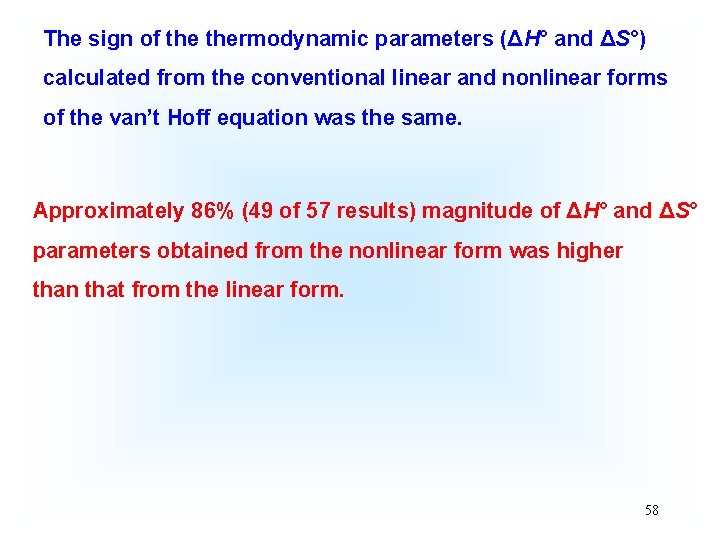
This value means that the magnitude of thermodynamic parameters (ΔH° and ΔS°) obtained from the nonlinear method is significantly different from that from the linear one. The results show that among collective 57 points of the experimental survey, the majority of ΔH° and ΔS° values (49, approximately 86. 0%) obtained from the nonlinear method had a slightly higher magnitude than that from the linear method. 57

The sign of thermodynamic parameters (ΔH° and ΔS°) calculated from the conventional linear and nonlinear forms of the van’t Hoff equation was the same. Approximately 86% (49 of 57 results) magnitude of ΔH° and ΔS° parameters obtained from the nonlinear form was higher than that from the linear form. 58

59

60

61

62

Link for the Chapter 3 as well as the Critical Review and Short communication about Thermodynamics of adsorption. http: //bit. ly/thermodynamics_Eder_Lima 63

Thank you for your special attention 64
 Divisao de log
Divisao de log Soma e produto
Soma e produto Equao
Equao Mecflu
Mecflu Forma logaritmica
Forma logaritmica Equao
Equao Aleta
Aleta Equao
Equao Thermodynamic
Thermodynamic Microstates in thermodynamics
Microstates in thermodynamics Bergeron process definition
Bergeron process definition Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thermodynamic property relations
Thermodynamic property relations What is enthalpy in thermodynamics
What is enthalpy in thermodynamics Second law of thermodynamic
Second law of thermodynamic Second law of thermodynamic
Second law of thermodynamic Volume expansivity and isothermal compressibility
Volume expansivity and isothermal compressibility Vanthoff equation
Vanthoff equation Curtin-hammett principle
Curtin-hammett principle Thermodynamic property relations
Thermodynamic property relations Cp-cv=r/m
Cp-cv=r/m Formula sheet thermodynamics
Formula sheet thermodynamics Space mean
Space mean Thermodynamic temperature
Thermodynamic temperature Change of entropy formula
Change of entropy formula Entropy in thermodynamics
Entropy in thermodynamics What is thermodynamic equilibrium constant
What is thermodynamic equilibrium constant Thermodynamic square
Thermodynamic square Etransl
Etransl Thermodynamic potentials
Thermodynamic potentials Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Thermodynamic
Thermodynamic Thermodynamic potentials
Thermodynamic potentials Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Extensive and intensive properties in thermodynamics
Extensive and intensive properties in thermodynamics Thermodynamic identity
Thermodynamic identity Gibbs free energy equation
Gibbs free energy equation Maxwell relations applications
Maxwell relations applications Zeroth law of thermo
Zeroth law of thermo Adsorption at solid gas interface
Adsorption at solid gas interface Chromatography mechanism
Chromatography mechanism Eosin adsorption indicator
Eosin adsorption indicator Adsorption technique in blood banking
Adsorption technique in blood banking Wet gum method ratio
Wet gum method ratio Slidetodoc.com
Slidetodoc.com Adsorption in physical pharmacy
Adsorption in physical pharmacy Bubble method for soft gelatin capsule
Bubble method for soft gelatin capsule Process equipment design solved problems
Process equipment design solved problems Langmuir isotherm
Langmuir isotherm Mechanism of adsorption chromatography
Mechanism of adsorption chromatography What is adsorption
What is adsorption Fajans titration
Fajans titration Adsorption introduction
Adsorption introduction Substance launcher
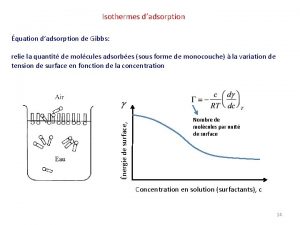
Substance launcher Isotherme de gibbs
Isotherme de gibbs What is adsorption isotherm
What is adsorption isotherm Adsorption technique in blood banking
Adsorption technique in blood banking Adsorption system
Adsorption system Chromatography mobile phase and stationary phase
Chromatography mobile phase and stationary phase Langmuir adsorption isotherm
Langmuir adsorption isotherm Primary adsorption layer
Primary adsorption layer Positive adsorption
Positive adsorption Adsorption phenomenon
Adsorption phenomenon