Personalized Therapy of Lung Cancer 2011 Winter Lung








- Slides: 25

Personalized Therapy of Lung Cancer 2011 Winter Lung Cancer Conference Thomas J. Lynch, Jr. , M. D. Jonathan and Richard Sackler Professor of Internal Medicine Director, Yale Cancer Center Physician-in-Chief, Smilow Cancer Hospital

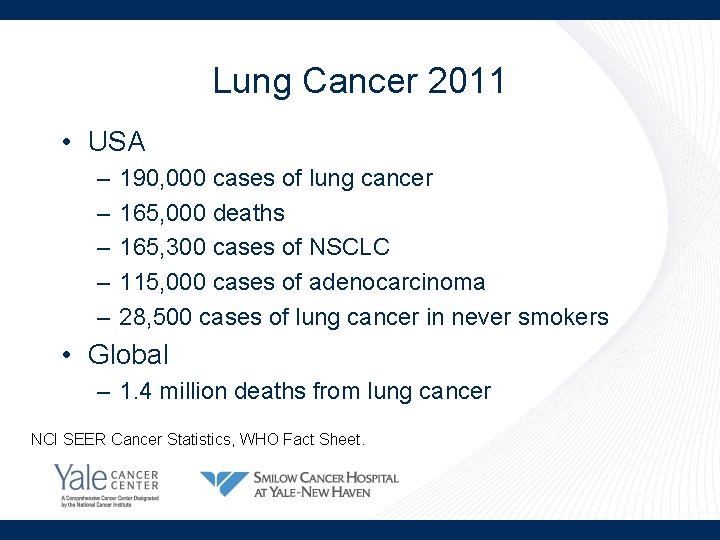
Lung Cancer 2011 • USA – – – 190, 000 cases of lung cancer 165, 000 deaths 165, 300 cases of NSCLC 115, 000 cases of adenocarcinoma 28, 500 cases of lung cancer in never smokers • Global – 1. 4 million deaths from lung cancer NCI SEER Cancer Statistics, WHO Fact Sheet.

Cancer 2011 • Cancer is a disease caused by abnormal genes that “drive” either excessive cell growth or inadequate cell destruction • Imbalance of growth and death signals leads to growth of cancer cells into tumors • Tumors then proceed to grow and metastasize • Understanding which genes drive which cancers will provide a “roadmap” to curing cancer

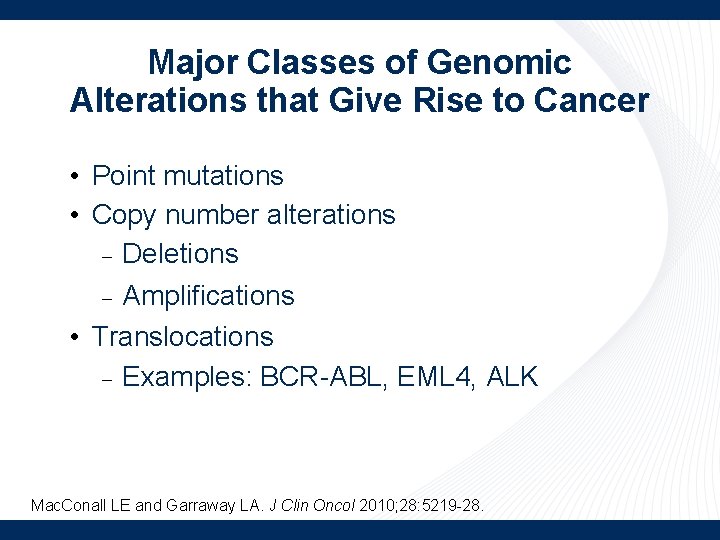
Major Classes of Genomic Alterations that Give Rise to Cancer • Point mutations • Copy number alterations Deletions Amplifications • Translocations Examples: BCR-ABL, EML 4, ALK Mac. Conall LE and Garraway LA. J Clin Oncol 2010; 28: 5219 -28.

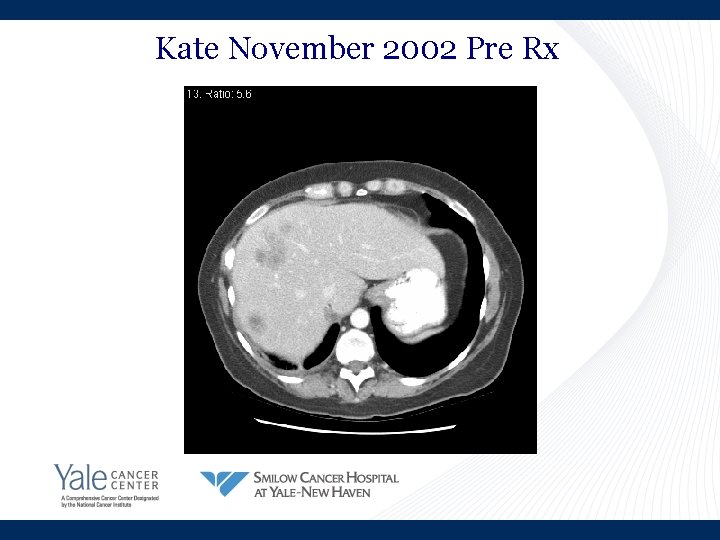
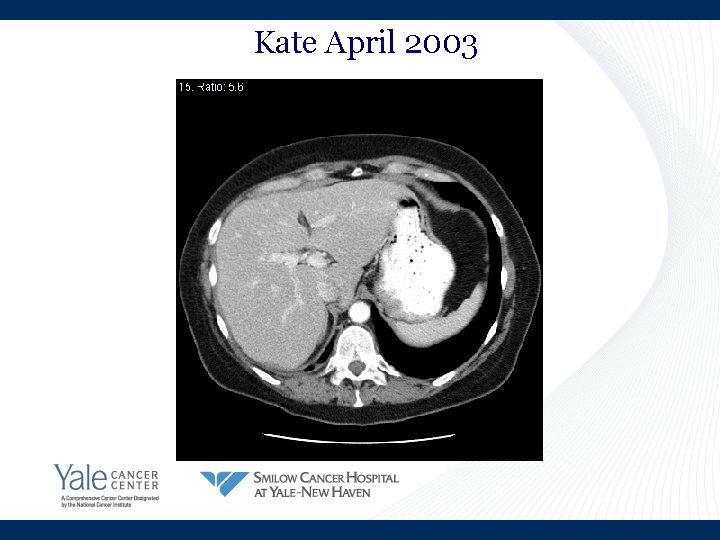
Kate’s Story • March 2002: Shortness of breath • April 2002: Lung cancer in brain, lung and lymph nodes • Summer 2002: Chemotherapy • November 2002: Progression in liver

Kate November 2002 Pre Rx

Kate April 2003

INTACT Trials: 1 -Year Overall Survival INTACT 1 • Gefitinib 500 mg + gemcitabine/cisplatin, 9. 9 months • Gefitinib 250 mg + gemcitabine/cisplatin, 9. 9 months • Placebo, 11. 1 months INTACT 2 • Gefitinib 500 mg + paclitaxel/carboplatin, 8. 7 months • Gefitinib 250 mg + paclitaxel/carboplatin, 9. 8 months • Placebo, 9. 9 months Giaccone G et al. J Clin Oncol 2004; 22: 777 -84. Herbst RS et al. J Clin Oncol 2004; 22: 785 -94.

First-Line Gefitinib in Patients with Advanced NSCLC Harboring Somatic EGFR Mutations • Mutations detected in 34/98 patients who underwent direct DNA sequencing of tumor tissue EGFR exons 18 to 21 – In-frame deletions of 11 -15 bp, 53% – L 858 R, 26% – Atypical, 21% • Objective response rate, 55% • Median progression-free survival, 9. 2 months Sequist LV et al. J Clin Oncol 2008; 26(15): 2442 -9.

Resistance § There are 2 robustly described TKI-resistance mechanisms: T 790 M in EGFR and MET amplification § 1 patient with both T 790 M and L 858 R had a best response of SD and remained on treatment for 55 days Source: Sequist LV et al. Proc ASCO 2007. Abstract 7504

T 790 M • Gatekeeper mutation present in nearly 60% of acquired resistance • Rare familial germline mutations found • Detected at diagnosis in CTCs and circulating DNA in 30% of patients using highly sensitive methods • Efforts to target T 790 M are emerging

T 790 M • Irreversible Dual (EGFR, Her-2) kinases have not yet proven effective in this setting • Novel T 790 M specific inhibitor (Nathanael Gray-DFCI) under development • Combination therapy might hold best hope

2011: Lung Adenoca. Multiple Molecular Subsets ALK fusion ROS fusion BRAF PIK 3 CAPDGFR amp MEK 1 KRAS HER 2 EGFR Squam Large Adeno Unknown Small Courtesy William Pao

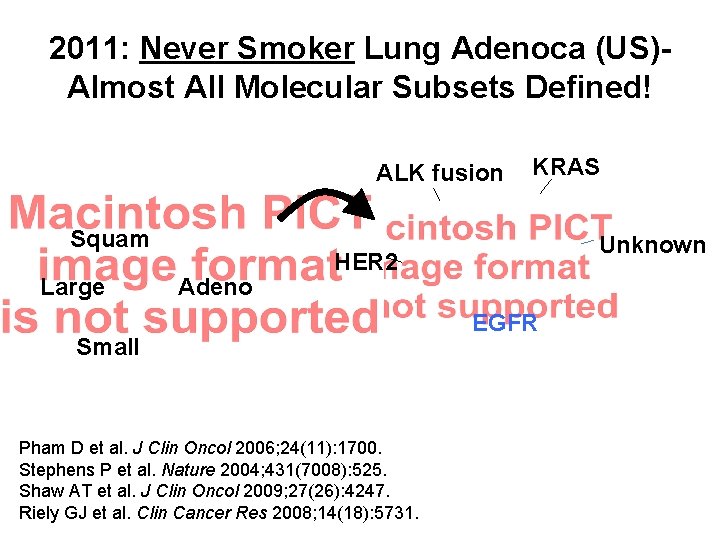
2011: Never Smoker Lung Adenoca (US)Almost All Molecular Subsets Defined! ALK fusion Squam Large Adeno KRAS Unknown HER 2 Small Pham D et al. J Clin Oncol 2006; 24(11): 1700. Stephens P et al. Nature 2004; 431(7008): 525. Shaw AT et al. J Clin Oncol 2009; 27(26): 4247. Riely GJ et al. Clin Cancer Res 2008; 14(18): 5731. EGFR

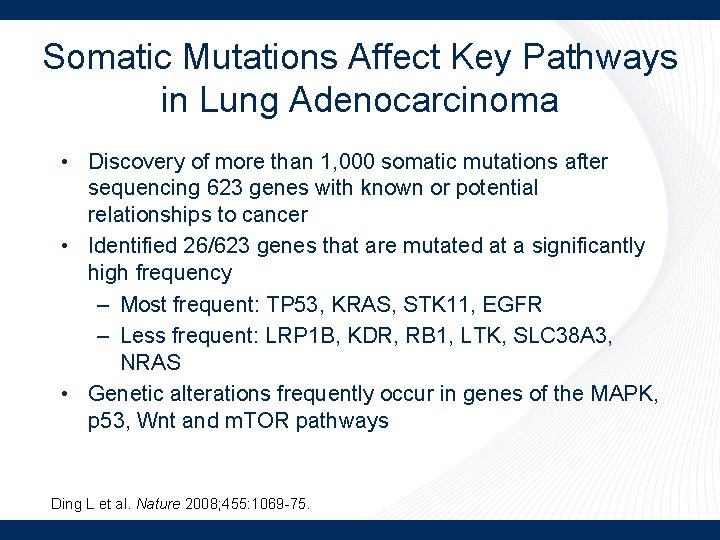
Somatic Mutations Affect Key Pathways in Lung Adenocarcinoma • Discovery of more than 1, 000 somatic mutations after sequencing 623 genes with known or potential relationships to cancer • Identified 26/623 genes that are mutated at a significantly high frequency – Most frequent: TP 53, KRAS, STK 11, EGFR – Less frequent: LRP 1 B, KDR, RB 1, LTK, SLC 38 A 3, NRAS • Genetic alterations frequently occur in genes of the MAPK, p 53, Wnt and m. TOR pathways Ding L et al. Nature 2008; 455: 1069 -75.

Mc. Dermott U et al. N Engl J Med 2011; 364: 340 -50.

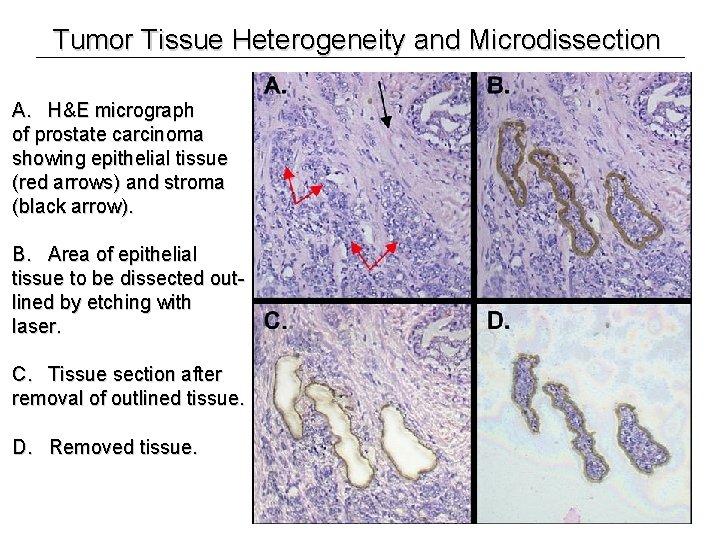
Tumor Tissue Heterogeneity and Microdissection A. H&E micrograph of prostate carcinoma showing epithelial tissue (red arrows) and stroma (black arrow). B. Area of epithelial tissue to be dissected outlined by etching with laser. C. Tissue section after removal of outlined tissue. D. Removed tissue.


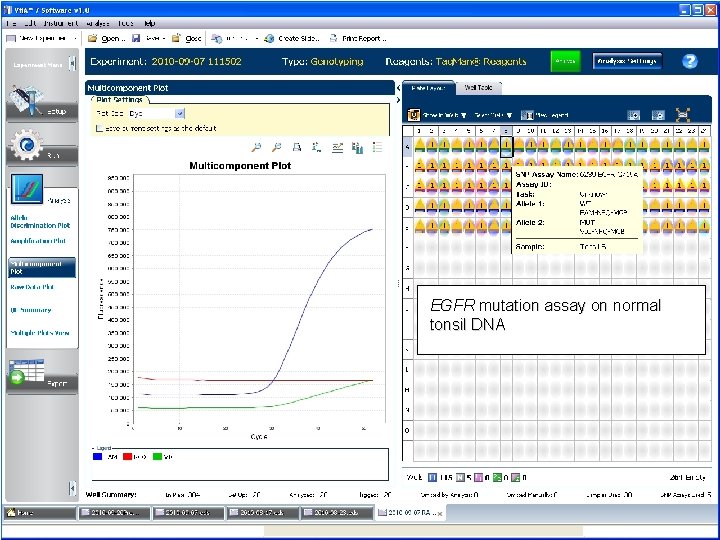
EGFR mutation assay on normal tonsil DNA

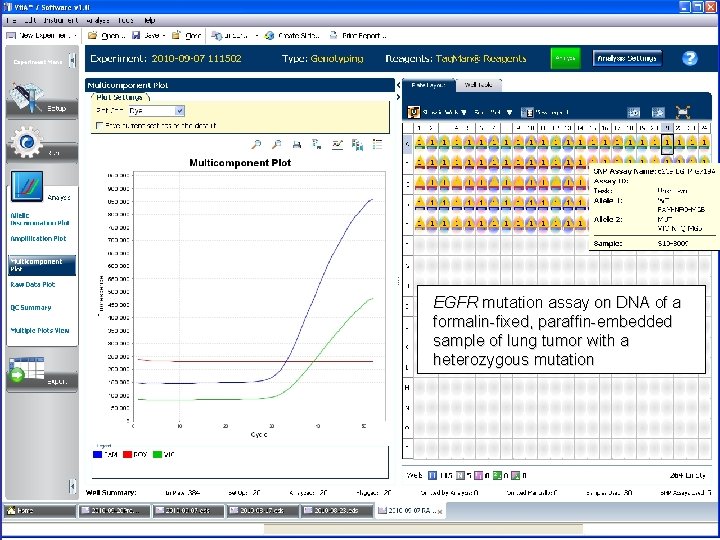
EGFR mutation assay on DNA of a formalin-fixed, paraffin-embedded sample of lung tumor with a heterozygous mutation

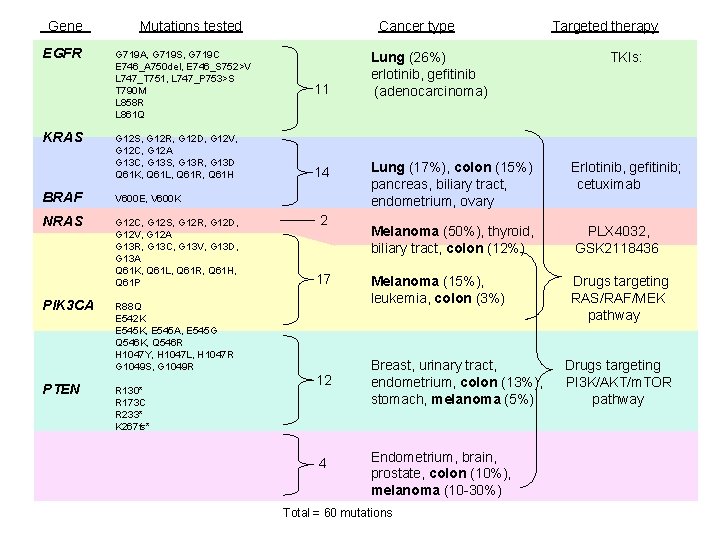
Gene EGFR KRAS Mutations tested G 719 A, G 719 S, G 719 C E 746_A 750 del, E 746_S 752>V L 747_T 751, L 747_P 753>S T 790 M L 858 R L 861 Q G 12 S, G 12 R, G 12 D, G 12 V, G 12 C, G 12 A G 13 C, G 13 S, G 13 R, G 13 D Q 61 K, Q 61 L, Q 61 R, Q 61 H BRAF V 600 E, V 600 K NRAS G 12 C, G 12 S, G 12 R, G 12 D, G 12 V, G 12 A G 13 R, G 13 C, G 13 V, G 13 D, G 13 A Q 61 K, Q 61 L, Q 61 R, Q 61 H, Q 61 P PIK 3 CA PTEN R 88 Q E 542 K E 545 K, E 545 A, E 545 G Q 546 K, Q 546 R H 1047 Y, H 1047 L, H 1047 R G 1049 S, G 1049 R R 130* R 173 C R 233* K 267 fs* Cancer type 11 14 2 17 12 4 Lung (26%) erlotinib, gefitinib (adenocarcinoma) Targeted therapy TKIs: Lung (17%), colon (15%) pancreas, biliary tract, endometrium, ovary Erlotinib, gefitinib; cetuximab Melanoma (50%), thyroid, biliary tract, colon (12%) PLX 4032, GSK 2118436 Melanoma (15%), leukemia, colon (3%) Drugs targeting RAS/RAF/MEK pathway Breast, urinary tract, endometrium, colon (13%), stomach, melanoma (5%) Endometrium, brain, prostate, colon (10%), melanoma (10 -30%) Total = 60 mutations Drugs targeting PI 3 K/AKT/m. TOR pathway

Genome Statistics • 29, 000 human genes • Average gene 3000 bases but wide variation exists • 99. 9% of our bases are exactly the same from person to person • Functions are unknown for 50% of the discovered genes

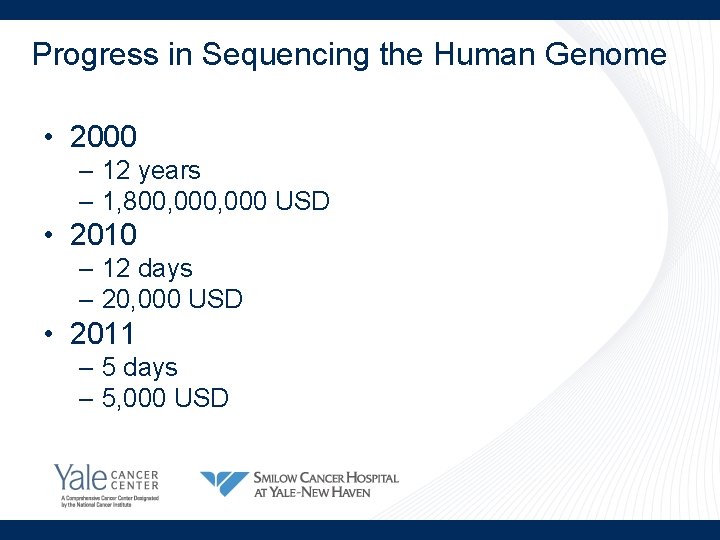
Progress in Sequencing the Human Genome • 2000 – 12 years – 1, 800, 000 USD • 2010 – 12 days – 20, 000 USD • 2011 – 5 days – 5, 000 USD

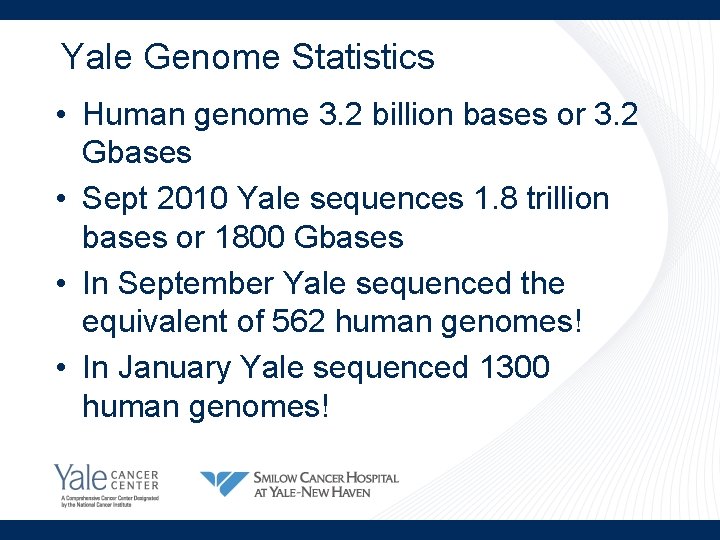
Yale Genome Statistics • Human genome 3. 2 billion bases or 3. 2 Gbases • Sept 2010 Yale sequences 1. 8 trillion bases or 1800 Gbases • In September Yale sequenced the equivalent of 562 human genomes! • In January Yale sequenced 1300 human genomes!

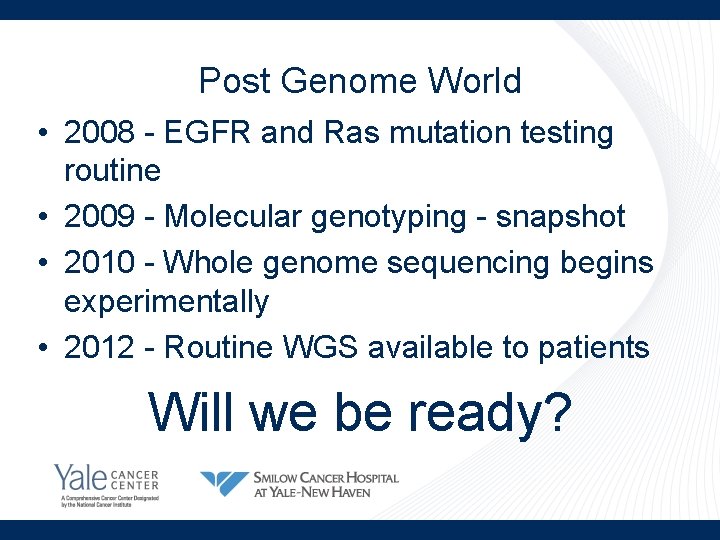
Post Genome World • 2008 - EGFR and Ras mutation testing routine • 2009 - Molecular genotyping - snapshot • 2010 - Whole genome sequencing begins experimentally • 2012 - Routine WGS available to patients Will we be ready?
 Lifetime risk of lung cancer
Lifetime risk of lung cancer Siadh vs diabetes insipidus
Siadh vs diabetes insipidus Smart goals for ineffective airway clearance
Smart goals for ineffective airway clearance Gesundheit central european lung cancer patient ne
Gesundheit central european lung cancer patient ne Optimal lung cancer pathway
Optimal lung cancer pathway Servier medical art
Servier medical art Optimal lung cancer pathway
Optimal lung cancer pathway Tnm stage lung cancer
Tnm stage lung cancer Lung cancer screening shared decision making tool
Lung cancer screening shared decision making tool Winter kommt winter kommt flocken fallen nieder
Winter kommt winter kommt flocken fallen nieder Winter kommt flocken fallen nieder
Winter kommt flocken fallen nieder Meine lieblingsjahreszeit ist der winter
Meine lieblingsjahreszeit ist der winter Proton therapy for breast cancer after mastectomy
Proton therapy for breast cancer after mastectomy Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress What are the major humanistic therapies
What are the major humanistic therapies Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy Personalized learning learner profile
Personalized learning learner profile Institute for personalized learning
Institute for personalized learning Contextual bandits for personalized recommendation
Contextual bandits for personalized recommendation Basic communication operations in parallel computing
Basic communication operations in parallel computing Personalised mobile search engine
Personalised mobile search engine Medicine
Medicine Personalized recommendations
Personalized recommendations Personalized power motive
Personalized power motive Pentaho personalized demo
Pentaho personalized demo Personalized cybersecurity
Personalized cybersecurity