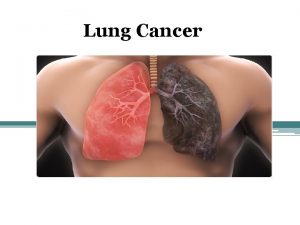
Lung Cancer John C Morris M D Professor

- Slides: 47

Lung Cancer John C. Morris, M. D. Professor Division of Hematology-Oncology Department of Internal Medicine University of Cincinnati February 3, 2018

• 1761 - “Lung tumors” reported in coal miners in Silesia • Prior to the 20 th century a rare disease: • 1878 - 1% of cancers seen at autopsy at the University of Dresden, but by 1927 it accounted for 14% of cancers (Witschi H. , 2001). • Prior to 1930 lung cancer was a rare diagnosis in the U. S. • 1986 - Lung cancer surpassed breast cancer as the most common fatal cancer in women • Leading cause of cancer death in the U. S. with an expected 222, 500 cases and 158, 870 deaths in 2017 (Siegel RL, et al. 2017)

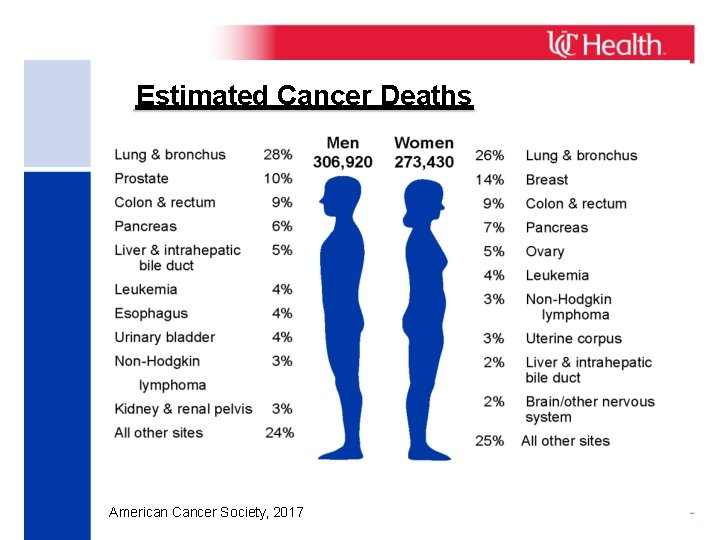
Estimated Cancer Deaths American Cancer Society, 2017

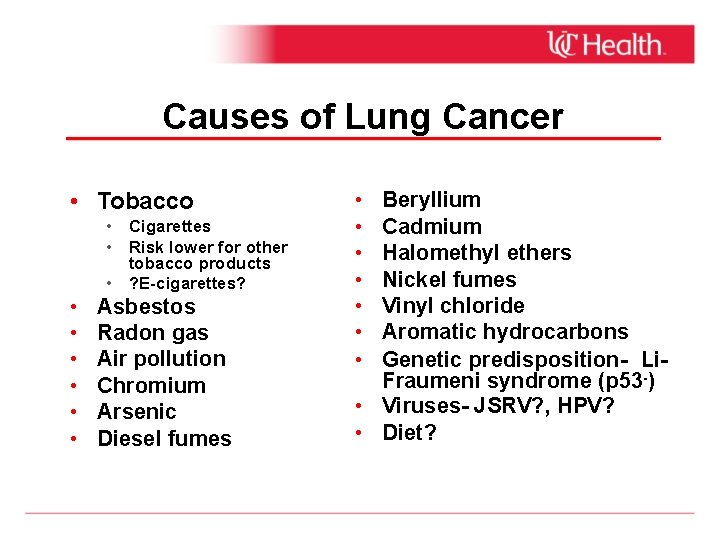
Causes of Lung Cancer • Tobacco • Cigarettes • Risk lower for other tobacco products • ? E-cigarettes? • • • Asbestos Radon gas Air pollution Chromium Arsenic Diesel fumes • • Beryllium Cadmium Halomethyl ethers Nickel fumes Vinyl chloride Aromatic hydrocarbons Genetic predisposition- Li. Fraumeni syndrome (p 53 -) • Viruses- JSRV? , HPV? • Diet?

Smoking and Lung Cancer Risk • WHO estimates 80 -90% of lung cancer is smoking-related: • • • 16 -18% of smokers will develop lung cancer. Lung cancer risk is 15 to 30 -fold higher for smokers than non-smokers. Passive smoking (2 nd hand smoke) may increase risk by 16 -24%. • More than 60 known EPA-designated carcinogens are found in cigarette smoke including: arsenic, benzene, benzo-[a]-pyrene, cadmium, chromium, complex hydrocarbons, nicotine, plutonium, polonium, radon, nitrosamines, etc. • Greatest reduction in lung cancer risk occurs if you quit smoking by age 30, but even quitting at age 50 results in a >60% risk reduction. • Patients who already suffer from advanced lung cancer that quit smoking have longer survivals.

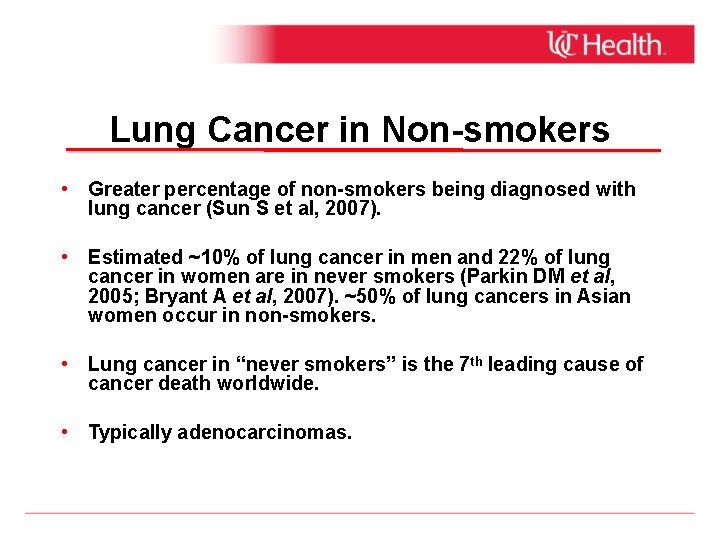
Lung Cancer in Non-smokers • Greater percentage of non-smokers being diagnosed with lung cancer (Sun S et al, 2007). • Estimated ~10% of lung cancer in men and 22% of lung cancer in women are in never smokers (Parkin DM et al, 2005; Bryant A et al, 2007). ~50% of lung cancers in Asian women occur in non-smokers. • Lung cancer in “never smokers” is the 7 th leading cause of cancer death worldwide. • Typically adenocarcinomas.

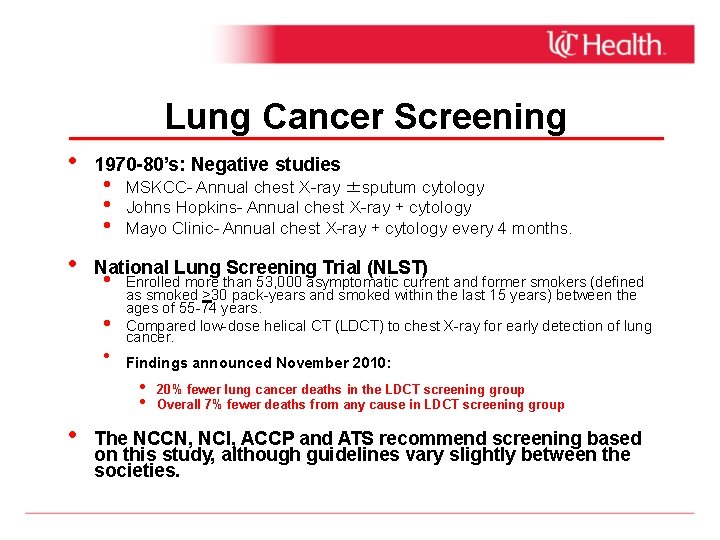
Lung Cancer Screening • • 1970 -80’s: Negative studies • • • MSKCC- Annual chest X-ray ±sputum cytology Johns Hopkins- Annual chest X-ray + cytology Mayo Clinic- Annual chest X-ray + cytology every 4 months. National Lung Screening Trial (NLST) • • • Enrolled more than 53, 000 asymptomatic current and former smokers (defined as smoked >30 pack-years and smoked within the last 15 years) between the ages of 55 -74 years. Compared low-dose helical CT (LDCT) to chest X-ray for early detection of lung cancer. Findings announced November 2010: • • • 20% fewer lung cancer deaths in the LDCT screening group Overall 7% fewer deaths from any cause in LDCT screening group The NCCN, NCI, ACCP and ATS recommend screening based on this study, although guidelines vary slightly between the societies.

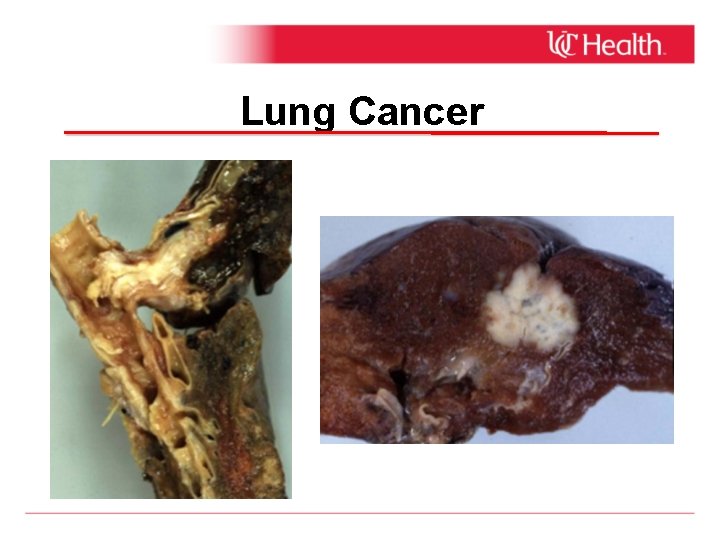
Lung Cancer

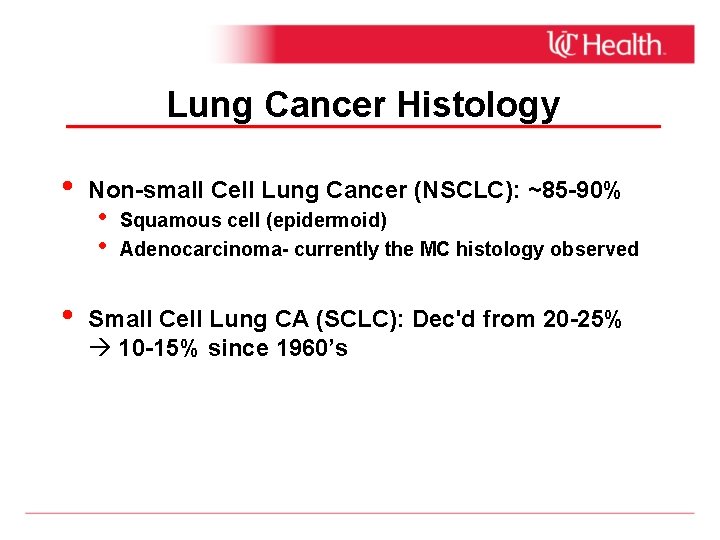
Lung Cancer Histology • • Non-small Cell Lung Cancer (NSCLC): ~85 -90% • • Squamous cell (epidermoid) Adenocarcinoma- currently the MC histology observed Small Cell Lung CA (SCLC): Dec'd from 20 -25% 10 -15% since 1960’s

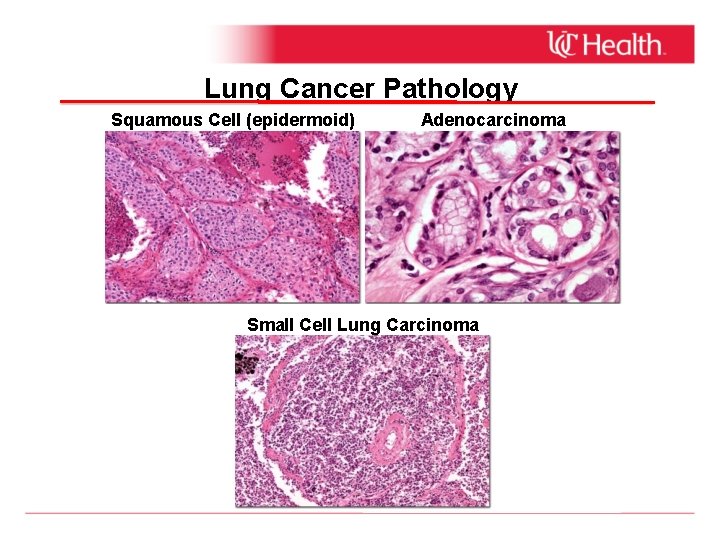
Lung Cancer Pathology Squamous Cell (epidermoid) Adenocarcinoma Small Cell Lung Carcinoma

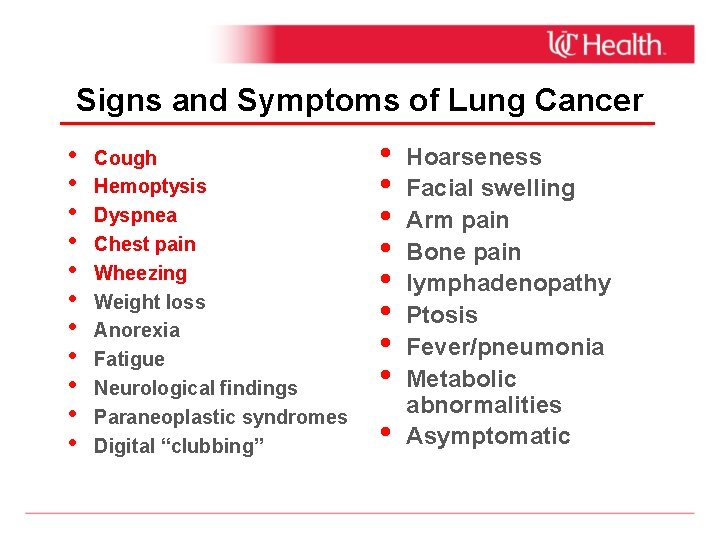
Signs and Symptoms of Lung Cancer • • • Cough Hemoptysis Dyspnea Chest pain Wheezing Weight loss Anorexia Fatigue Neurological findings Paraneoplastic syndromes Digital “clubbing” • • • Hoarseness Facial swelling Arm pain Bone pain lymphadenopathy Ptosis Fever/pneumonia Metabolic abnormalities Asymptomatic

Medical Evaluation of the Pt w/ Suspected Lung Cancer • • • H&P Serum chemistries, LDH, LFTs, CBC with differential and platelet count, routine coagulation studies. High resolution CT-scan of chest and upper abdomen with contrast to include liver and adrenal glands Biopsy Positron emission tomography (PET)/CT Brain MRI Bone scan-has been replaced by PET/CT Pulmonary function testing (PFTs) if contemplating surgery Additional lung functions studies if PFTs marginal

Lung Cancer: Tissue is the issue! • • • Sputum cytology Bronchoscopy • • Lavage Brushings Forceps biopsy Transbronchial biopsy Fine needle aspiration (FNA) biopsy Endobronchial ultrasound (EBUS) FNA • Accuracy for mediastinal LN- 84 -96. 3% Bone marrow biopsy/aspirate (SCLC)- not recommended Open biopsy or resection specimen

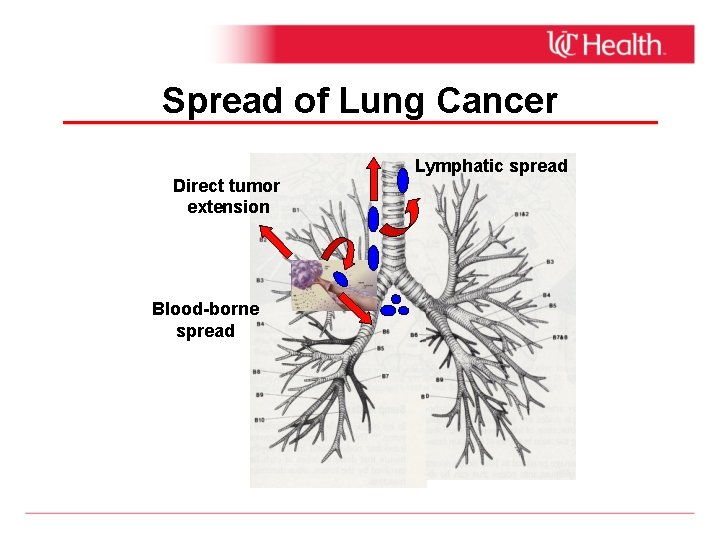
Spread of Lung Cancer Direct tumor extension Blood-borne spread Lymphatic spread

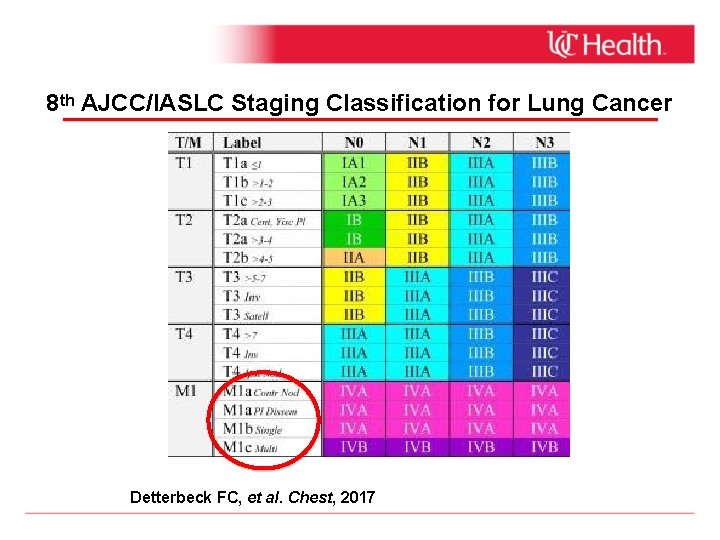
8 th AJCC/IASLC Staging Classification for Lung Cancer Detterbeck FC, et al. Chest, 2017

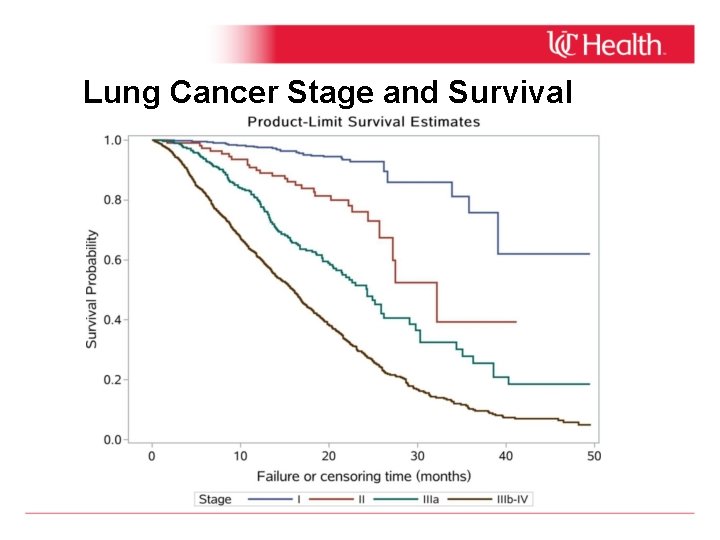
Lung Cancer Stage and Survival

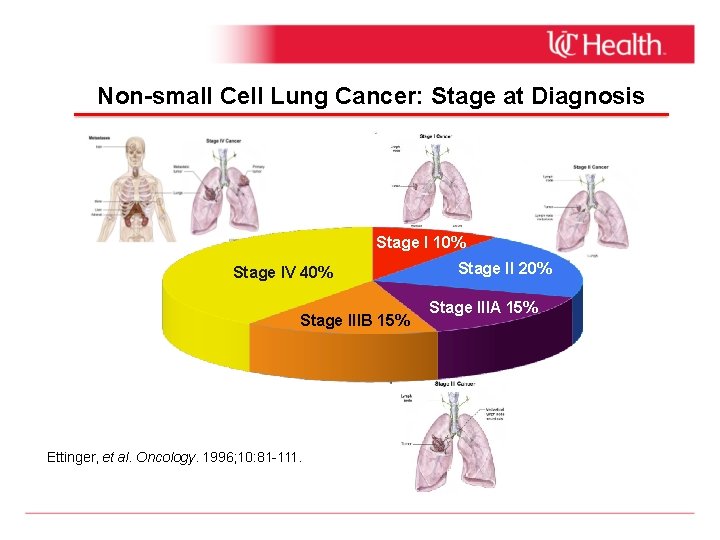
Non-small Cell Lung Cancer: Stage at Diagnosis Stage I 10% Stage IV 40% Stage IIIB 15% Ettinger, et al. Oncology. 1996; 10: 81 -111. Stage II 20% Stage IIIA 15%

Treatment of Lung Cancer • Surgery • Radiation • Chemotherapy • Antineoplastic agents • Targeted therapies • Immunotherapy

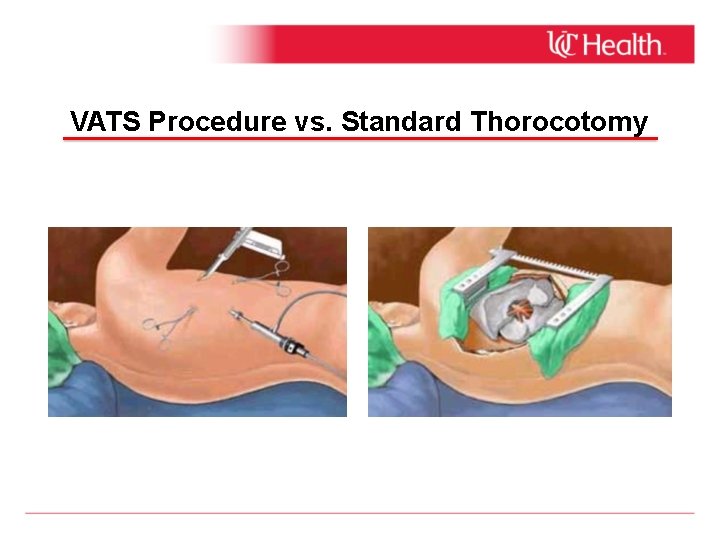
VATS Procedure vs. Standard Thorocotomy

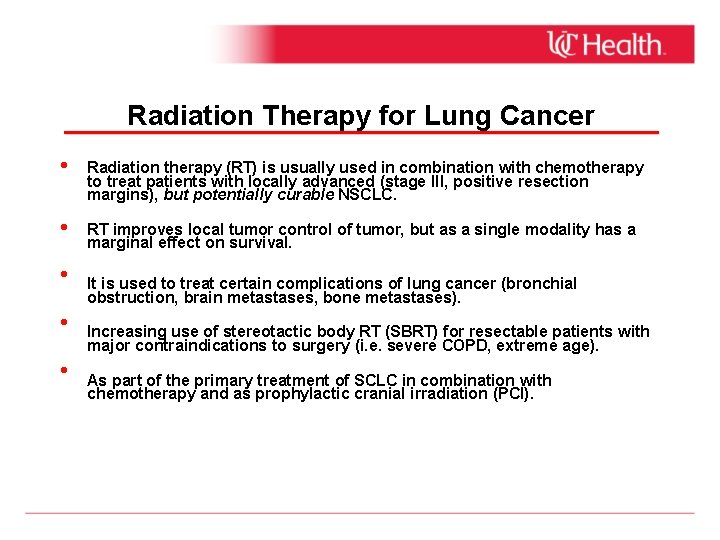
Radiation Therapy for Lung Cancer • Radiation therapy (RT) is usually used in combination with chemotherapy to treat patients with locally advanced (stage III, positive resection margins), but potentially curable NSCLC. • RT improves local tumor control of tumor, but as a single modality has a marginal effect on survival. • • • It is used to treat certain complications of lung cancer (bronchial obstruction, brain metastases, bone metastases). Increasing use of stereotactic body RT (SBRT) for resectable patients with major contraindications to surgery (i. e. severe COPD, extreme age). As part of the primary treatment of SCLC in combination with chemotherapy and as prophylactic cranial irradiation (PCI).

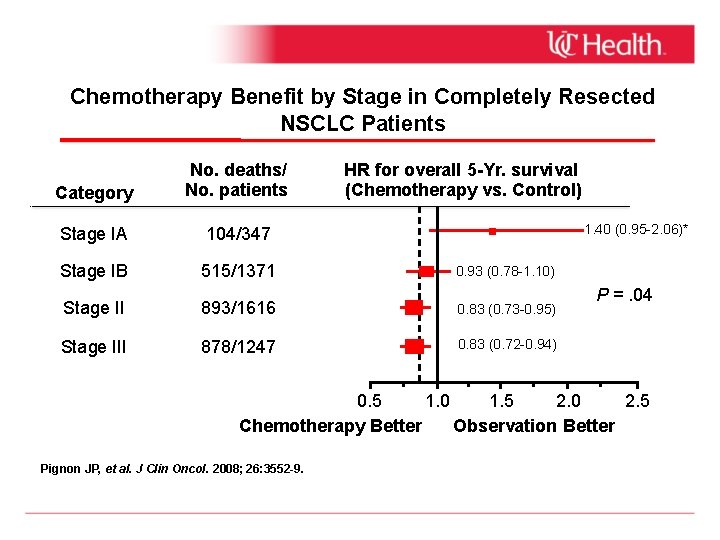
Chemotherapy Benefit by Stage in Completely Resected NSCLC Patients Category No. deaths/ No. patients HR for overall 5 -Yr. survival (Chemotherapy vs. Control) Stage IA 104/347 Stage IB 515/1371 0. 93 (0. 78 -1. 10) Stage II 893/1616 0. 83 (0. 73 -0. 95) Stage III 878/1247 0. 83 (0. 72 -0. 94) 1. 40 (0. 95 -2. 06)* P =. 04 0. 5 1. 0 1. 5 2. 0 2. 5 Chemotherapy Better Observation Better Pignon JP, et al. J Clin Oncol. 2008; 26: 3552 -9.

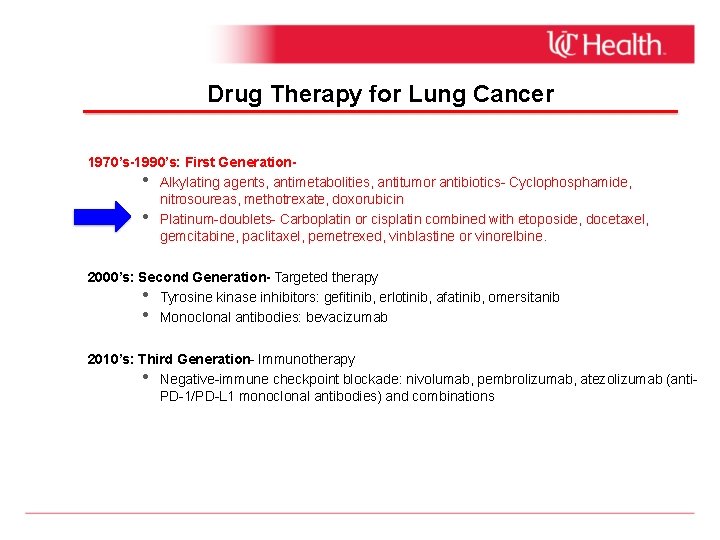
Drug Therapy for Lung Cancer 1970’s-1990’s: First Generation • Alkylating agents, antimetabolities, antitumor antibiotics- Cyclophosphamide, nitrosoureas, methotrexate, doxorubicin • Platinum-doublets- Carboplatin or cisplatin combined with etoposide, docetaxel, gemcitabine, paclitaxel, pemetrexed, vinblastine or vinorelbine. 2000’s: Second Generation- Targeted therapy • Tyrosine kinase inhibitors: gefitinib, erlotinib, afatinib, omersitanib • Monoclonal antibodies: bevacizumab 2010’s: Third Generation- Immunotherapy • Negative-immune checkpoint blockade: nivolumab, pembrolizumab, atezolizumab (anti. PD-1/PD-L 1 monoclonal antibodies) and combinations

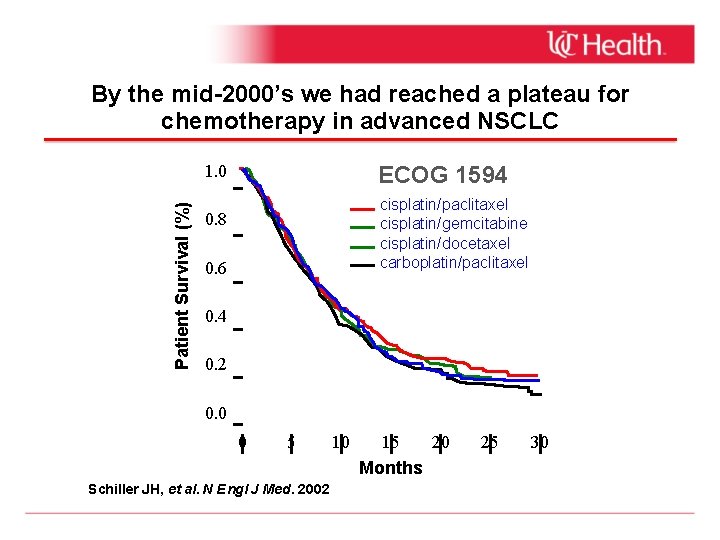
By the mid-2000’s we had reached a plateau for chemotherapy in advanced NSCLC ECOG 1594 Patient Survival (%) 1. 0 cisplatin/paclitaxel cisplatin/gemcitabine cisplatin/docetaxel carboplatin/paclitaxel 0. 8 0. 6 0. 4 0. 2 0. 0 0 5 Schiller JH, et al. N Engl J Med. 2002 10 15 20 Months 25 30

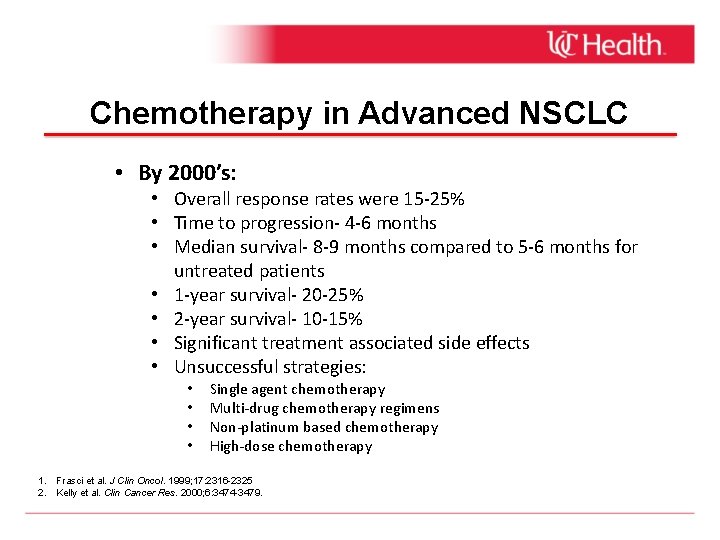
Chemotherapy in Advanced NSCLC • By 2000’s: • Overall response rates were 15 -25% • Time to progression- 4 -6 months • Median survival- 8 -9 months compared to 5 -6 months for untreated patients • 1 -year survival- 20 -25% • 2 -year survival- 10 -15% • Significant treatment associated side effects • Unsuccessful strategies: • • Single agent chemotherapy Multi-drug chemotherapy regimens Non-platinum based chemotherapy High-dose chemotherapy 1. Frasci et al. J Clin Oncol. 1999; 17: 2316 -2325 2. Kelly et al. Clin Cancer Res. 2000; 6: 3474 -3479.

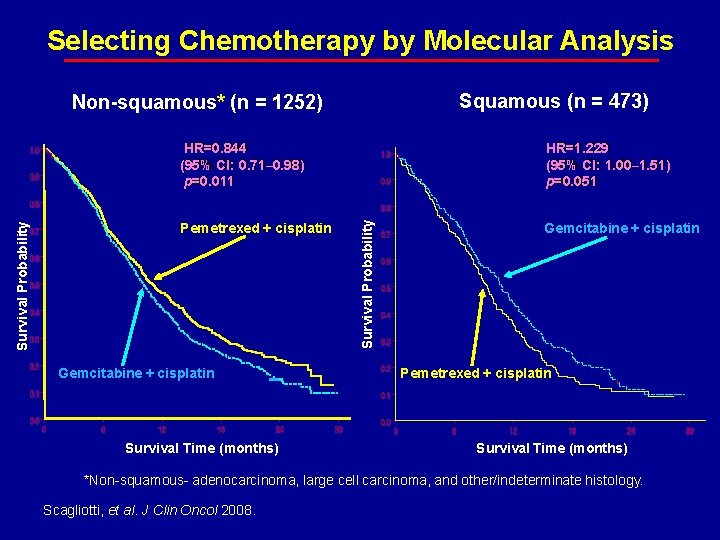
Selecting Chemotherapy by Molecular Analysis Squamous (n = 473) Non-squamous* (n = 1252) Pemetrexed + cisplatin Gemcitabine + cisplatin Survival Time (months) HR=1. 229 (95% CI: 1. 00– 1. 51) p=0. 051 Survival Probability HR=0. 844 (95% CI: 0. 71– 0. 98) p=0. 011 Gemcitabine + cisplatin Pemetrexed + cisplatin Survival Time (months) *Non-squamous- adenocarcinoma, large cell carcinoma, and other/indeterminate histology. Scagliotti, et al. J Clin Oncol 2008.

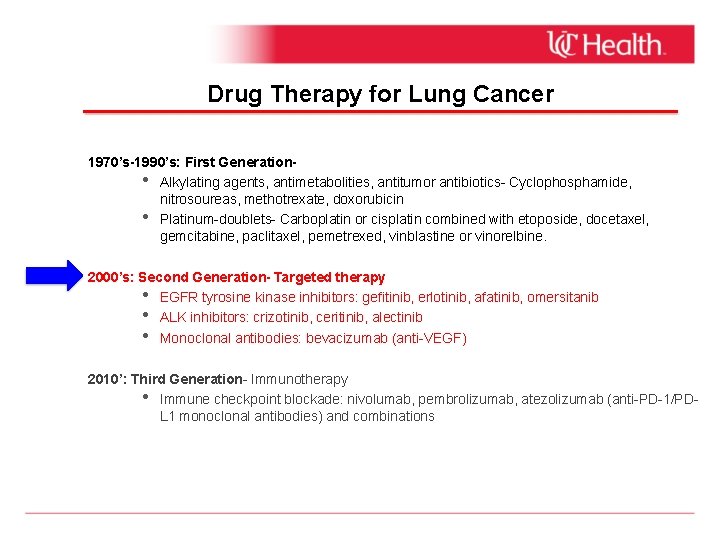
Drug Therapy for Lung Cancer 1970’s-1990’s: First Generation • Alkylating agents, antimetabolities, antitumor antibiotics- Cyclophosphamide, nitrosoureas, methotrexate, doxorubicin • Platinum-doublets- Carboplatin or cisplatin combined with etoposide, docetaxel, gemcitabine, paclitaxel, pemetrexed, vinblastine or vinorelbine. 2000’s: Second Generation- Targeted therapy • EGFR tyrosine kinase inhibitors: gefitinib, erlotinib, afatinib, omersitanib • ALK inhibitors: crizotinib, ceritinib, alectinib • Monoclonal antibodies: bevacizumab (anti-VEGF) 2010’: Third Generation- Immunotherapy • Immune checkpoint blockade: nivolumab, pembrolizumab, atezolizumab (anti-PD-1/PDL 1 monoclonal antibodies) and combinations

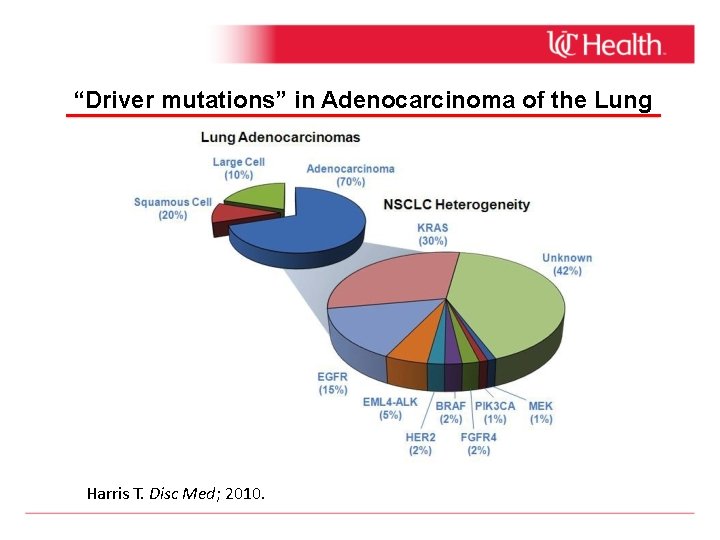
“Driver mutations” in Adenocarcinoma of the Lung Harris T. Disc Med; 2010.

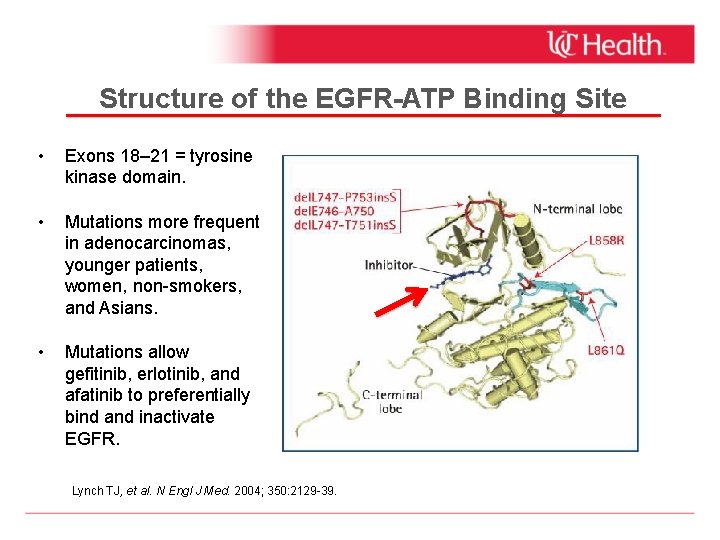
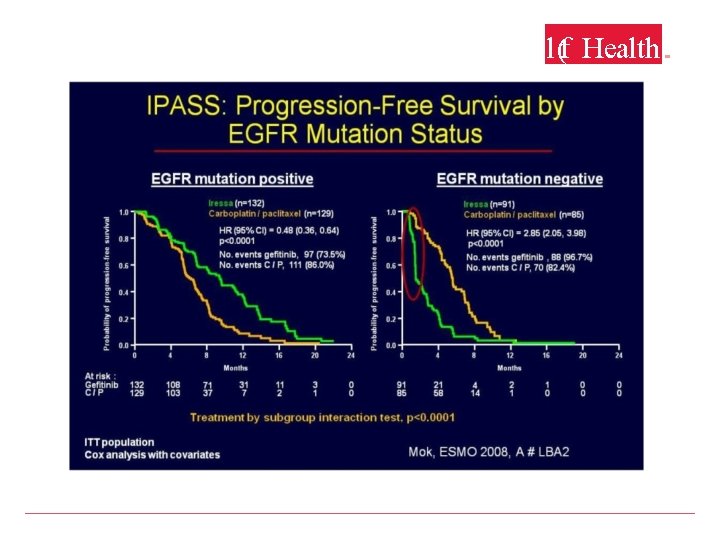
Structure of the EGFR-ATP Binding Site • Exons 18– 21 = tyrosine kinase domain. • Mutations more frequent in adenocarcinomas, younger patients, women, non-smokers, and Asians. • Mutations allow gefitinib, erlotinib, and afatinib to preferentially bind and inactivate EGFR. Lynch TJ, et al. N Engl J Med. 2004; 350: 2129 -39.

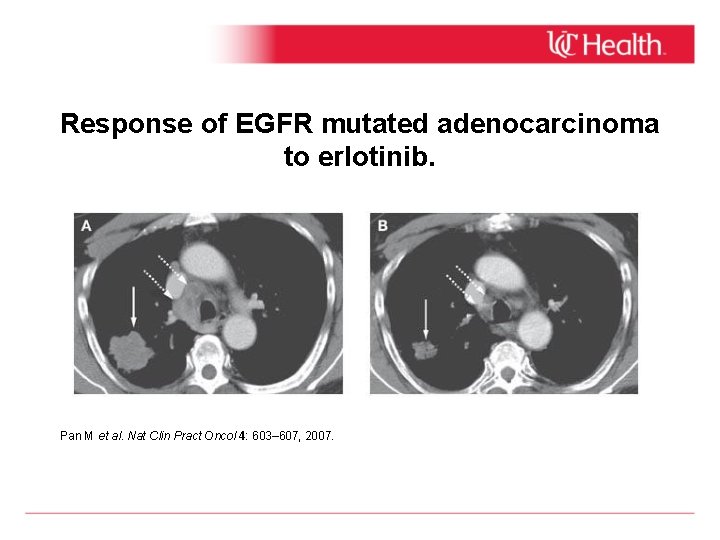
Response of EGFR mutated adenocarcinoma to erlotinib. Pan M et al. Nat Clin Pract Oncol 4: 603– 607, 2007.

l(f Health. .

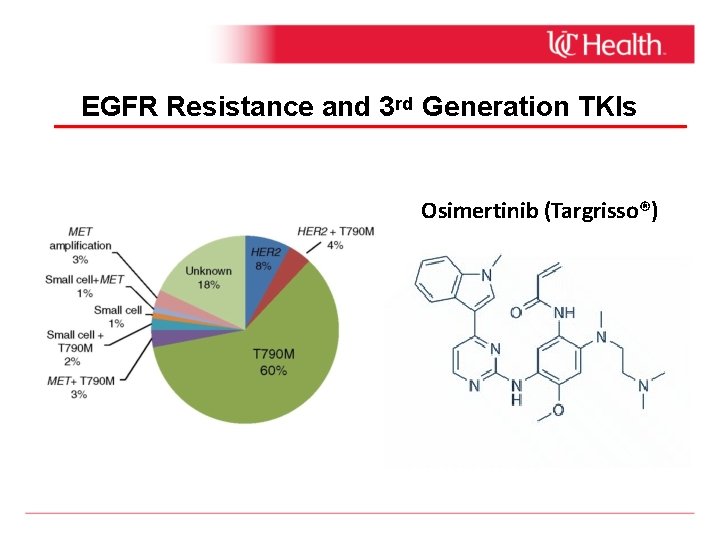
EGFR Resistance and 3 rd Generation TKIs Osimertinib (Targrisso®)

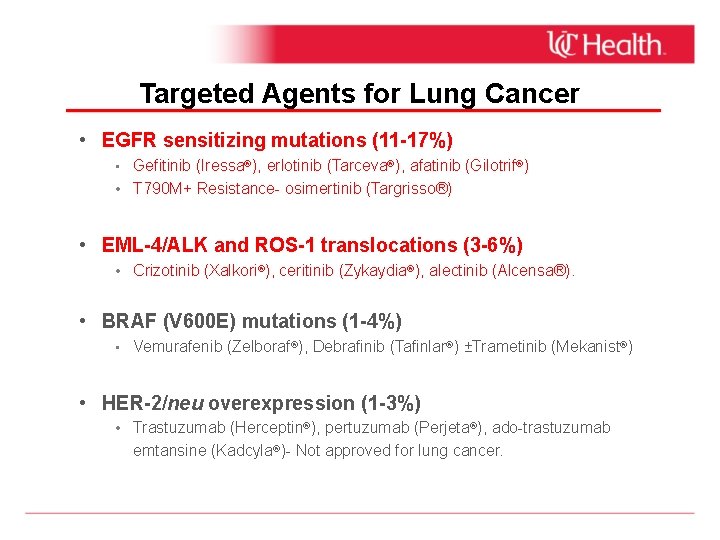
Targeted Agents for Lung Cancer • EGFR sensitizing mutations (11 -17%) • Gefitinib (Iressa®), erlotinib (Tarceva®), afatinib (Gilotrif®) • T 790 M+ Resistance- osimertinib (Targrisso®) • EML-4/ALK and ROS-1 translocations (3 -6%) • Crizotinib (Xalkori®), ceritinib (Zykaydia®), alectinib (Alcensa®). • BRAF (V 600 E) mutations (1 -4%) • Vemurafenib (Zelboraf®), Debrafinib (Tafinlar®) ±Trametinib (Mekanist®) • HER-2/neu overexpression (1 -3%) • Trastuzumab (Herceptin®), pertuzumab (Perjeta®), ado-trastuzumab emtansine (Kadcyla®)- Not approved for lung cancer.

Drug Therapy for Lung Cancer 1970’s-1990’s: First Generation • Alkylating agents, antimetabolities, antitumor antibiotics- Cyclophosphamide, nitrosoureas, methotrexate, doxorubicin • Platinum-doublets- Carboplatin or cisplatin combined with etoposide, docetaxel, gemcitabine, paclitaxel, pemetrexed, vinblastine or vinorelbine. 2000’s: Second Generation- Targeted therapy • Tyrosine kinase inhibitors: gefitinib, erlotinib, afatinib, omersitanib • Monoclonal antibodies: bevacizumab Today: Third Generation- Immunotherapy • Negative-immune checkpoint blockade: nivolumab, pembrolizumab, atezolizumab (anti. PD-1/PD-L 1 monoclonal antibodies) and chemoimmunotherapy combinations

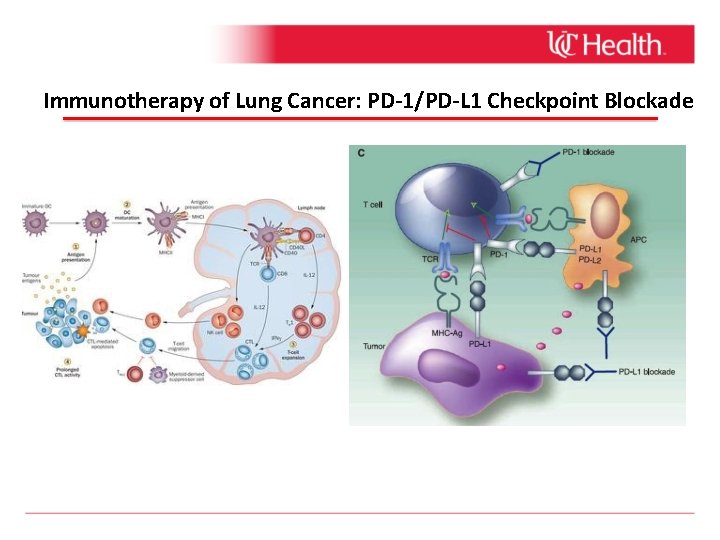
Immunotherapy of Lung Cancer: PD-1/PD-L 1 Checkpoint Blockade

Immunotherapy: Checkpoint Inhibitors in Lung Cancer • Nivolumab (Opdivo®) • Administered I. V. every 2 weeks • No testing for PD-L 1 expression on the tumor required for use in lung cancer • 2 nd-line treatment • Pembrolizumab (Keytruda®) • Administered I. V. every 3 weeks • Testing for PD-L 1 expression on the tumor required for use in lung cancer • Approved for 1 st-line treatment

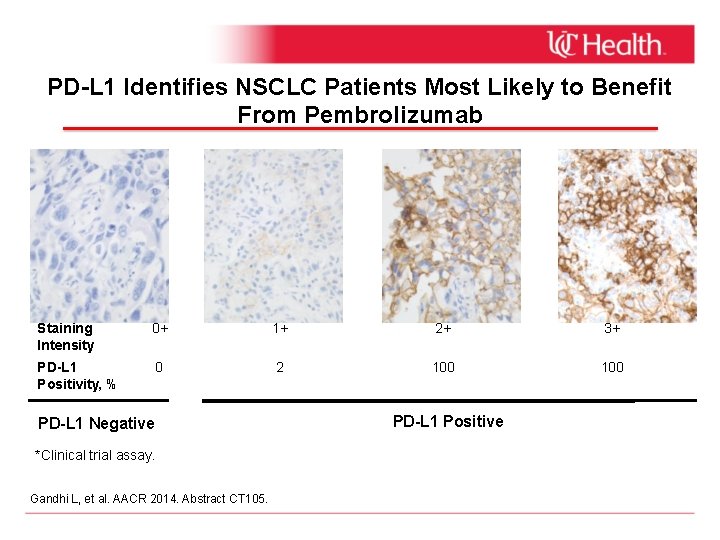
PD-L 1 Identifies NSCLC Patients Most Likely to Benefit From Pembrolizumab Staining Intensity 0+ 1+ 2+ 3+ PD-L 1 Positivity, % 0 2 100 PD-L 1 Negative *Clinical trial assay. Gandhi L, et al. AACR 2014. Abstract CT 105. PD-L 1 Positive

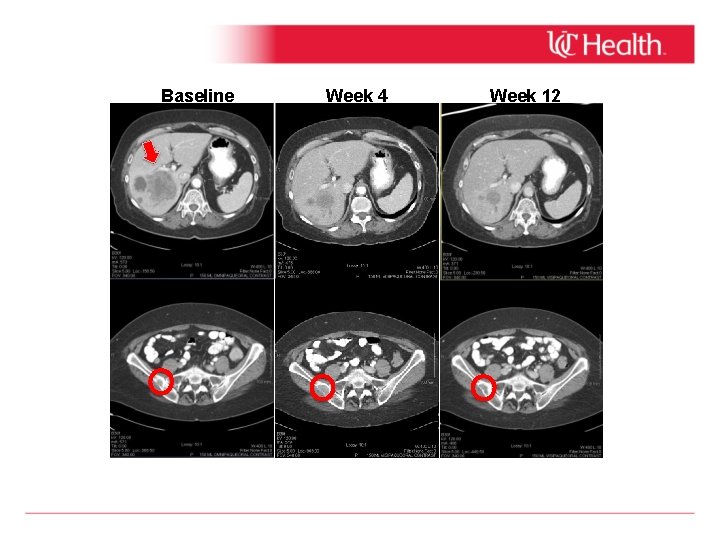
Baseline Week 4 Week 12

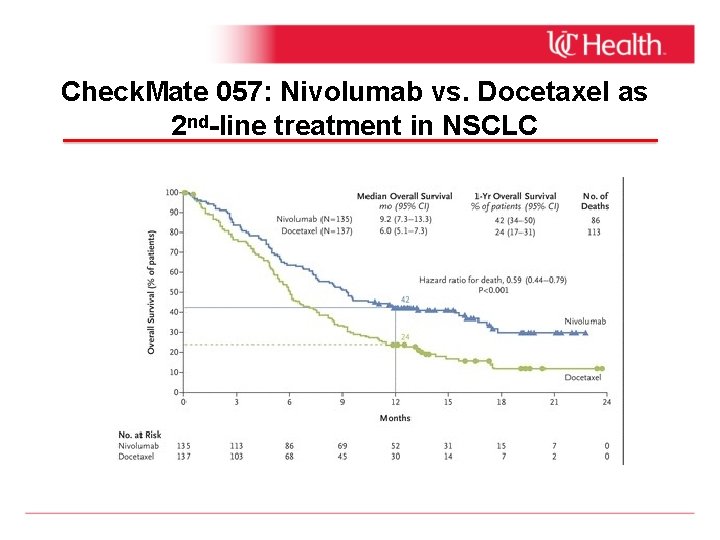
Check. Mate 057: Nivolumab vs. Docetaxel as 2 nd-line treatment in NSCLC

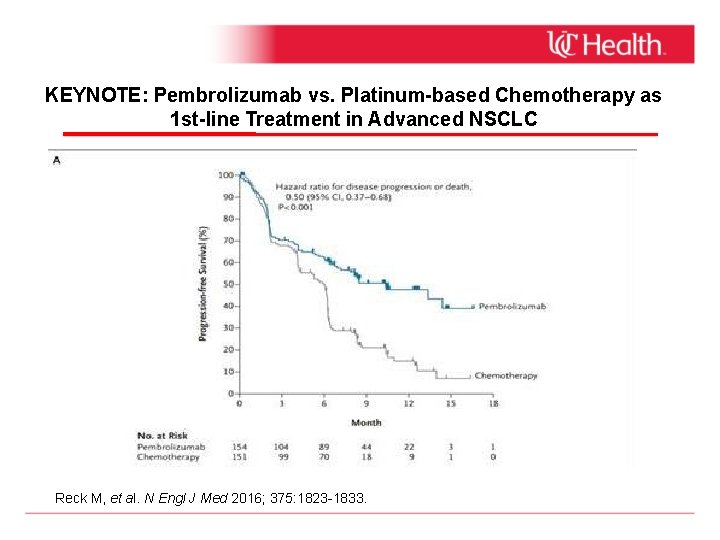
KEYNOTE: Pembrolizumab vs. Platinum-based Chemotherapy as 1 st-line Treatment in Advanced NSCLC Reck M, et al. N Engl J Med 2016; 375: 1823 -1833.

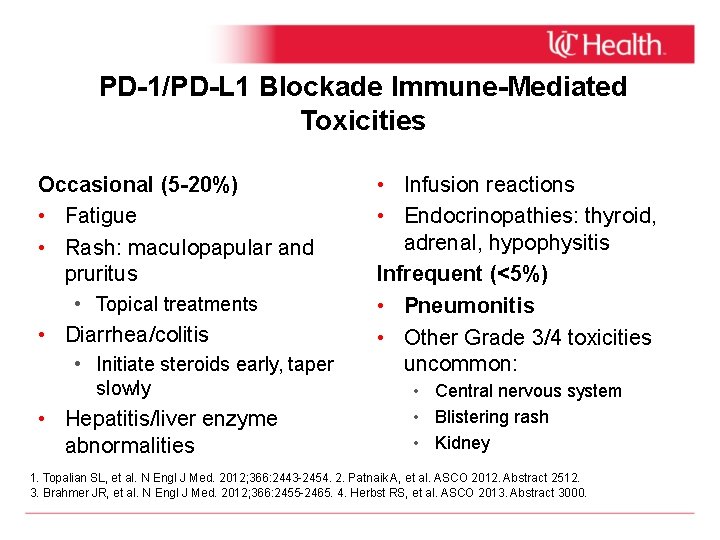
PD-1/PD-L 1 Blockade Immune-Mediated Toxicities Occasional (5 -20%) • Fatigue • Rash: maculopapular and pruritus • Topical treatments • Diarrhea/colitis • Initiate steroids early, taper slowly • Hepatitis/liver enzyme abnormalities • Infusion reactions • Endocrinopathies: thyroid, adrenal, hypophysitis Infrequent (<5%) • Pneumonitis • Other Grade 3/4 toxicities uncommon: • Central nervous system • Blistering rash • Kidney 1. Topalian SL, et al. N Engl J Med. 2012; 366: 2443 -2454. 2. Patnaik A, et al. ASCO 2012. Abstract 2512. 3. Brahmer JR, et al. N Engl J Med. 2012; 366: 2455 -2465. 4. Herbst RS, et al. ASCO 2013. Abstract 3000.

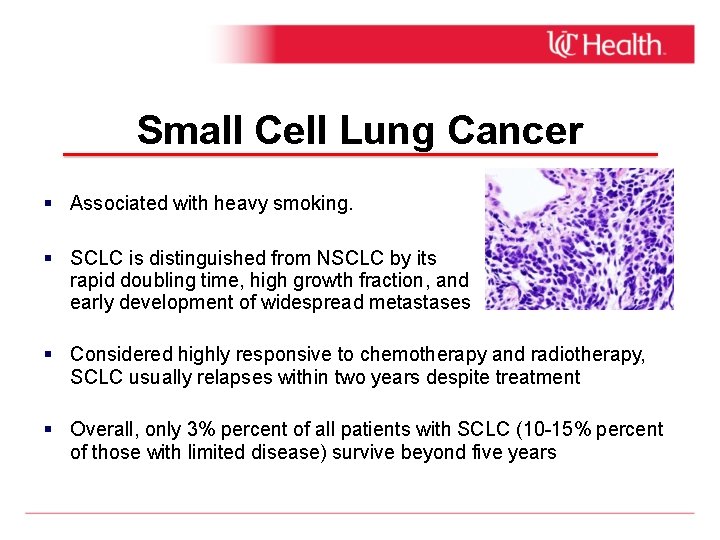
Small Cell Lung Cancer Associated with heavy smoking. SCLC is distinguished from NSCLC by its rapid doubling time, high growth fraction, and early development of widespread metastases Considered highly responsive to chemotherapy and radiotherapy, SCLC usually relapses within two years despite treatment Overall, only 3% percent of all patients with SCLC (10 -15% percent of those with limited disease) survive beyond five years

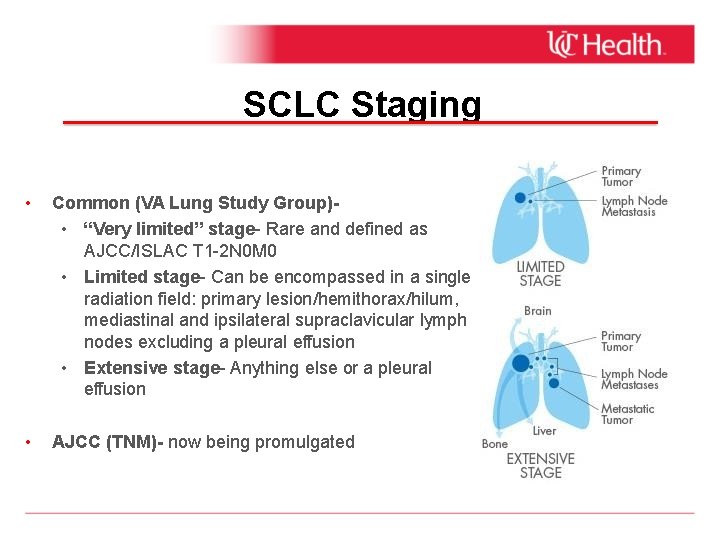
SCLC Staging • Common (VA Lung Study Group) • “Very limited” stage- Rare and defined as AJCC/ISLAC T 1 -2 N 0 M 0 • Limited stage- Can be encompassed in a single radiation field: primary lesion/hemithorax/hilum, mediastinal and ipsilateral supraclavicular lymph nodes excluding a pleural effusion • Extensive stage- Anything else or a pleural effusion • AJCC (TNM)- now being promulgated

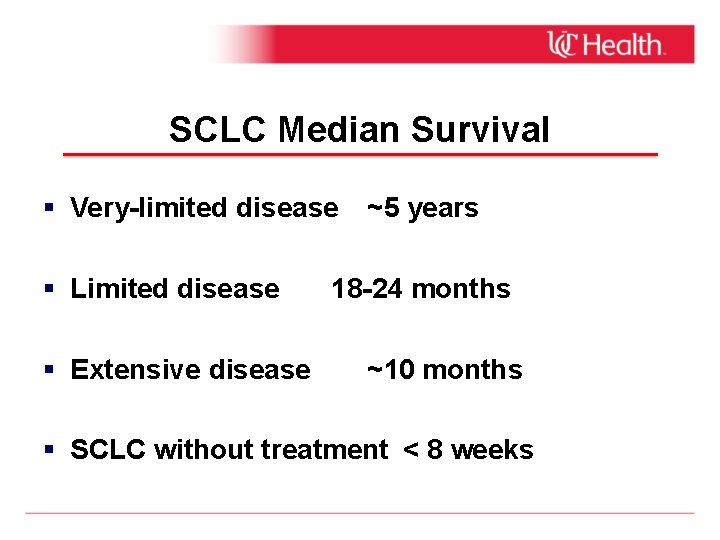
SCLC Median Survival Very-limited disease ~5 years Limited disease Extensive disease 18 -24 months ~10 months SCLC without treatment < 8 weeks

Treatment of Limited Stage SCLC In addition to chemotherapy, there is a significant role for radiation therapy (XRT) in the treatment of LS-SCLC. Local tumor progression occurs in up to 80 % of such patients treated with chemotherapy alone. High local recurrence rate can be significantly reduced by the addition of thoracic XRT. Survival is improved when thoracic XRT is added to chemotherapy compared with chemotherapy alone Prophylactic cranial irradiation (PCI)- Indicated for patients with a complete or partial response to their chemotherapy treatment.

Treatment of Limited Stage SCLC Current standard of care for patients with LS-SCLC: Four cycles of combination chemotherapy (typically cisplatin/carboplatin plus etoposide [EP]) + concurrent thoracic radiotherapy during the chemotherapy treatment. Prophylactic cranial irradiation (PCI) Generally recommended for patients with a complete response or significant tumor regression at the completion of chemotherapy.

Treatment of Extensive Stage SCLC The majority of patients with SCLC have extensive stage disease. Definition - tumor that includes distant metastases, malignant pericardial or pleural effusions, and/or contralateral supraclavicular or contralateral hilar lymph node involvement. Primary therapeutic modality is systemic chemotherapy. Usually etoposide/cisplatin or carboplatin. Good response to chemotherapy- XRT may additional benefit.

Paraneoplastic syndromes • • Lambert-Eaton: myesthenia-like syndrome Limbic encephalitis Cerebellar degeneration Hypercalcemia - very rare with SCLC, most often with SQUAMOUS CELL CARCINOMA • Cushing’s syndrome- due to ectopic ACTH secretion, NOT cortisol • SIADH • Hypertrophic pulmonary osteoarthropathy
 Tnm stage lung cancer
Tnm stage lung cancer Cerebral salt wasting
Cerebral salt wasting Central european lung cancer patient network
Central european lung cancer patient network Lung cancer location
Lung cancer location Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Nursing process sequence
Nursing process sequence Optimal lung cancer pathway
Optimal lung cancer pathway Optimal lung cancer pathway
Optimal lung cancer pathway Lifetime risk of lung cancer
Lifetime risk of lung cancer Promotion from associate professor to professor
Promotion from associate professor to professor Ocps school board meeting
Ocps school board meeting Professor john forsythe
Professor john forsythe Professor john forsythe
Professor john forsythe Professor john wood
Professor john wood Sds holland food bv
Sds holland food bv Feedback hattie effect size
Feedback hattie effect size Professor john hughes
Professor john hughes Professor john stanley
Professor john stanley Shayna morris
Shayna morris V5-7gzofadq -site:youtube.com
V5-7gzofadq -site:youtube.com Morris fuller benton
Morris fuller benton Morris yock
Morris yock Horace morris but mostly dolores
Horace morris but mostly dolores Morris bitrochanteric test
Morris bitrochanteric test Abigail morris save water
Abigail morris save water Frank morris alcatraz
Frank morris alcatraz Golden type william morris
Golden type william morris Wallpaper - hyacinth, pattern #480
Wallpaper - hyacinth, pattern #480 Morris art nouveau
Morris art nouveau Font matching
Font matching Skyline elementary tacoma
Skyline elementary tacoma Jeff morris rpi
Jeff morris rpi May morris decorative needlework
May morris decorative needlework Morris badminton racket
Morris badminton racket History of volleyball
History of volleyball James nickel morris
James nickel morris Knuth morris pratt pronunciation
Knuth morris pratt pronunciation The nature of gothic william morris
The nature of gothic william morris Computer system architecture by morris mano
Computer system architecture by morris mano Morris water maze video
Morris water maze video Robert morris document 1963
Robert morris document 1963 Tv middleware
Tv middleware Tsotsi literature essay on decency
Tsotsi literature essay on decency The reason for the seasons
The reason for the seasons Kazuo shiraga
Kazuo shiraga Esc of morris county
Esc of morris county Morris louis saraband
Morris louis saraband Signo de morris
Signo de morris