SURFACE CHEMISTRY Adsorption The phenomenon of higher concentration










- Slides: 21

SURFACE CHEMISTRY


Adsorption The phenomenon of higher concentration of any molecular species at the surface than in the bulk Adsorbent The substance on the surface of which adsorption takes place is called adsorbent Adsorbate The substance which is being adsorbed on the surface of another substance. Desorption The process of removal of an adsorbed substance from the surface on which it is absorbed

Adsorbent Materials • Activated Carbon • Activated Alumina • Silica Gel • Molecular Sieves (Zeolites) • Polar and Non-polar adsorbents

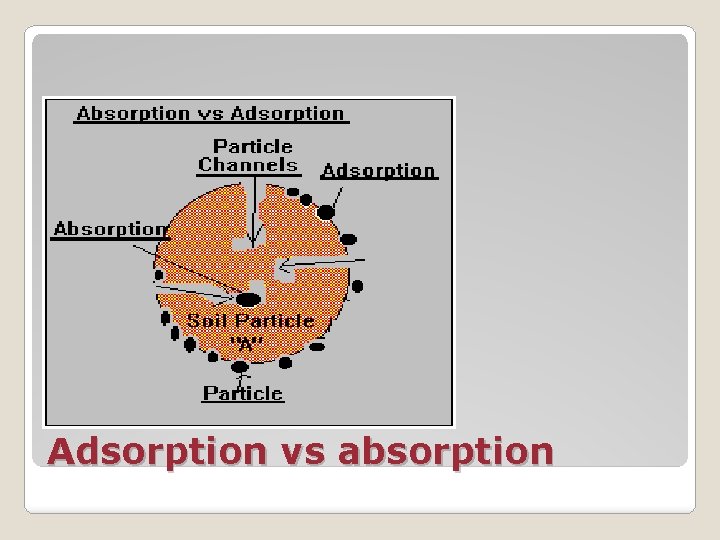
Adsorption vs absorption

Types of Adsorption Positive adsorption occurs when the concentration of adsorbate is higher on the surface of adsorbent than in the bulk. Negative adsorption occurs when the concentration of adsorbate is less on the surface of adsorbent than in the bulk.

Types of adsorption 1. Physical adsorption 2. Chemical adsorption

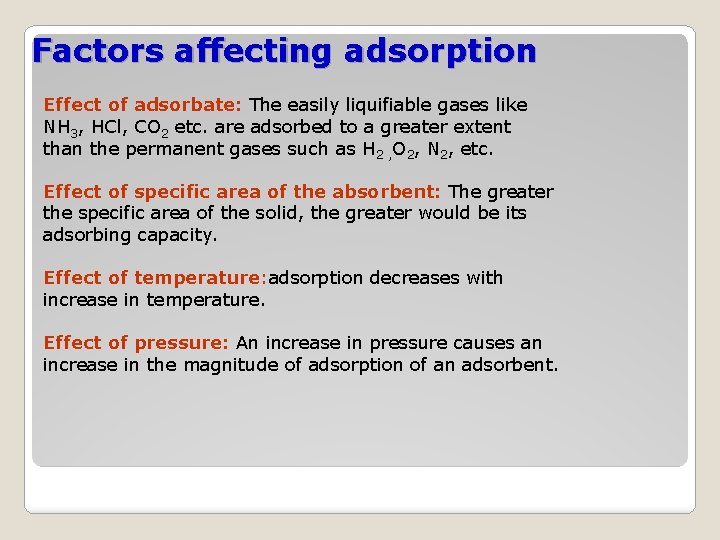
Factors affecting adsorption Effect of adsorbate: The easily liquifiable gases like NH 3, HCl, CO 2 etc. are adsorbed to a greater extent than the permanent gases such as H 2 , O 2, N 2, etc. Effect of specific area of the absorbent: The greater the specific area of the solid, the greater would be its adsorbing capacity. Effect of temperature: adsorption decreases with increase in temperature. Effect of pressure: An increase in pressure causes an increase in the magnitude of adsorption of an adsorbent.

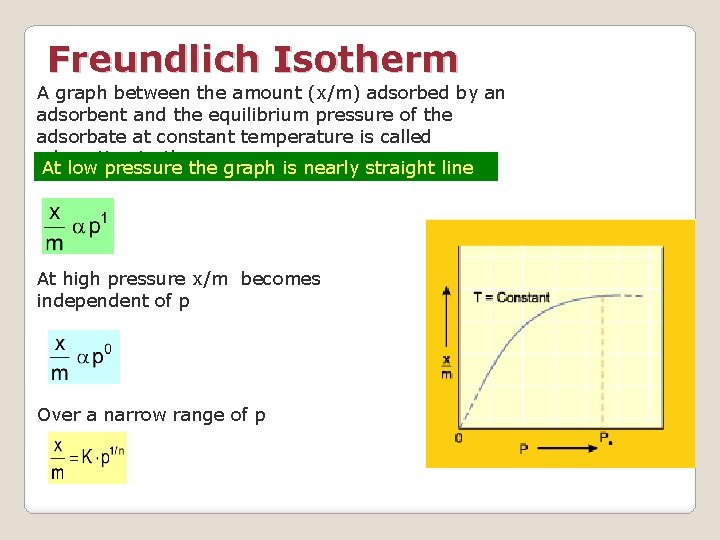
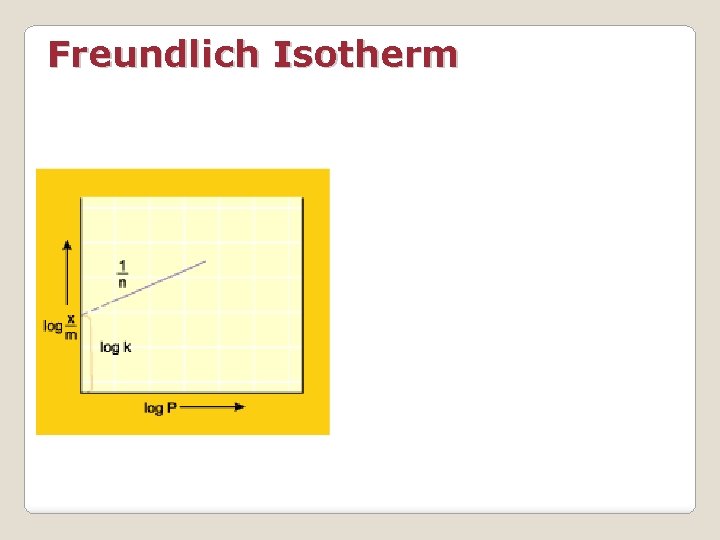
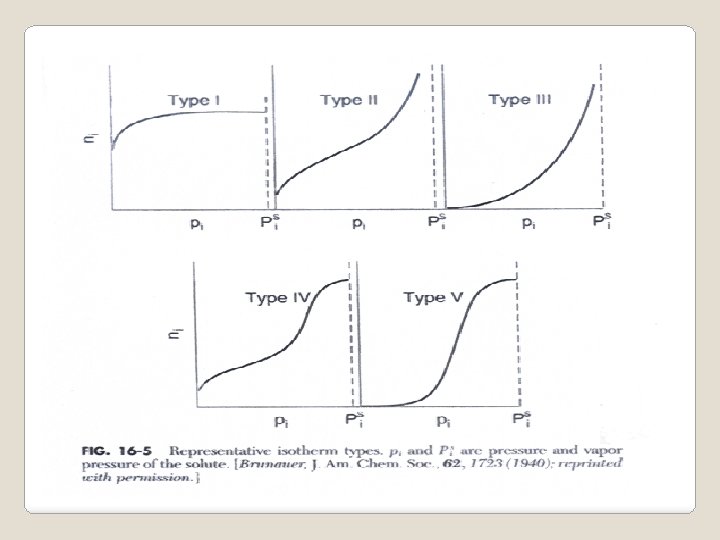
Freundlich Isotherm A graph between the amount (x/m) adsorbed by an adsorbent and the equilibrium pressure of the adsorbate at constant temperature is called adsorption isotherm At low pressure the graph is nearly straight line At high pressure x/m becomes independent of p Over a narrow range of p

Freundlich Isotherm

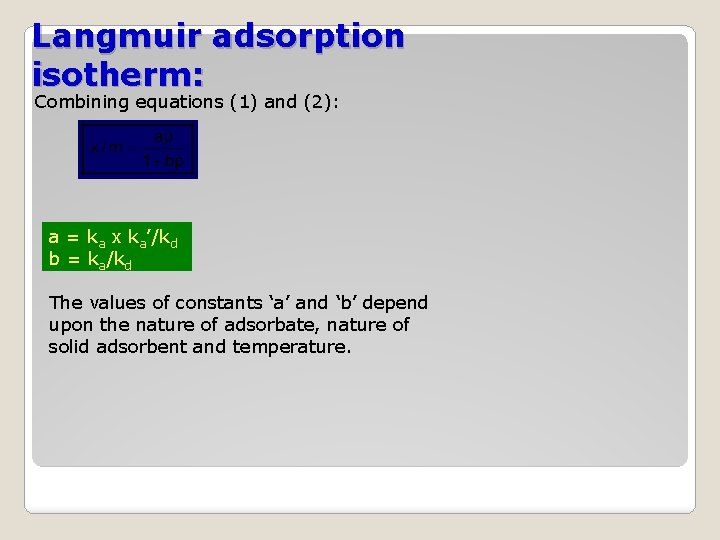
Langmuir adsorption isotherm: Combining equations (1) and (2): a = ka x ka’/kd b = ka/kd The values of constants ‘a’ and ‘b’ depend upon the nature of adsorbate, nature of solid adsorbent and temperature.

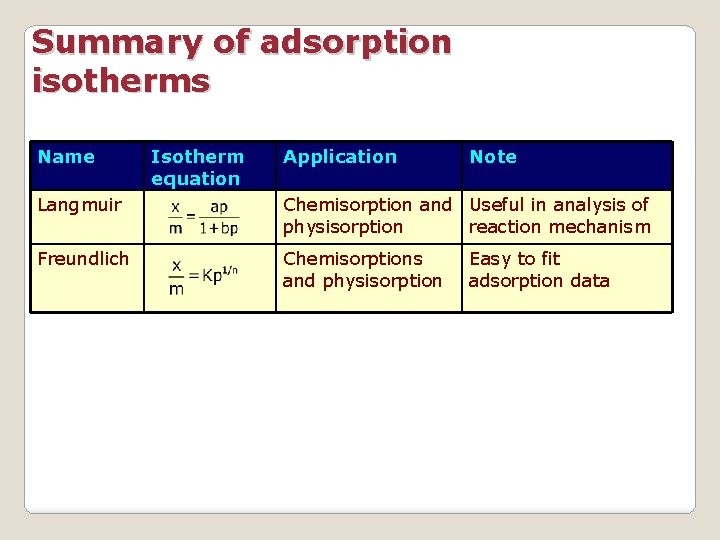
Summary of adsorption isotherms Name Isotherm equation Application Note Langmuir Chemisorption and Useful in analysis of physisorption reaction mechanism Freundlich Chemisorptions and physisorption Easy to fit adsorption data

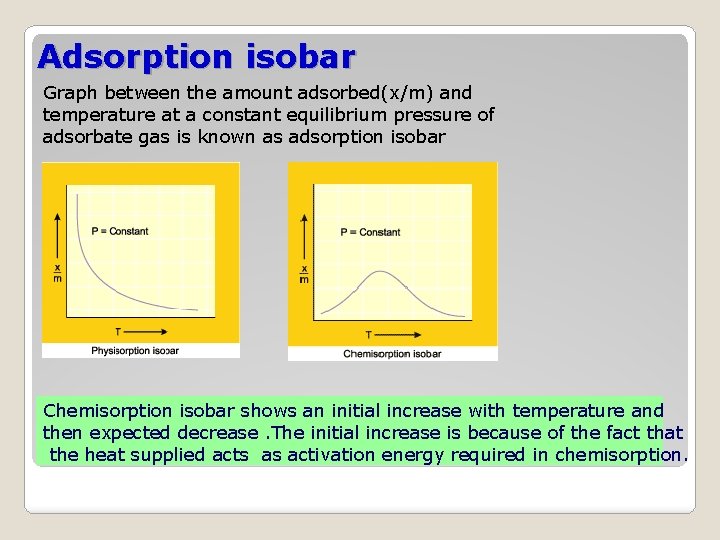
Adsorption isobar Graph between the amount adsorbed(x/m) and temperature at a constant equilibrium pressure of adsorbate gas is known as adsorption isobar Chemisorption isobar shows an initial increase with temperature and then expected decrease. The initial increase is because of the fact that the heat supplied acts as activation energy required in chemisorption.

Application of Adsorption ü In clarification of sugar ü In gas masks ü In catalysis ü In adsorption indicators ü In chromatographic analysis ü In softening of hard water ü In preserving vacuum ü In paint industry ü In removing moisture from air in the storage of delicate instruments


DIFFERENT PHYSICAL FORMS OF ACTIVATED CARBON

Need to make chemicals faster Most Reactions are too slow to be useful. . .

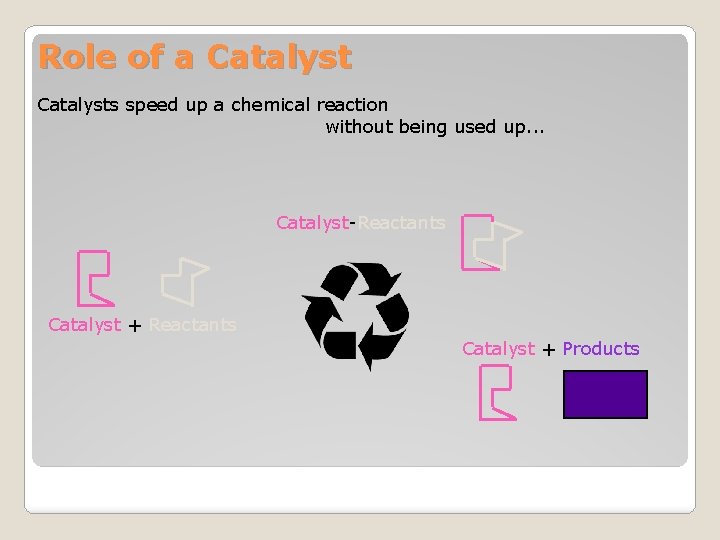
Role of a Catalysts speed up a chemical reaction without being used up. . . Catalyst-Reactants Catalyst + Products

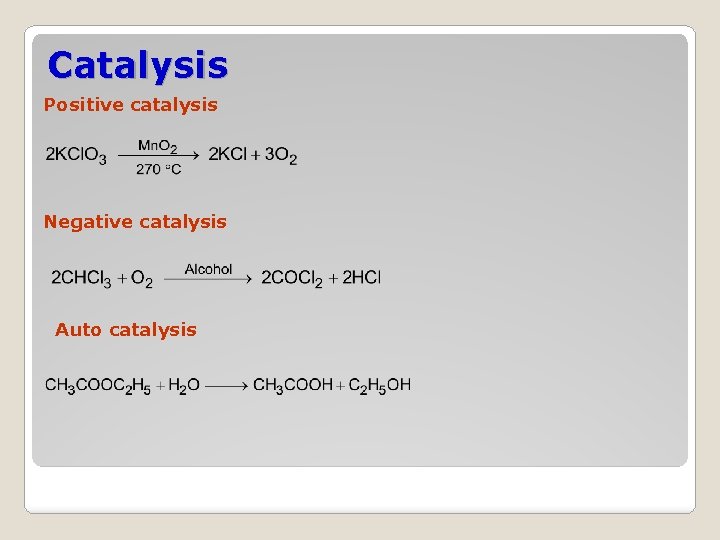
Catalysis Positive catalysis Negative catalysis Auto catalysis

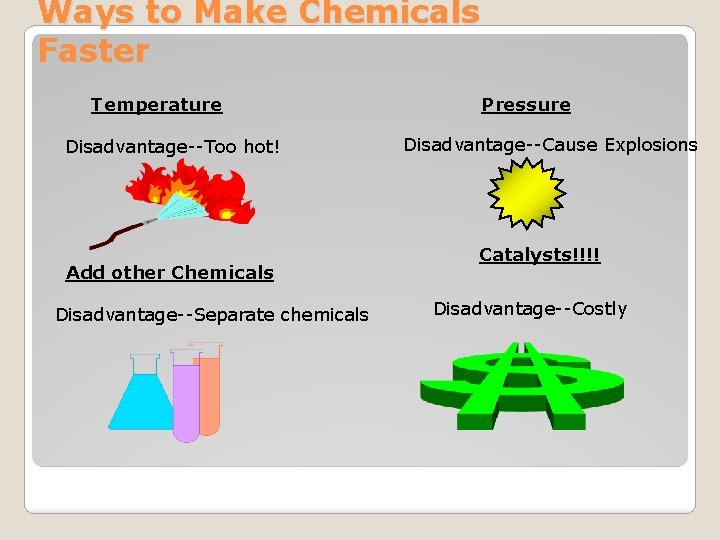
Ways to Make Chemicals Faster Temperature Disadvantage--Too hot! Add other Chemicals Disadvantage--Separate chemicals Pressure Disadvantage--Cause Explosions Catalysts!!!! Disadvantage--Costly

Thank you
 Negative adsorption
Negative adsorption Adsorption phenomenon
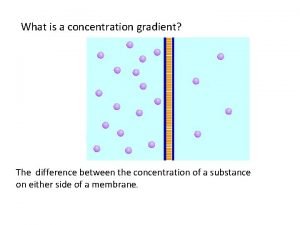
Adsorption phenomenon Whats a concentration gradient
Whats a concentration gradient Movement of high concentration to low concentration
Movement of high concentration to low concentration Borchert's epochs ap human geography definition
Borchert's epochs ap human geography definition A computer system that stores organizes retrieves
A computer system that stores organizes retrieves Molal units
Molal units Higher chemistry questions
Higher chemistry questions Cfe higher chemistry
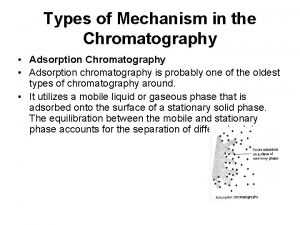
Cfe higher chemistry Mechanism of adsorption chromatography
Mechanism of adsorption chromatography Langmuir adsorption isotherm equation derivation
Langmuir adsorption isotherm equation derivation What is partition coefficient
What is partition coefficient Adsorption technique in blood banking
Adsorption technique in blood banking Adsorption in physical pharmacy
Adsorption in physical pharmacy Fajans titration
Fajans titration Which detector used in hplc
Which detector used in hplc Define precipitation titration
Define precipitation titration Freundlich adsorption isotherm formula
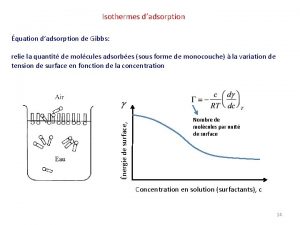
Freundlich adsorption isotherm formula Isotherme de gibbs
Isotherme de gibbs What is post precipitation
What is post precipitation Methods of emulsion
Methods of emulsion What is adsorption
What is adsorption