SOFT GELATIN CAPSULES DEPARTMENT OF PHARMACEUTICS CHALAPATHI INSTITUTE





















- Slides: 51

SOFT GELATIN CAPSULES DEPARTMENT OF PHARMACEUTICS CHALAPATHI INSTITUTE OF PHARMACEUTICAL SCIENCES

ØINTRODUCTION ØFORMULATION OF SOFT GELS ØMANUFACTURE OF SOFT GELS ØQUALITY CONTROL TESTS ØREFERENCES

q. INTRODUCTION ØCapsules are solid dosage forms in which medicinal agents are enclosed within hard or soft soluble shell. The shells are generally formed from gelatin. ØThe capsule is regarded as container drug delivery system that provides a tasteless/ odorless dosage form without need for secondary coating step. ØCapsule may classified depending on nature of shell Hard gelatin Soft gelatin capsules, also called as soft gels

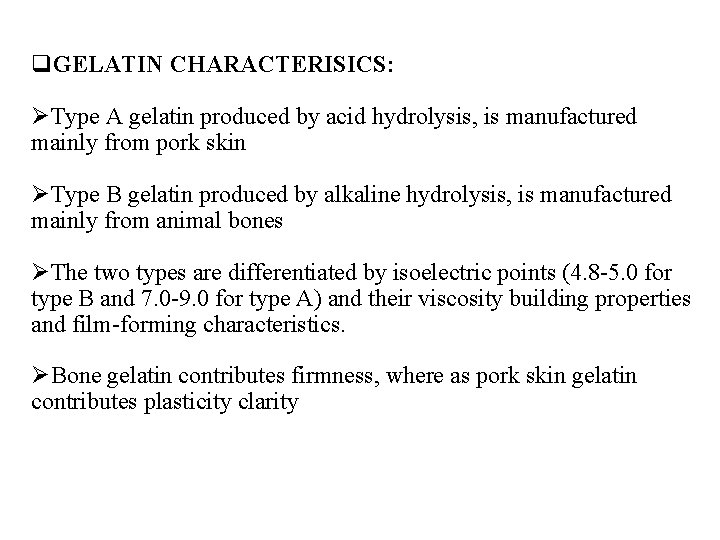
q. GELATIN CHARACTERISICS: ØType A gelatin produced by acid hydrolysis, is manufactured mainly from pork skin ØType B gelatin produced by alkaline hydrolysis, is manufactured mainly from animal bones ØThe two types are differentiated by isoelectric points (4. 8 -5. 0 for type B and 7. 0 -9. 0 for type A) and their viscosity building properties and film-forming characteristics. ØBone gelatin contributes firmness, where as pork skin gelatin contributes plasticity clarity

q. SOFT GELATIN CAPSULES • Soft Gelatin Capsules are one-piece forms that encase a predetermined dose of liquid or semi liquid fill. • Filled and hermetically sealed in a single operation • Soft gelatin capsules offer the capability of delivering a liquid in a solid dosage form. • Soft gels ability to enhance the bioavailability not only makes it the preferred dosage form with poor bioavailability owing to poor aqueous solubility but also for reformulation of marketed drugs with the purpose of life cycle extension

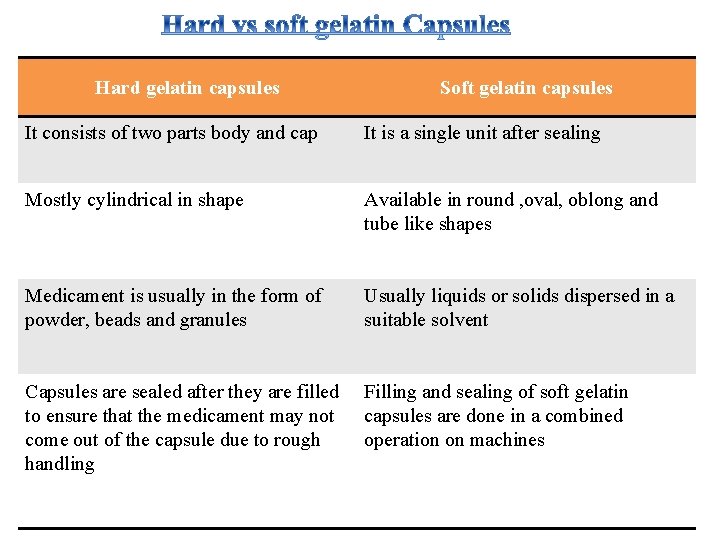
Hard gelatin capsules Soft gelatin capsules It consists of two parts body and cap It is a single unit after sealing Mostly cylindrical in shape Available in round , oval, oblong and tube like shapes Medicament is usually in the form of powder, beads and granules Usually liquids or solids dispersed in a suitable solvent Capsules are sealed after they are filled to ensure that the medicament may not come out of the capsule due to rough handling Filling and sealing of soft gelatin capsules are done in a combined operation on machines

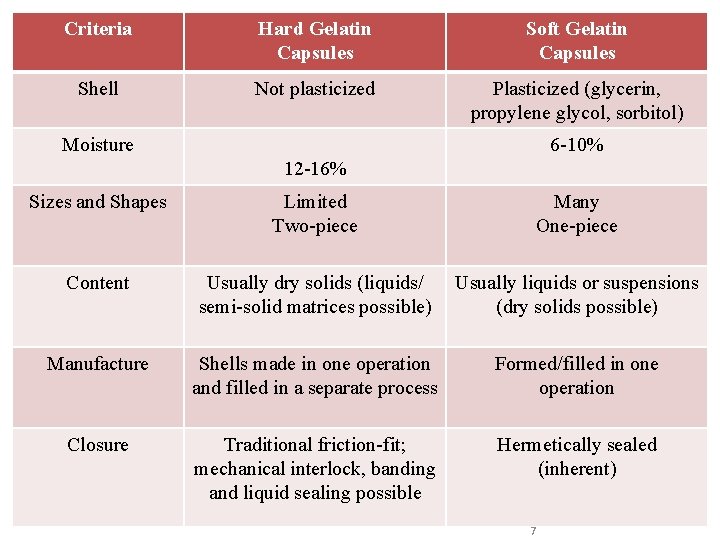
Criteria Hard Gelatin Capsules Soft Gelatin Capsules Shell Not plasticized Plasticized (glycerin, propylene glycol, sorbitol) Moisture 6 -10% 12 -16% Sizes and Shapes Limited Two-piece Many One-piece Content Usually dry solids (liquids/ semi-solid matrices possible) Usually liquids or suspensions (dry solids possible) Manufacture Shells made in one operation and filled in a separate process Formed/filled in one operation Closure Traditional friction-fit; mechanical interlock, banding and liquid sealing possible Hermetically sealed (inherent) 7

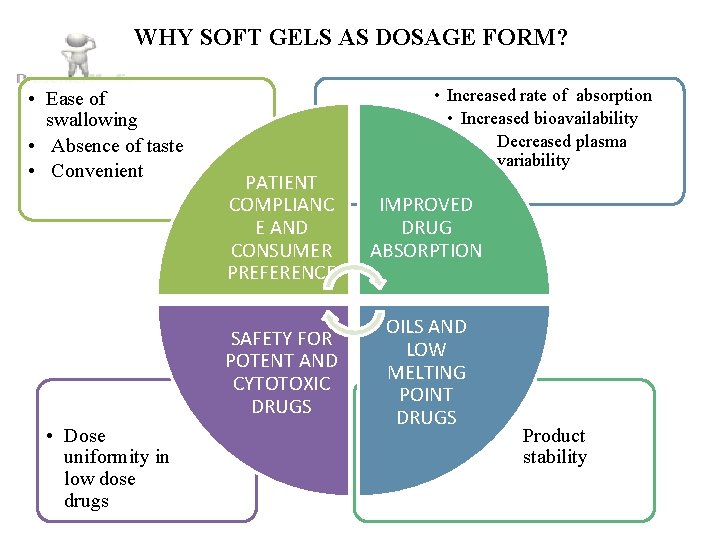
WHY SOFT GELS AS DOSAGE FORM? • Ease of swallowing • Absence of taste • Convenient • Dose uniformity in low dose drugs • Increased rate of absorption • Increased bioavailability • Decreased plasma variability PATIENT COMPLIANC E AND CONSUMER PREFERENCE IMPROVED DRUG ABSORPTION SAFETY FOR POTENT AND CYTOTOXIC DRUGS OILS AND LOW MELTING POINT DRUGS • Product stability

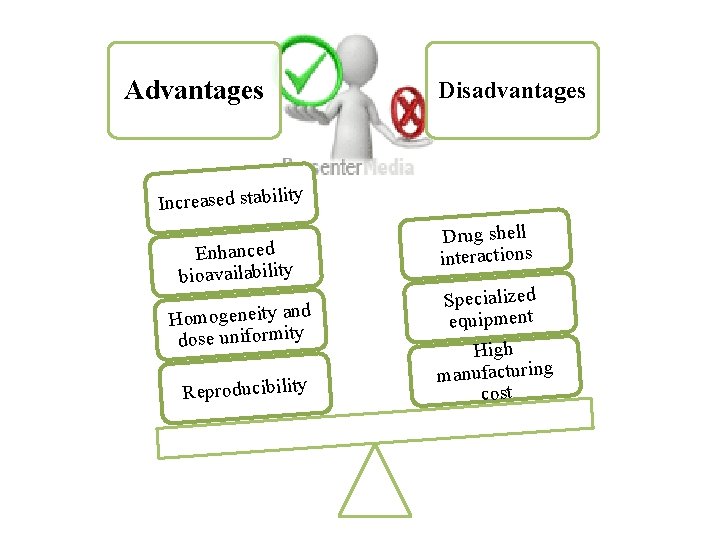
Advantages Disadvantages Increased stability Enhanced bioavailability Homogeneity and dose uniformity Reproducibility Drug shell interactions Specialized equipment High manufacturing cost


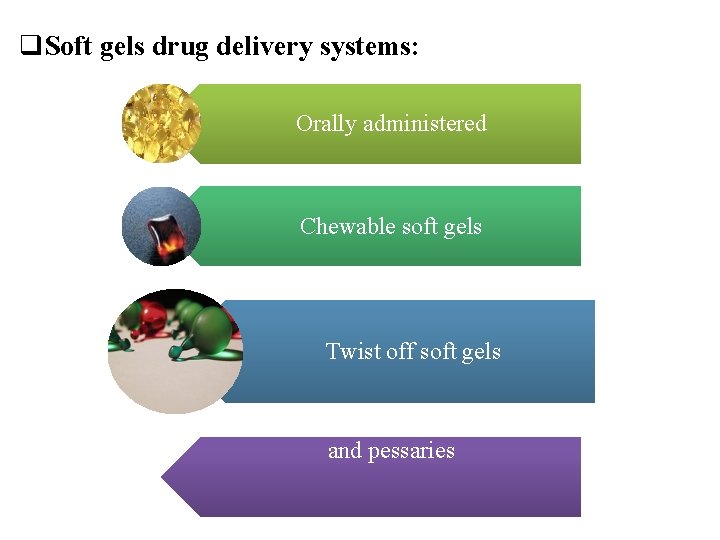
q. Soft gels drug delivery systems: Orally administered Chewable soft gels Twist off soft gels Meltable soft gels like suppositories and pessaries

q. FORMULATION OF SOFT GELS Excipients of Soft gels a. GELATIN b. SOFTENER (plasticizer): sorbitol, xylose, maltitol, glycerin, PEG, water) c. PRESERVATIVES: Methyl paraben, propyl paraben, butylated hydroxyaniline, EDTA, sodium benzoate d. DYES AND PIGMENTS e. SOLVENT Polar: glycerin, PEG 400, PEG 3350, ethanol, propylene glycol, water Nonpolar: beeswax, coconut oil, triglycerin, corn oil, mineral oil, soybean oil, D, L-α-tocopherol f. FLAVOR AND FRAGRANCE g. PIGMENT: Titanium oxide, ferric oxide h. ANTICAKING AGENT: Silicone dioxide i. HUMECTANTS: Polyol, glycerin

q. NATURE OF CAPSULE (GELATIN) SHELL Bloom Strength: ümeasure of the cohesive strength of the cross linking that occurs between gelatin molecules üproportional to molecular weight of gelatin Determined by measuring weight in grams required to move a plastic plunger i. e. 0. 5 inches in diameter 4 mm into a 62/3 % gelatin gel that has been held at 10°C for 17 hours v. Value ranges from 150 – 250 g v Higher the bloom strength - more physical stability to the capsule shell - more costly

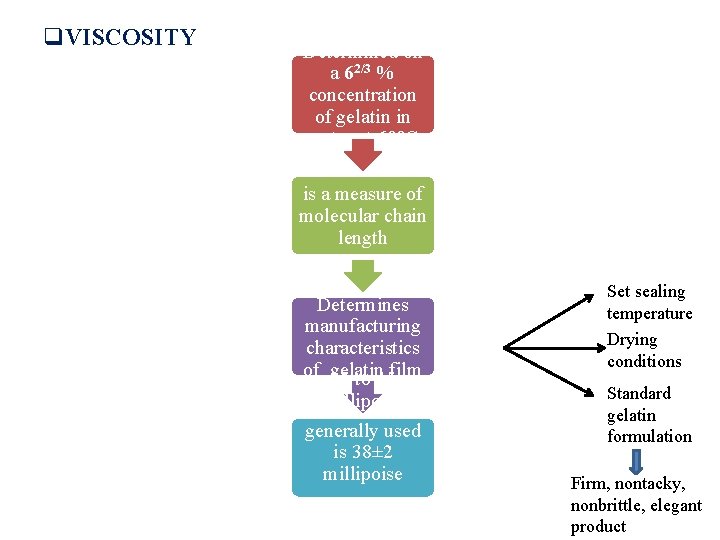
q. VISCOSITY Determined on a 62/3 % concentration of gelatin in water at 60°C is a measure of molecular chain length Determines manufacturing characteristics of gelatin 25 to 45 film millipoise generally used is 38± 2 millipoise Set sealing temperature Drying conditions Standard gelatin formulation Firm, nontacky, nonbrittle, elegant product

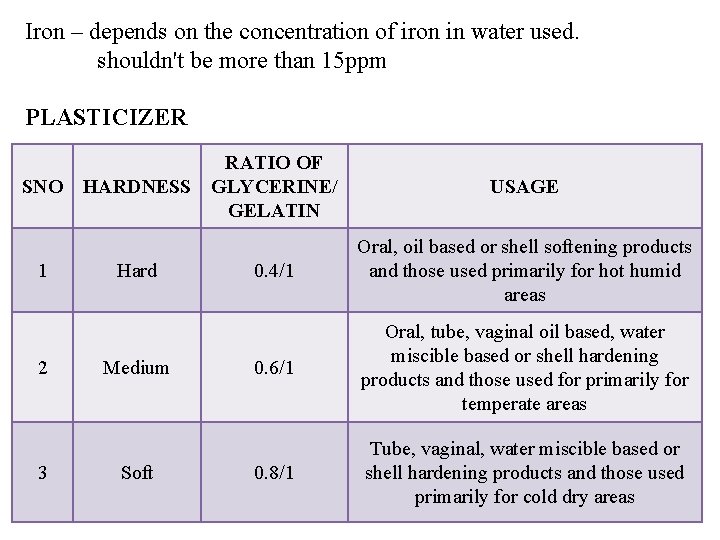
Iron – depends on the concentration of iron in water used. shouldn't be more than 15 ppm PLASTICIZER RATIO OF SNO HARDNESS GLYCERINE/ GELATIN 1 2 3 Hard Medium Soft USAGE 0. 4/1 Oral, oil based or shell softening products and those used primarily for hot humid areas 0. 6/1 Oral, tube, vaginal oil based, water miscible based or shell hardening products and those used for primarily for temperate areas 0. 8/1 Tube, vaginal, water miscible based or shell hardening products and those used primarily for cold dry areas

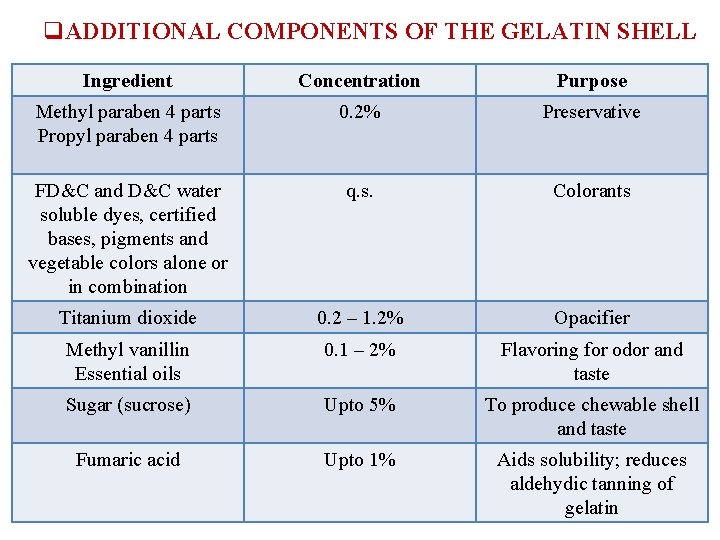
q. ADDITIONAL COMPONENTS OF THE GELATIN SHELL Ingredient Concentration Purpose Methyl paraben 4 parts Propyl paraben 4 parts 0. 2% Preservative FD&C and D&C water soluble dyes, certified bases, pigments and vegetable colors alone or in combination q. s. Colorants Titanium dioxide 0. 2 – 1. 2% Opacifier Methyl vanillin Essential oils 0. 1 – 2% Flavoring for odor and taste Sugar (sucrose) Upto 5% To produce chewable shell and taste Fumaric acid Upto 1% Aids solubility; reduces aldehydic tanning of gelatin

q. DYES AND PIGMENTS: üColor of the capsule shell should never be lighter in hue than capsulated material. üDarker colors are more appropriate for large size (14 to 20 minim oblong) üInteraction between colorants and shell additives must be studies. üClear colors are employed for clear type fill materials. üOpaque colors are used for suspensions.

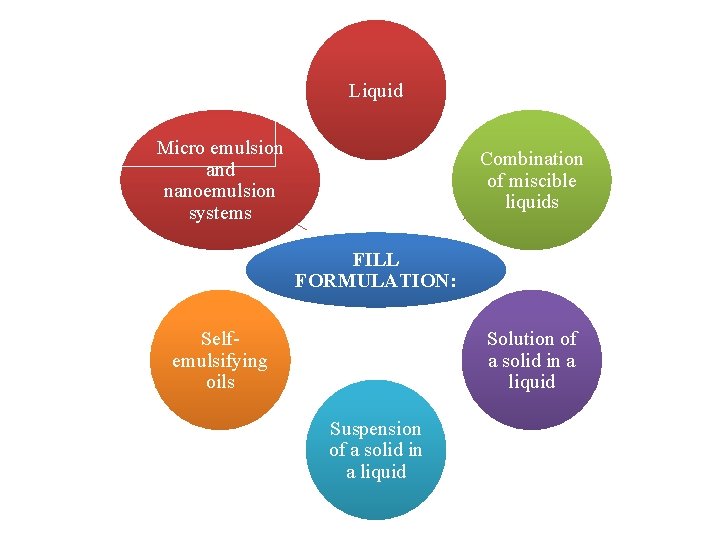
Liquid Micro emulsion and nanoemulsion systems Combination of miscible liquids FILL FORMULATION: Selfemulsifying oils Solution of a solid in a liquid Suspension of a solid in a liquid

q. CRITERIA OF A FORMULATION: üCapacity to dissolve the drug üRate of dispersion in the gastro intestinal tract after rupture of the soft gel shell üRuptures and releases the fill matrix üCapacity to retain the drug in solution in the gastro intestinal tract üCompatibility with the soft gel shell üAbility to optimize the rate , extent and consistency of drug absorbed.

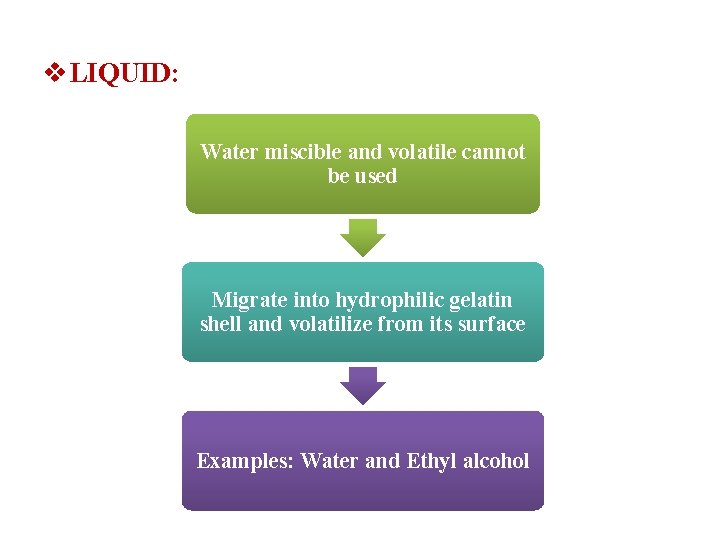
v. LIQUID: Water miscible and volatile cannot be used Migrate into hydrophilic gelatin shell and volatilize from its surface Examples: Water and Ethyl alcohol

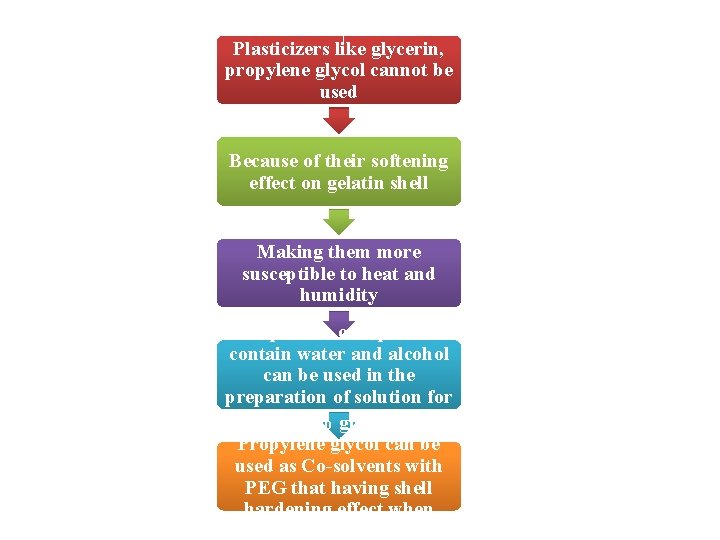
Plasticizers like glycerin, propylene glycol cannot be used Because of their softening effect on gelatin shell Making them more susceptible to heat and humidity Upto 5% of capsule contain water and alcohol can be used in the preparation of solution for Upto capsulation 10% glycerin or Propylene glycol can be used as Co-solvents with PEG that having shell hardening effect when capsulated alone

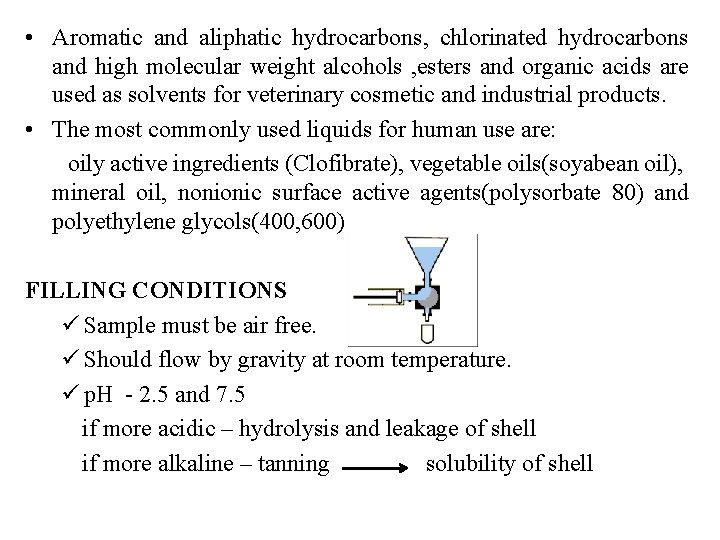
• Aromatic and aliphatic hydrocarbons, chlorinated hydrocarbons and high molecular weight alcohols , esters and organic acids are used as solvents for veterinary cosmetic and industrial products. • The most commonly used liquids for human use are: oily active ingredients (Clofibrate), vegetable oils(soyabean oil), mineral oil, nonionic surface active agents(polysorbate 80) and polyethylene glycols(400, 600) FILLING CONDITIONS ü Sample must be air free. ü Should flow by gravity at room temperature. ü p. H - 2. 5 and 7. 5 if more acidic – hydrolysis and leakage of shell if more alkaline – tanning solubility of shell

q. CAPSULATION OF SUSPENSIONS Ingredient stability Bio pharmaceutical properties Flow characteristics Drug substance

BASE ADSORPTION is expressed as the no of grams of liquid base required to produce a capsulatable mixture when mixed with 1 g of solid. Base adsorption is influenced by üSize and shape üPhysical state – fibrous, amorphous or crystalline üDensity üMoisture content üOleophilic or hydrophilic nature

Procedure for determining base adsorption 40 g of solid in 150 ml beaker 100 g of liquid base in 150 ml beaker Add small equivalents of base to solid with spatula Stir the base into the solid until the solid is properly wetted Continue to add base until a free flowing mixture is obtained

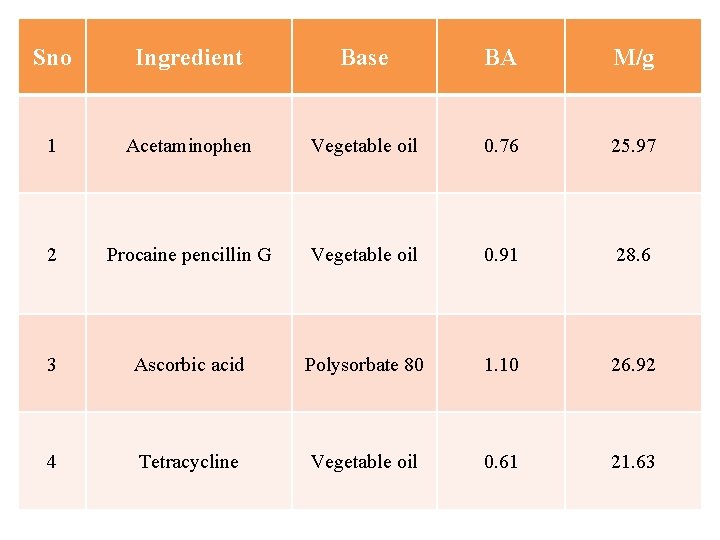
Sno Ingredient Base BA M/g 1 Acetaminophen Vegetable oil 0. 76 25. 97 2 Procaine pencillin G Vegetable oil 0. 91 28. 6 3 Ascorbic acid Polysorbate 80 1. 10 26. 92 4 Tetracycline Vegetable oil 0. 61 21. 63

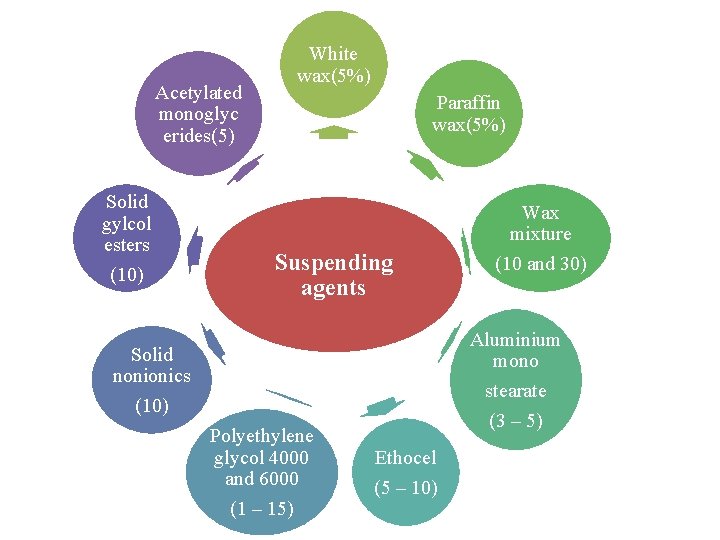
Acetylated monoglyc erides(5) Solid gylcol esters (10) White wax(5%) Paraffin wax(5%) Suspending agents Aluminium mono stearate (3 – 5) Solid nonionics (10) Polyethylene glycol 4000 and 6000 (1 – 15) Wax mixture (10 and 30) Ethocel (5 – 10)

SELF EMULSIFYING OILS: Combinations of pharmaceutical oil and a non-ionic surfactant such as Polyoxyethylene sorbitan mono oleate can provide an oily formulation which disperses rapidly in the gastro intestinal fluid. The resulting oil/surfactant Droplets enable rapid transfer of drug to the absorbing mucosa and subsequent drug absorption. MICROEMULSION AND NANOEMULSION SYSTEMS: §A micro emulsion of a lipid-surfactant-polar liquid system is a characterized by its translucent single phase appearance. §A nanoemulsion is a similar system but contains the emulsion droplets in the 100 nm size range. §Micro emulsion and nanoemulsion systems have the advantage of a high capacity to solubilize drug compounds and to retain the drug in solution even after dilution in gastrointestinal fluids

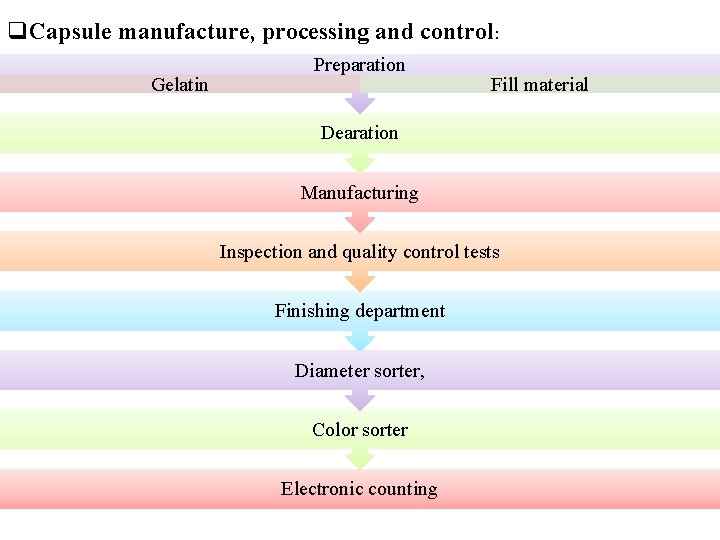
q. Capsule manufacture, processing and control: Gelatin Preparation Fill material Dearation Manufacturing Inspection and quality control tests Finishing department Diameter sorter, Color sorter Electronic counting

q. OPERATING CONDITIONS Plant temperature – 20 to 22°C Operating areas – 40% RH Drying areas – 20 -30% RH Gelatin film Preparation Required gelatin weighed Chilled to 7°C In melting tanks 29. 5 Hg at 93°C (for 3 hrs) Mixing for 25 mins Colorants added and maintained at 57 - 60°C

Fill preparation Stainless steel jacketed tanks (10 – 450 gallons) (for initial blending with liquid base) Milling or homogenized (not to reduce the size but to break the agglomerates) Wetted with liquid carrier to form smooth homogenous mixture Dearation (to achieve uniform capsule fill weight and to prevent loss of potency by oxidation) If ingredients are volatile, added after dearation and if suspensions/ liquids, vacuum 29. 5 Hg Dearation can be achieved by heating at 60°C

IN PROCESS TESTING üIngredient assay üSpecific gravity üTest for homogeneity üMoisture content üAir entrapment

Manufacturing of soft gels: Plate process Accogel process Bubble method Rotary die process

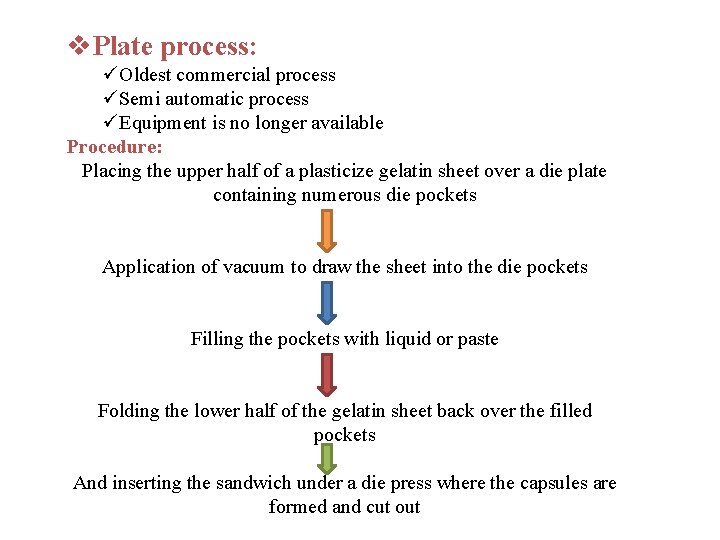
v. Plate process: üOldest commercial process üSemi automatic process üEquipment is no longer available Procedure: Placing the upper half of a plasticize gelatin sheet over a die plate containing numerous die pockets Application of vacuum to draw the sheet into the die pockets Filling the pockets with liquid or paste Folding the lower half of the gelatin sheet back over the filled pockets And inserting the sandwich under a die press where the capsules are formed and cut out

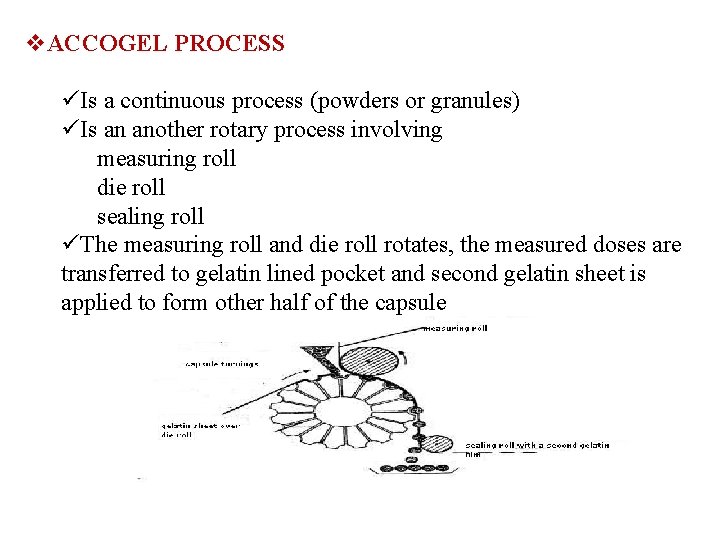
v. ACCOGEL PROCESS üIs a continuous process (powders or granules) üIs an another rotary process involving measuring roll die roll sealing roll üThe measuring roll and die roll rotates, the measured doses are transferred to gelatin lined pocket and second gelatin sheet is applied to form other half of the capsule

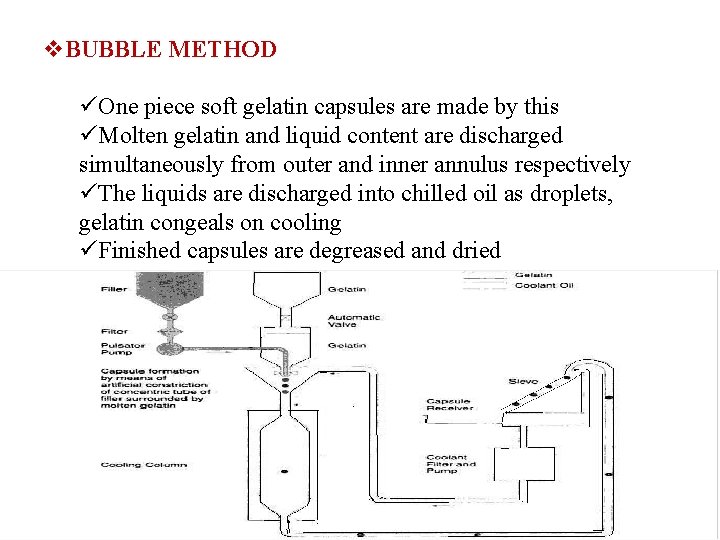
v. BUBBLE METHOD üOne piece soft gelatin capsules are made by this üMolten gelatin and liquid content are discharged simultaneously from outer and inner annulus respectively üThe liquids are discharged into chilled oil as droplets, gelatin congeals on cooling üFinished capsules are degreased and dried

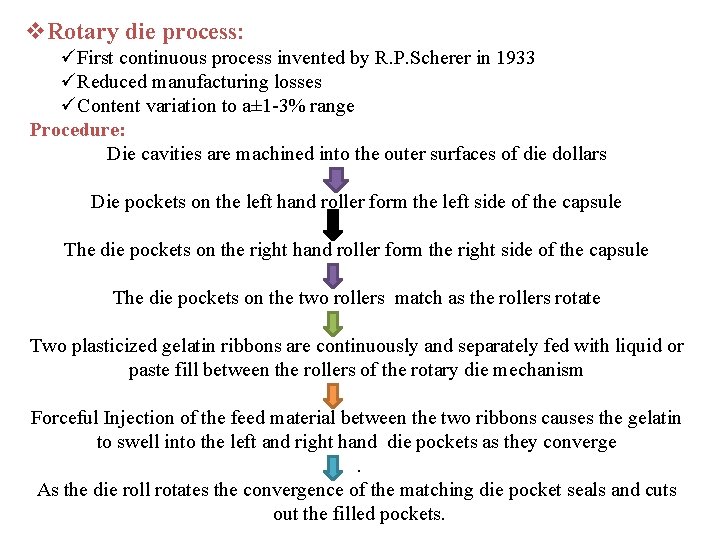
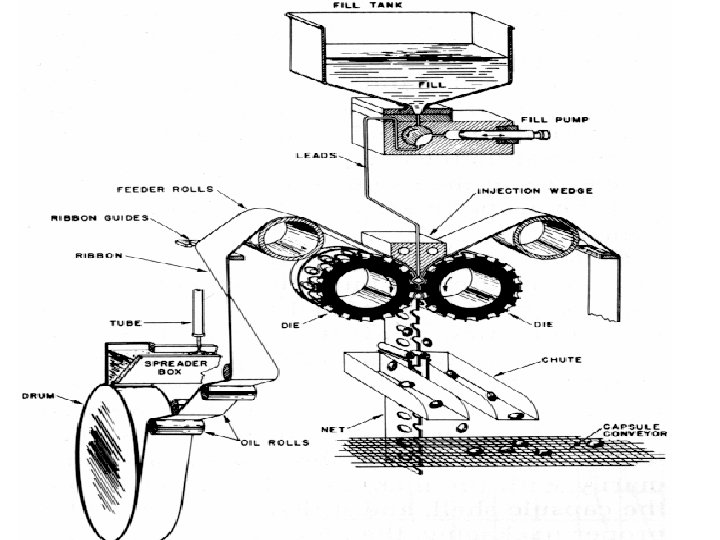
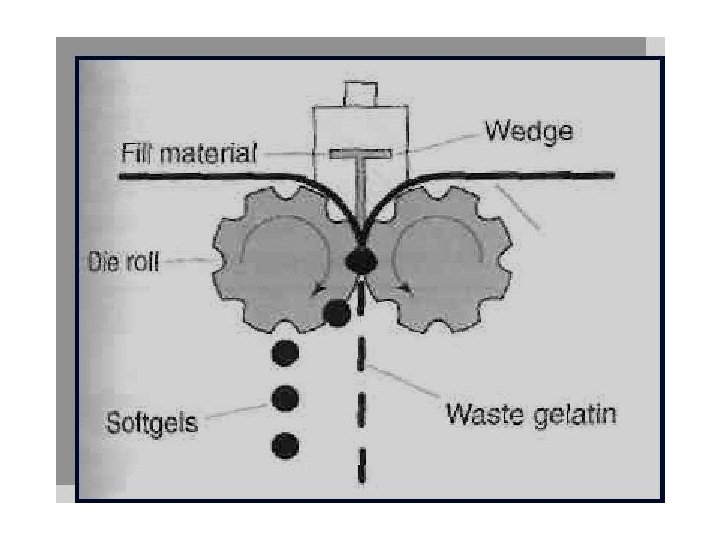
v. Rotary die process: üFirst continuous process invented by R. P. Scherer in 1933 üReduced manufacturing losses üContent variation to a± 1 -3% range Procedure: Die cavities are machined into the outer surfaces of die dollars Die pockets on the left hand roller form the left side of the capsule The die pockets on the right hand roller form the right side of the capsule The die pockets on the two rollers match as the rollers rotate Two plasticized gelatin ribbons are continuously and separately fed with liquid or paste fill between the rollers of the rotary die mechanism Forceful Injection of the feed material between the two ribbons causes the gelatin to swell into the left and right hand die pockets as they converge. As the die roll rotates the convergence of the matching die pocket seals and cuts out the filled pockets.





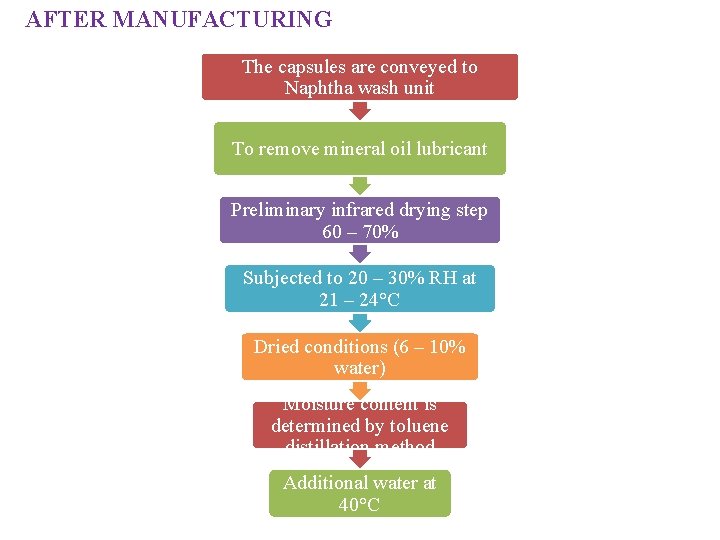
AFTER MANUFACTURING The capsules are conveyed to Naphtha wash unit To remove mineral oil lubricant Preliminary infrared drying step 60 – 70% Subjected to 20 – 30% RH at 21 – 24°C Dried conditions (6 – 10% water) Moisture content is determined by toluene distillation method Additional water at 40°C

q. INSPECTION AND QC STEPS üSeal thickness üTotal or shell moisture tests üCapsule fragility or rupture tests üDetermining freezing and high temperature effects q. FINISHING DEPARTMENT v. Heat branding or ink painting for identification purpose. v. Diameter sorting: Capsules with ± 0. 020 inch of theoretical diameter of particular capsule is tested. Over fills, under fills, foreign capsules are discarded

Color sorter ØPneumatic conveyor – capsule color that doesn't conform to reference color standard is discarded Passed to electronic counting and packaging unit Electronic counting can count as many as 8000 capsules/min

Preparation room Gelatin Melting Part Capsule Cleaning Process Capsule making process Capsule Air Drying Capsule Inspection Process Shape Fixing And Drying Process Inner Packed Process

Outside Packing Process Storage

Quality control tests ØContent uniformity ØWeight variation ØContents of the packaged dosage form ØDisintegration test ØDissolution test

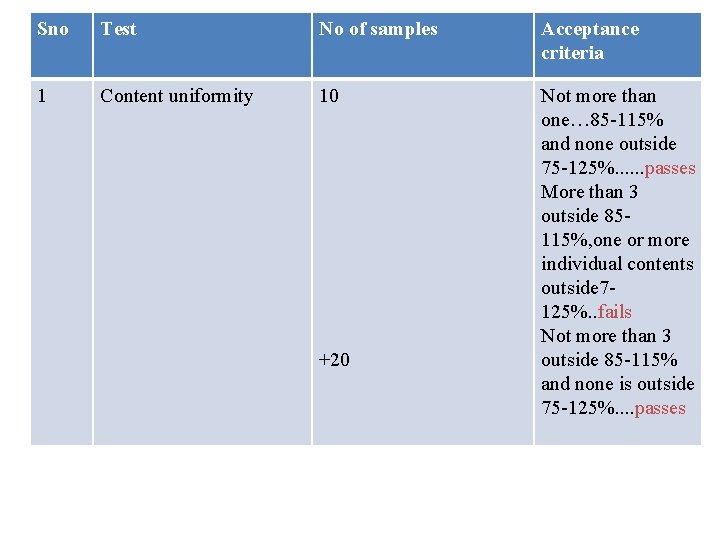
Sno Test No of samples Acceptance criteria 1 Content uniformity 10 Not more than one… 85 -115% and none outside 75 -125%. . . passes More than 3 outside 85115%, one or more individual contents outside 7125%. . fails Not more than 3 outside 85 -115% and none is outside 75 -125%. . passes +20

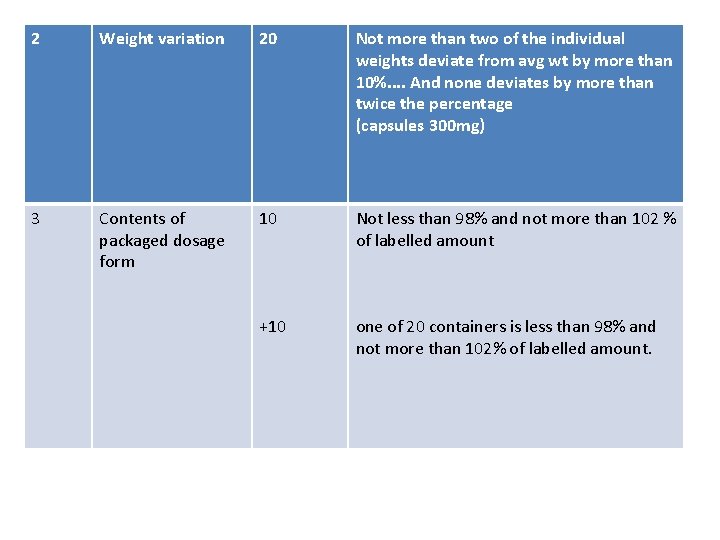
2 Weight variation 20 Not more than two of the individual weights deviate from avg wt by more than 10%. . And none deviates by more than twice the percentage (capsules 300 mg) 3 Contents of packaged dosage form 10 Not less than 98% and not more than 102 % of labelled amount +10 one of 20 containers is less than 98% and not more than 102% of labelled amount.

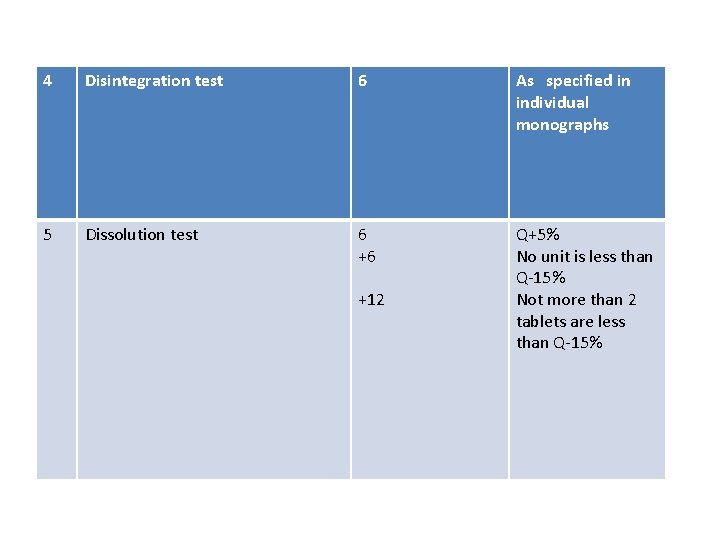
4 Disintegration test 6 As specified in individual monographs 5 Dissolution test 6 +6 Q+5% No unit is less than Q-15% Not more than 2 tablets are less than Q-15% +12

q. References : Ø Remington: The Science and Practice of Pharmacy Vol II 20 th edition. Ø Theory and Practice of Industrial Pharmacy By Leon Lachmann. Ø Modern pharmaceutics, fourth edition, edited by Gilbert S. Banker, Christopher Rhodes. Ø Pharmaceutical Dosage Forms and Drug Delivery systems By Howard. C. Ansel, Loyd V. Allen, Jr. Nicholas G. Popovich Ø Pharmaceutics: The science of dosage form design, edited by M. E. Aulton. 2 nd edition.
 Hard gelatin capsules definition
Hard gelatin capsules definition Minim/gram factor for soft gelatin capsules
Minim/gram factor for soft gelatin capsules Accogel process
Accogel process Punch method of capsule filling
Punch method of capsule filling N chalapathi
N chalapathi Tidal percussion
Tidal percussion Applications of molality in pharmacy
Applications of molality in pharmacy Upward creaming
Upward creaming Viscosity application in pharmacy
Viscosity application in pharmacy What's surface tension
What's surface tension Paste dosage form
Paste dosage form Size separation advantages and disadvantages
Size separation advantages and disadvantages Define geometric dilution
Define geometric dilution Finely divided, bulk effervescent
Finely divided, bulk effervescent Vanishing cream pharmaceutics
Vanishing cream pharmaceutics Example of physical incompatibility
Example of physical incompatibility Example of physical incompatibility in pharmaceutics
Example of physical incompatibility in pharmaceutics Dusting powder
Dusting powder Effervescent granules label
Effervescent granules label Suppository definition pharmacy
Suppository definition pharmacy Ideal properties of suppositories base
Ideal properties of suppositories base Pharmaceutical dosage form definition
Pharmaceutical dosage form definition Freely flowing powders
Freely flowing powders Pharmaceutics unit 1
Pharmaceutics unit 1 Examples of galenicals
Examples of galenicals Tablet definition pharmacy
Tablet definition pharmacy What is cmc in physical pharmacy
What is cmc in physical pharmacy Method of preparation of suspension
Method of preparation of suspension Phase rule physical pharmacy
Phase rule physical pharmacy Eye support vestige
Eye support vestige Capsule solid dosage form
Capsule solid dosage form Blautix capsules where to buy
Blautix capsules where to buy Beast super test powder
Beast super test powder Pan polishing capsules
Pan polishing capsules Vestige coconut oil
Vestige coconut oil Tiens cell rejuvenation capsules side effects
Tiens cell rejuvenation capsules side effects Vestige spirulina capsules benefits
Vestige spirulina capsules benefits Matrix capsules with em routing
Matrix capsules with em routing Oral medication calculation formula
Oral medication calculation formula Vestige fish oil capsules
Vestige fish oil capsules How to make fake skin with vaseline and cornstarch
How to make fake skin with vaseline and cornstarch Inkompatibilitas gelatin
Inkompatibilitas gelatin Index of refraction of gelatin
Index of refraction of gelatin Gelatin in italian
Gelatin in italian Slidetodoc.com
Slidetodoc.com Gelatin test for tannins
Gelatin test for tannins Glycero gelatin suppository
Glycero gelatin suppository Gelatin hydrolysis test
Gelatin hydrolysis test Example of cynophoric
Example of cynophoric Diffusion through gelatin experiment
Diffusion through gelatin experiment Soft loan schemes for services sector (slsss)
Soft loan schemes for services sector (slsss) Provider gap
Provider gap