Matter Matter is anything that has mass and













- Slides: 31

Matter: Matter is anything that has mass and takes up space. It’s what the world is made of.

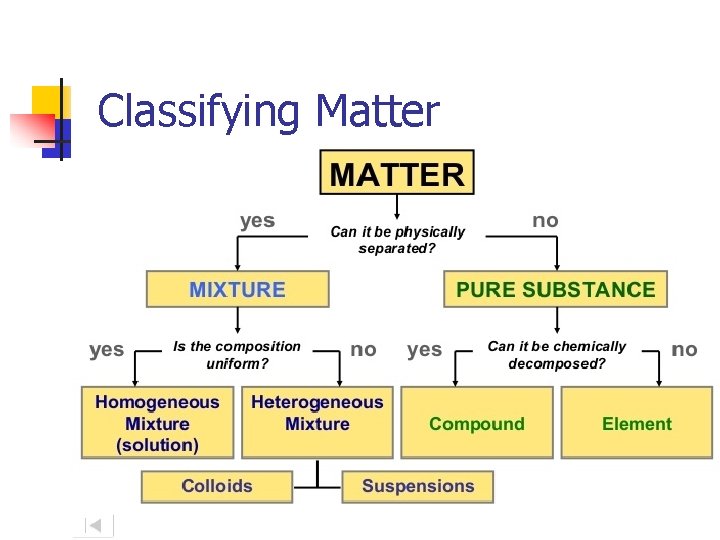
Classifying Matter

Types of Mixtures Heterogeneous- not uniform throughout Ex. -Wet Sand -Lucky Charms -Oil and water - Colloids- on a microscopic scale Homogenous- uniform throughout Ex. -Solutions -Tea -Salt water -Colloids on a macroscopic scale

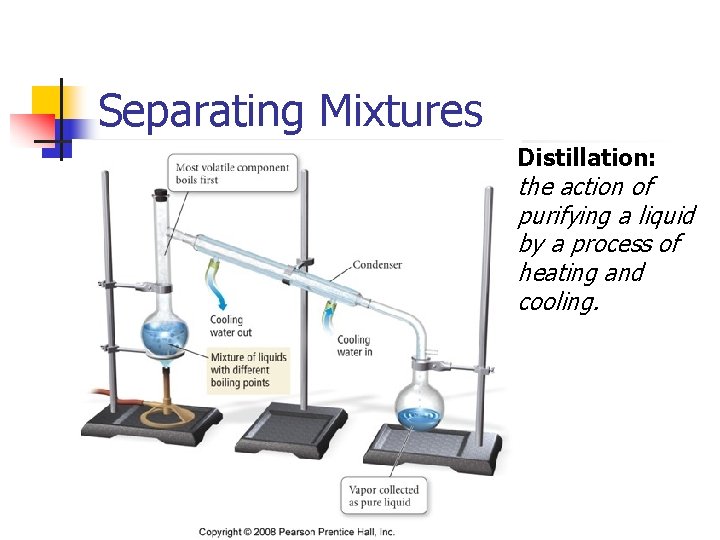
Separating Mixtures Distillation: the action of purifying a liquid by a process of heating and cooling.

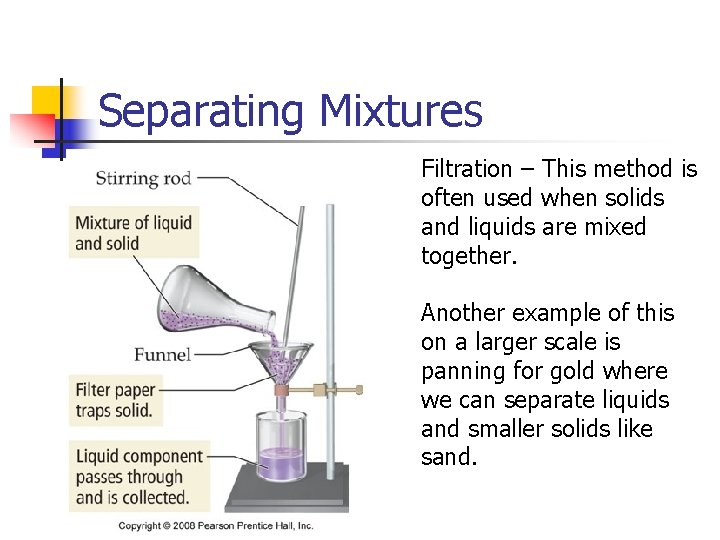
Separating Mixtures Filtration – This method is often used when solids and liquids are mixed together. Another example of this on a larger scale is panning for gold where we can separate liquids and smaller solids like sand.

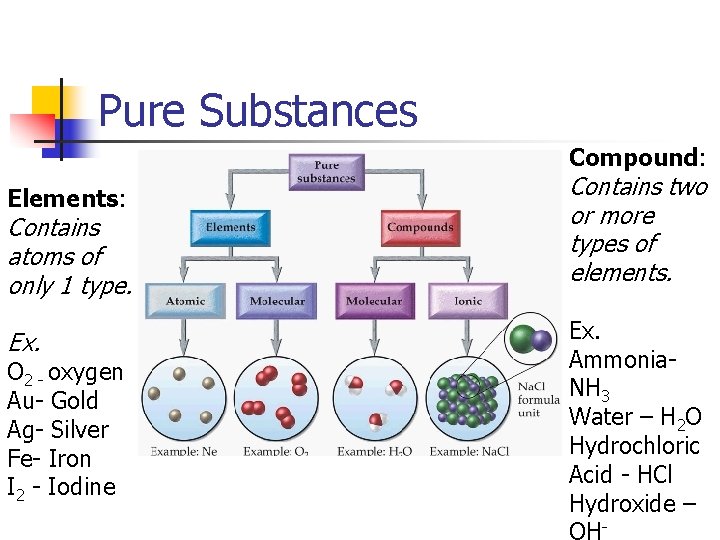
Pure Substances Compound: Elements: Contains atoms of only 1 type. Ex. O 2 - oxygen Au- Gold Ag- Silver Fe- Iron I 2 - Iodine Contains two or more types of elements. Ex. Ammonia. NH 3 Water – H 2 O Hydrochloric Acid - HCl Hydroxide – -

States and Phases of Matter

Solids n n n Solids have definite shape and definite volume. Solids have mass. Solids take up space. Read more!

Particles in Solids: n Are packed tightly together n Have very little energy n Vibrate in place

Liquids n n n Liquids take the shape of their container and have definite volume. Liquids have mass. Liquids take up space. Read more!

Particles in Liquids: n n n Are loosely packed Have medium energy levels Particles flow around each other

Gases n n n Gases spread out to fill the entire space given and do not have definite volume. Gases have mass. Gases take up space. Read more!

Particles in Gases: n n Move freely Have LOTS of energy

Plasma n n n Lightning is a plasma. Used in fluorescent light bulbs and Neon lights. Plasma is a lot like a gas, but the particles are electrically charged. Read more!

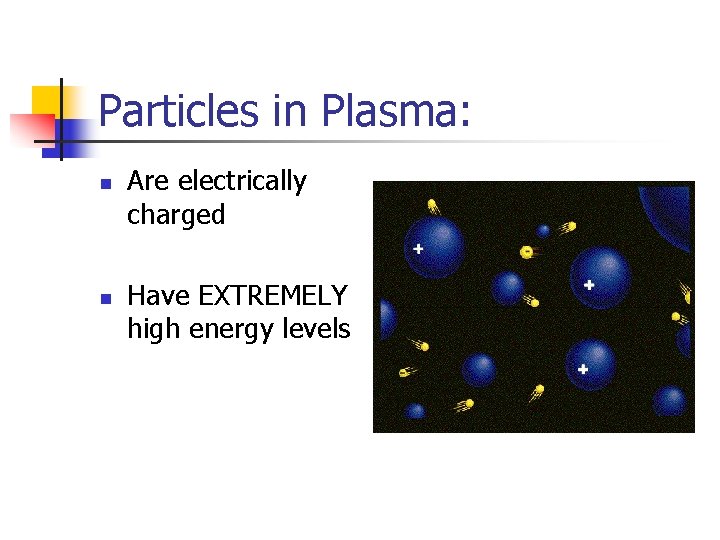
Particles in Plasma: n n Are electrically charged Have EXTREMELY high energy levels

Phases in Matter vs. States Phase- each section of matter that is the same throughout n State- Tells whether a substance is solid liquid or gas Ex. Oil and Water n 2 phases- there are 2 distinct layers even though they are both liquid 1 state- oil and water are both liquid

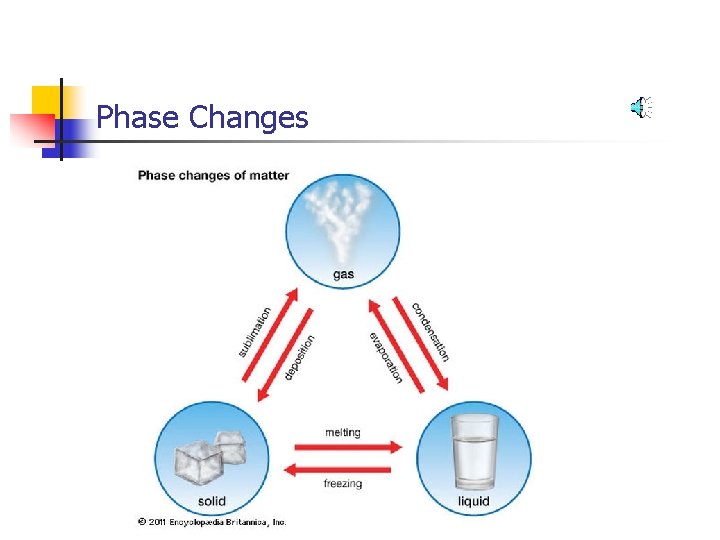
Phase Changes

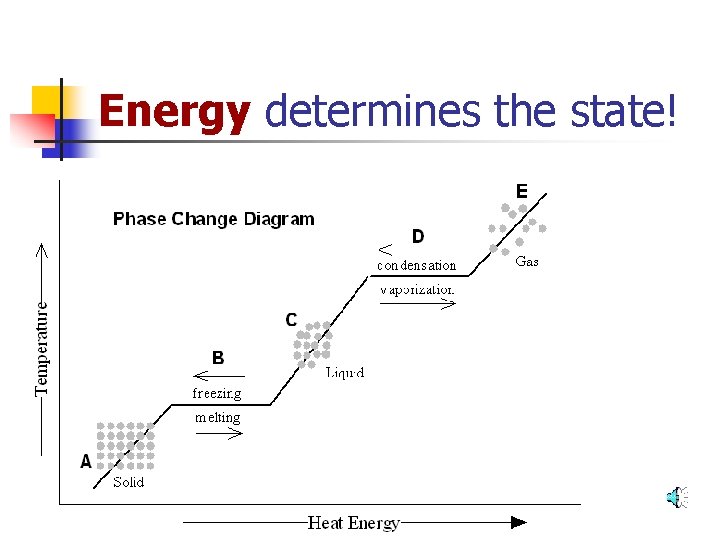
Add or Subtract Energy. . . When energy is added, particles move faster! When energy is taken away, particles move slower!

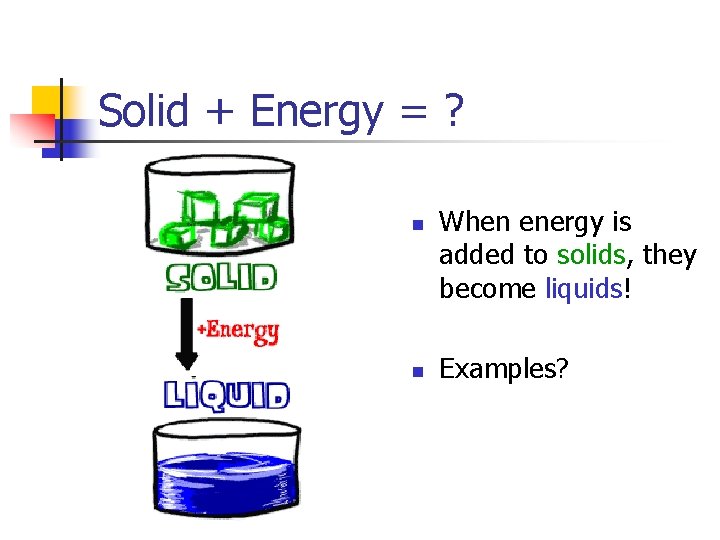
Solid + Energy = ? n n When energy is added to solids, they become liquids! Examples?

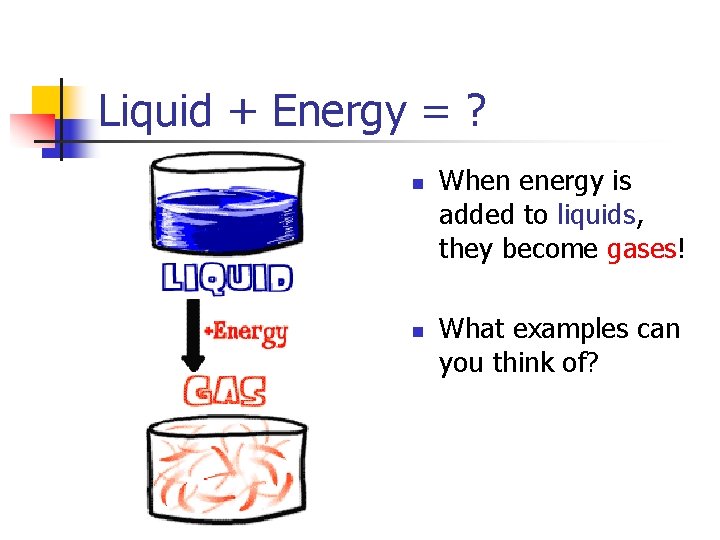
Liquid + Energy = ? n n When energy is added to liquids, they become gases! What examples can you think of?

Energy determines the state!

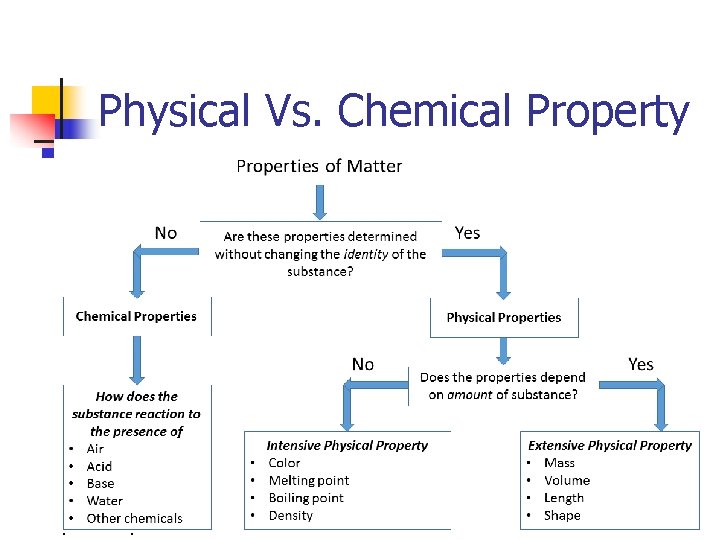
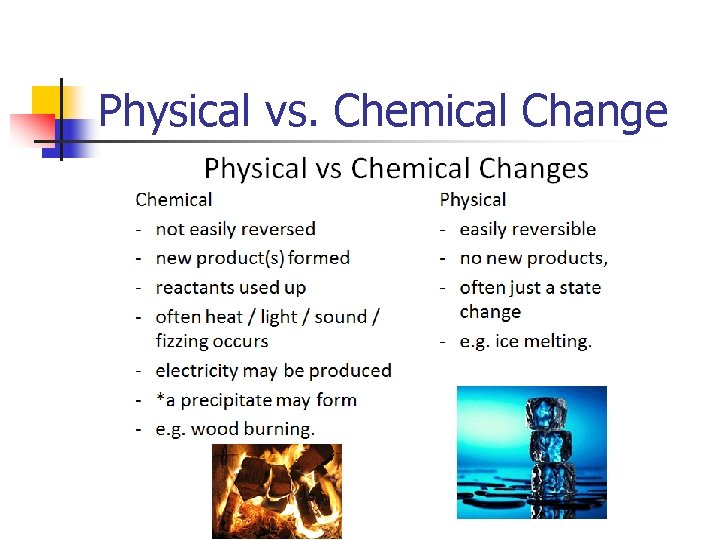
Physical Vs. Chemical Property Physical Propertycan be observed or measured without changing the composition of matter Ex. appearance, texture, color, odor, melting point, boiling point, density, solubility, polarity Chemical Property- is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity Ex: Reactivity, p. H, Combustibility

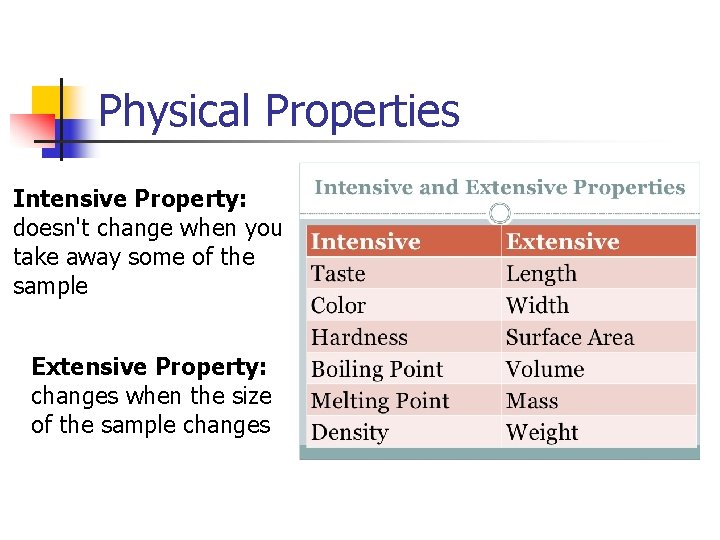
Physical Properties Intensive Property: doesn't change when you take away some of the sample Extensive Property: changes when the size of the sample changes


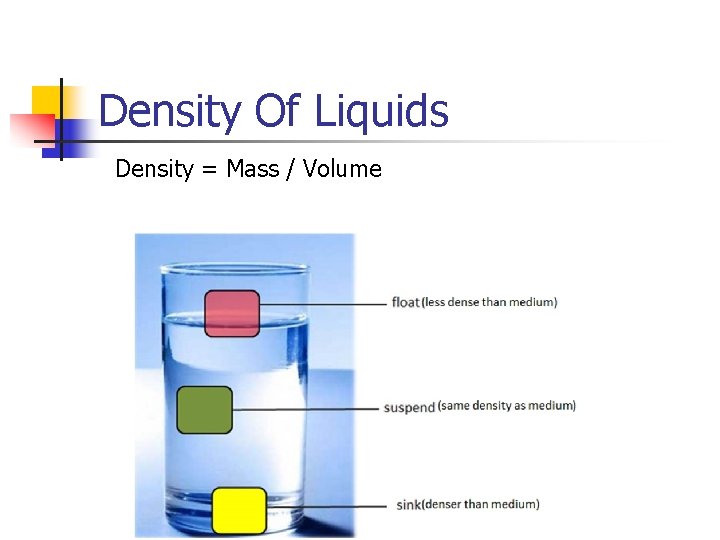
Density Of Liquids Density = Mass / Volume

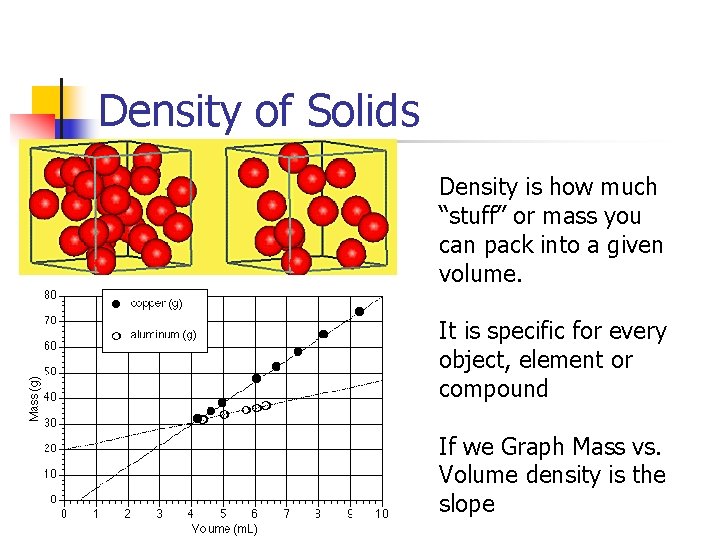
Density of Solids Density is how much “stuff” or mass you can pack into a given volume. It is specific for every object, element or compound If we Graph Mass vs. Volume density is the slope

Density Depends on Temp As temperature increases density usually decreases. ex. Metal expands with heat D=M/V increase V decreases D Water is weird because Ice due to its structure has a lower density then water even though it is colder.

Physical vs. Chemical Change

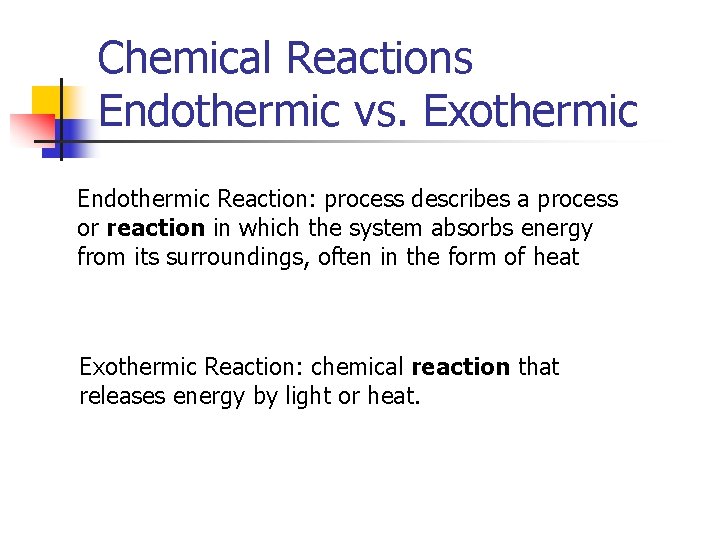
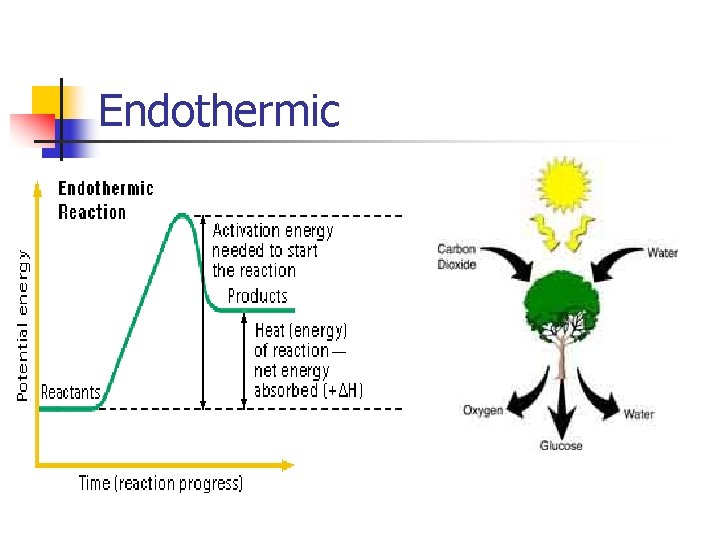
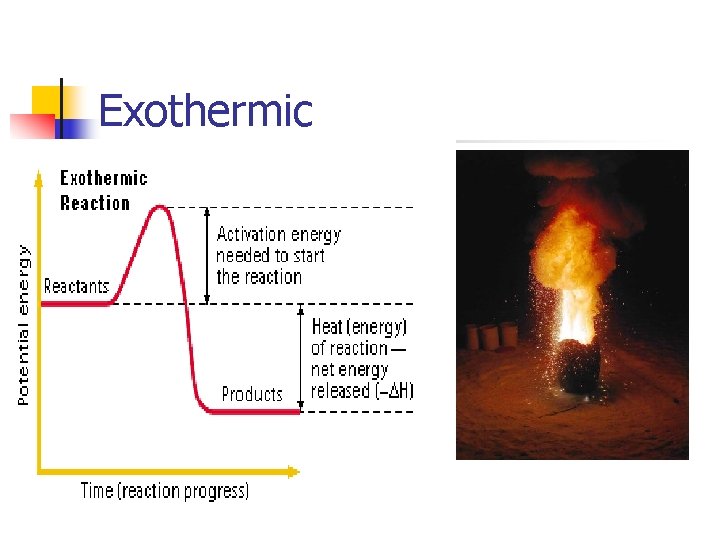
Chemical Reactions Endothermic vs. Exothermic Endothermic Reaction: process describes a process or reaction in which the system absorbs energy from its surroundings, often in the form of heat Exothermic Reaction: chemical reaction that releases energy by light or heat.

Endothermic

Exothermic
 Anything that has mass and occupies space.
Anything that has mass and occupies space. Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Matter is anything that has
Matter is anything that has A glass sinker has a mass m in air
A glass sinker has a mass m in air Matter is anything that...
Matter is anything that... It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Matter is anything that has
Matter is anything that has Is anything that has mass and volume
Is anything that has mass and volume Matter is anything that has and takes up
Matter is anything that has and takes up Anything that takes up space and has mass is
Anything that takes up space and has mass is Whats anything that has mass and takes up space
Whats anything that has mass and takes up space Anthing that takes up space and has mass is called?
Anthing that takes up space and has mass is called? Malleability defintion
Malleability defintion Anything that has matter and takes up space
Anything that has matter and takes up space Matter is anything that
Matter is anything that Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Block nhĩ thất cao độ
Block nhĩ thất cao độ Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Walmart thất bại ở nhật
Walmart thất bại ở nhật Tìm vết của đường thẳng
Tìm vết của đường thẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Frank has an eraser it has a mass of 4g
Frank has an eraser it has a mass of 4g Matter is defined as anything that
Matter is defined as anything that Matter anything that
Matter anything that Use of density
Use of density Ionized matter
Ionized matter Matter is anything that
Matter is anything that