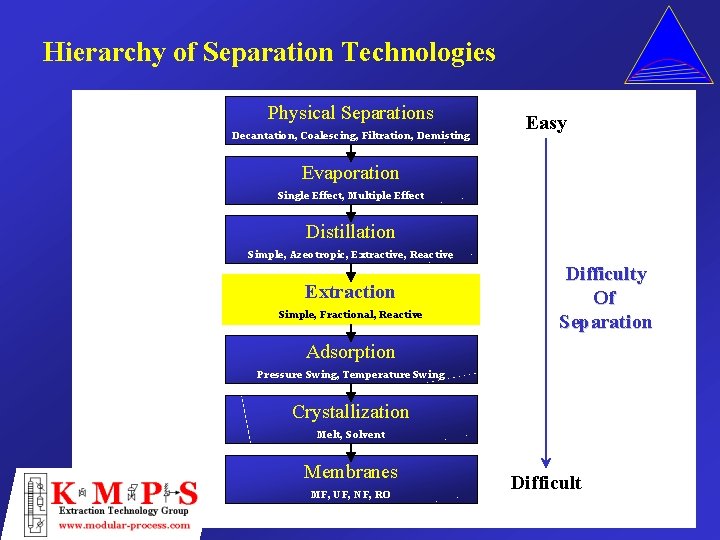
LiquidLiquid Extraction Hierarchy of Separation Technologies Physical Separations

![Removal of Organics From Water Distillation vs. Extraction BP [°C] Water Solu. [%] Azeotrope Removal of Organics From Water Distillation vs. Extraction BP [°C] Water Solu. [%] Azeotrope](https://slidetodoc.com/presentation_image_h2/2752c93b938715fed9ebf1ea30468e1e/image-5.jpg)














- Slides: 76

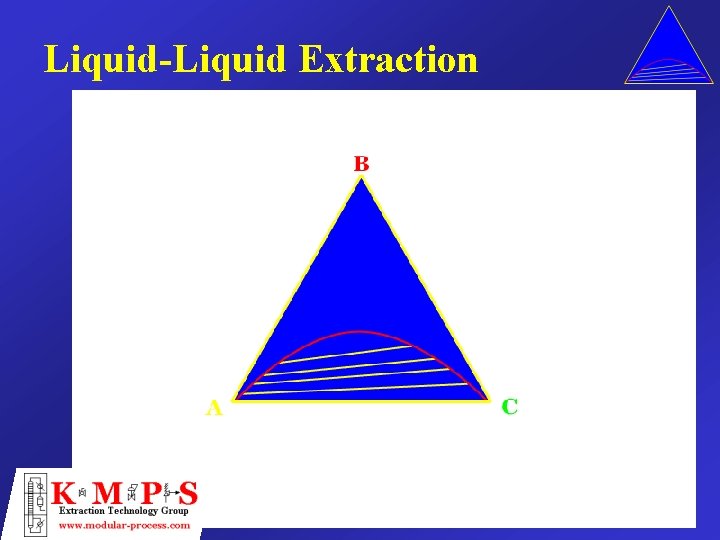
Liquid-Liquid Extraction

Hierarchy of Separation Technologies Physical Separations Decantation, Coalescing, Filtration, Demisting Easy Evaporation Single Effect, Multiple Effect Distillation Simple, Azeotropic, Extractive, Reactive Extraction Simple, Fractional, Reactive Difficulty Of Separation Adsorption Pressure Swing, Temperature Swing Crystallization Melt, Solvent Membranes MF, UF, NF, RO Difficult

Typical Applications • Remove products and pollutants from dilute aqueous streams • Wash polar compounds or acids/bases from organic streams • Heat sensitive products • Non-volatile materials • Azeotropic and close boiling mixtures • Alternative to high cost distillations

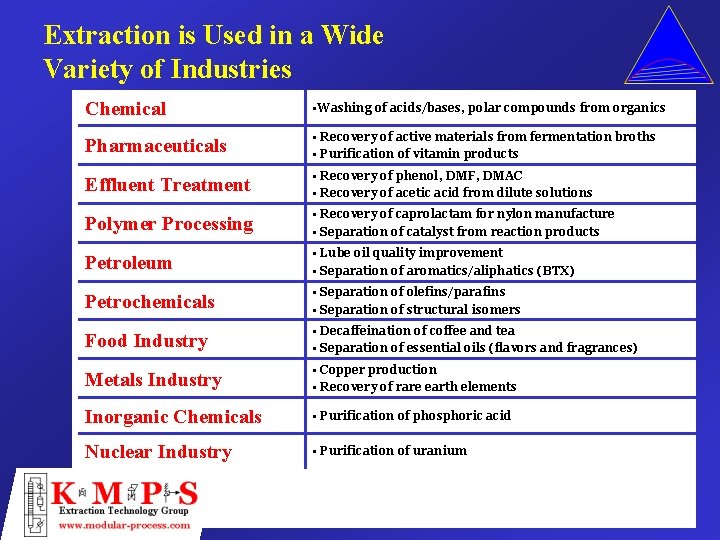
Extraction is Used in a Wide Variety of Industries Chemical • Washing of acids/bases, polar compounds from organics Pharmaceuticals • Recovery of active materials from fermentation broths • Purification of vitamin products Effluent Treatment • Recovery of phenol, DMF, DMAC • Recovery of acetic acid from dilute solutions Polymer Processing • Recovery of caprolactam for nylon manufacture • Separation of catalyst from reaction products Petroleum • Lube oil quality improvement • Separation of aromatics/aliphatics (BTX) Petrochemicals • Separation of olefins/parafins • Separation of structural isomers Food Industry • Decaffeination of coffee and tea • Separation of essential oils (flavors and fragrances) Metals Industry • Copper production • Recovery of rare earth elements Inorganic Chemicals • Purification of phosphoric acid Nuclear Industry • Purification of uranium
![Removal of Organics From Water Distillation vs Extraction BP C Water Solu Azeotrope Removal of Organics From Water Distillation vs. Extraction BP [°C] Water Solu. [%] Azeotrope](https://slidetodoc.com/presentation_image_h2/2752c93b938715fed9ebf1ea30468e1e/image-5.jpg)
Removal of Organics From Water Distillation vs. Extraction BP [°C] Water Solu. [%] Azeotrope B. P. [°C] Azeotrope Water [%] 40 2. 0 38. 1 1. 5 Acetone 56. 2 Infinite Non Azeotropic < 50 ppb Methanol 64. 5 Infinite Non Azeotropic < 50 ppb Benzene 80. 18 69. 4 8. 9 < 50 ppb Toluene 110. 8 0. 05 85. 0 20. 2 < 50 ppb -21 Infinite Formic Acid 100. 8 Infinite Acetic Acid 118. 0 Infinite Pyridine 115. 5 57 92. 6 43 < 10 ppm Aniline 181. 4 3. 60 99. 0 80. 8 < 10 ppm Phenol 181. 4 8. 20 99. 5 90. 8 < 10 ppm Nitrobenzene 210. 9 0. 04 98. 6 88. 0 < 10 ppm Dinitrotoluene (2, 4) 300. 03 99 – 100 > 90 < 10 ppm Dimethyl Formamide 153. 0 Infinite Non Azeotropic < 10 ppm Dimethyl Acetamide 166. 1 Infinite Non Azeotropic < 10 ppm n-Methylpyrrolidone 202. 0 Infinite Non Azeotropic < 10 ppm Distillation Organic Compound Methylene Chloride Extraction Formaldehyde Non Azeotropic 107. 1 22. 5 Non Azeotropic Typical Reduction Level < 50 ppb < 1, 000 ppm < 500 ppm

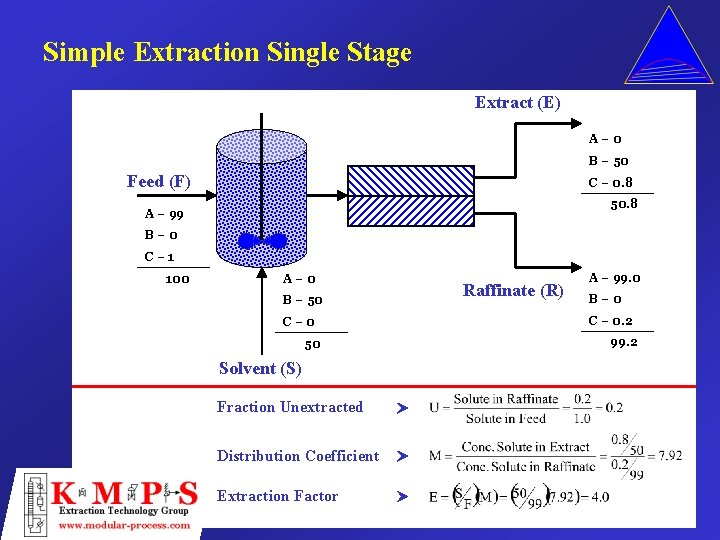
Simple Extraction Single Stage Extract (E) A– 0 B – 50 Feed (F) C – 0. 8 50. 8 A – 99 B– 0 C– 1 100 A– 0 B – 50 C– 0 50 Solvent (S) Fraction Unextracted Distribution Coefficient Extraction Factor Raffinate (R) A – 99. 0 B– 0 C – 0. 2 99. 2

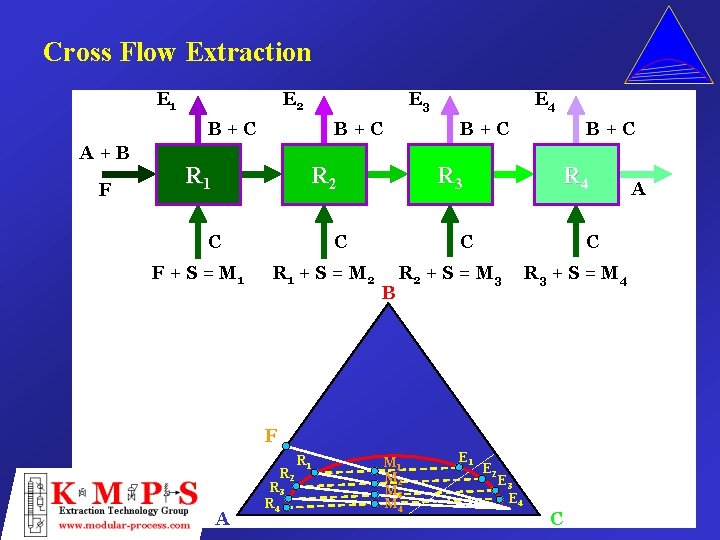
Cross Flow Extraction E 1 E 2 B+C A+B F E 3 B+C R 1 B+C R 2 C F + S = M 1 B+C R 3 C R 1 + S = M 2 E 4 R 4 C B C R 2 + S = M 3 R 3 + S = M 4 F R 2 A R 3 R 4 R 1 M 2 M 3 M 4 E 1 E 2 E 3 E 4 C A

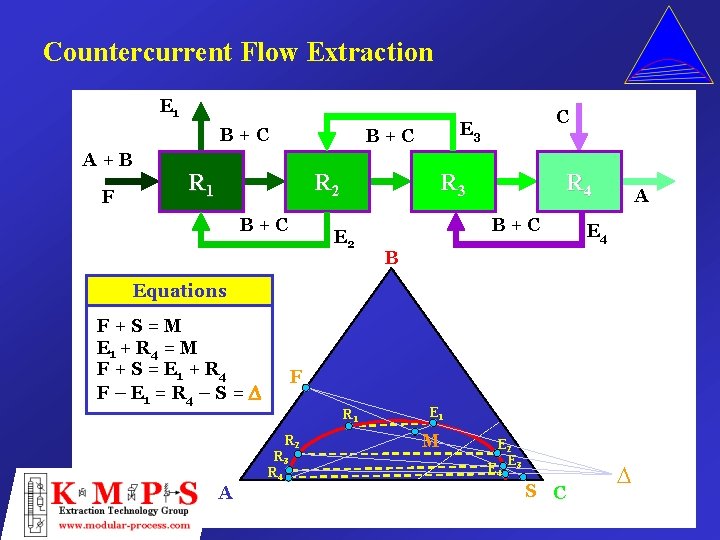
Countercurrent Flow Extraction E 1 B+C A+B F B+C R 1 R 2 B+C C E 3 R 3 E 2 R 4 B+C A E 4 B Equations F+S=M E 1 + R 4 = M F + S = E 1 + R 4 F – E 1 = R 4 – S = D F R 1 R 2 R 3 R 4 A E 1 M E 2 E 3 E 4 S C D

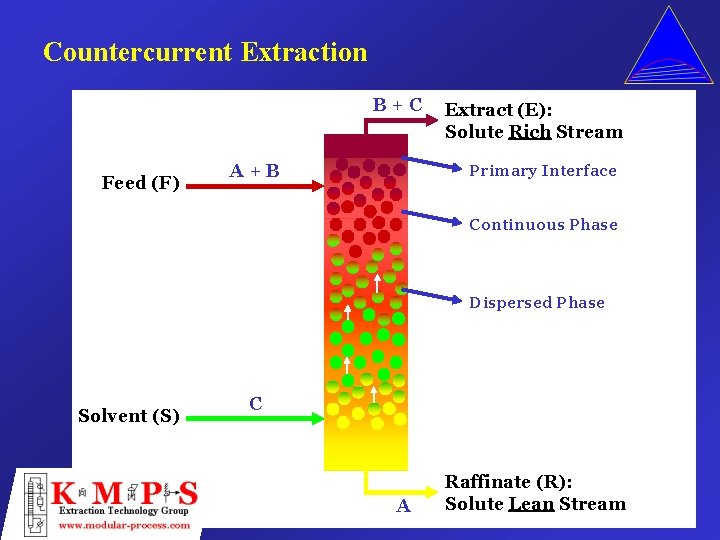
Countercurrent Extraction B+C Feed (F) A+B Extract (E): Solute Rich Stream Primary Interface Continuous Phase Dispersed Phase Solvent (S) C A Raffinate (R): Solute Lean Stream

Bench Scale Test Apparatus Variable Speed Drive Baffle Thermometer Tempered Water Out 1 – Liter Flask Tempered Water In Drain

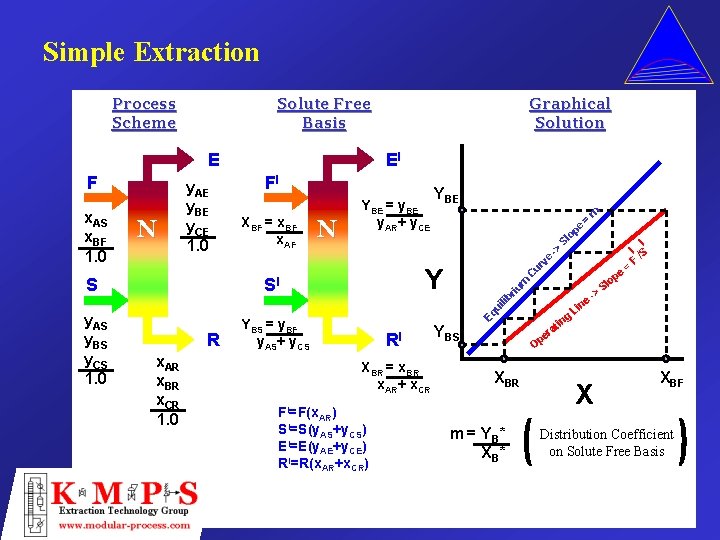
Simple Extraction Process Scheme m = Sl -> ve Y SI ur N YBE e op y. AS y. BS y. CS 1. 0 R x. AR x. BR x. CR 1. 0 Eq ui lib S XBF = x. BF x. AF YBE = y. BE y. AR+ y. CE C N FI riu m x. AS x. BF 1. 0 y. AE y. BE y. CE 1. 0 EI op e E F Graphical Solution Solute Free Basis YBS = y. BF y. AS+ y. CS RI XBR = x. BR x. AR+ x. CR AR) I S =S(y. AS+y. CS) EI=E(y. AE+y. CE) RI=R(x. AR+x. CR) YBS g tin ne Li -> = I I /S F Sl ra pe O XBR FI=F(x m = Y B* X XBF Distribution Coefficient on Solute Free Basis

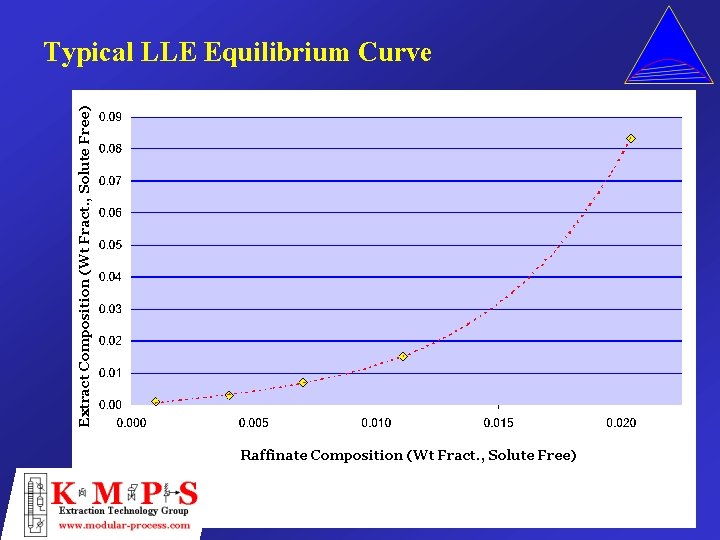
Extract Composition (Wt Fract. , Solute Free) Typical LLE Equilibrium Curve Raffinate Composition (Wt Fract. , Solute Free)

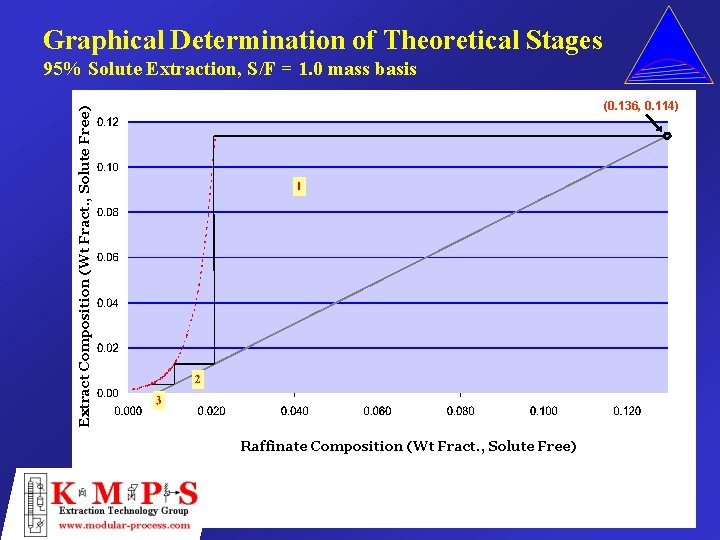
Graphical Determination of Theoretical Stages 95% Solute Extraction, S/F = 1. 0 mass basis Extract Composition (Wt Fract. , Solute Free) (0. 136, 0. 114) Raffinate Composition (Wt Fract. , Solute Free)

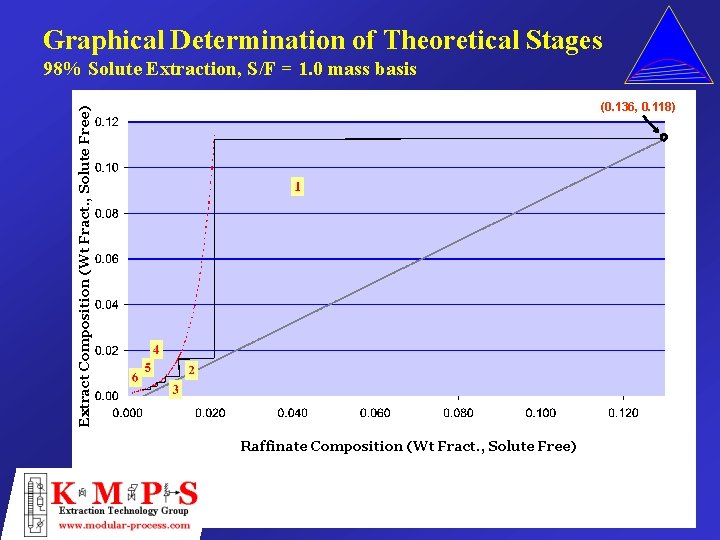
Graphical Determination of Theoretical Stages 98% Solute Extraction, S/F = 1. 0 mass basis Extract Composition (Wt Fract. , Solute Free) (0. 136, 0. 118) Raffinate Composition (Wt Fract. , Solute Free)

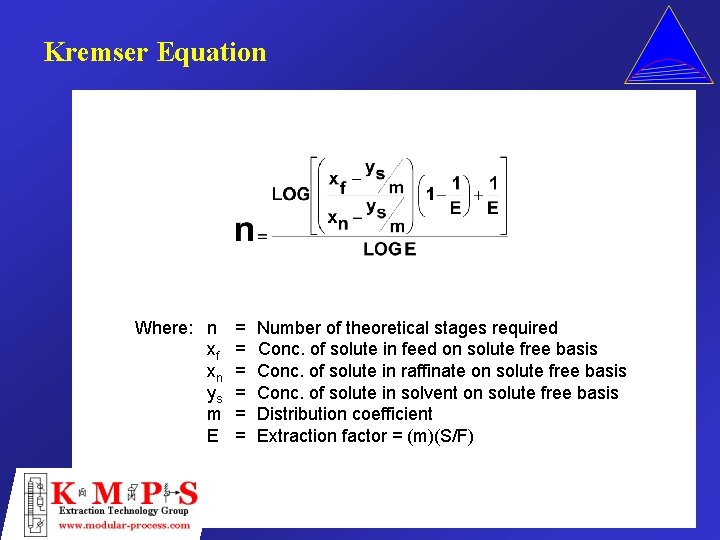
Kremser Equation Where: n xf xn ys m E = = = Number of theoretical stages required Conc. of solute in feed on solute free basis Conc. of solute in raffinate on solute free basis Conc. of solute in solvent on solute free basis Distribution coefficient Extraction factor = (m)(S/F)

Engineering Calculations Kremser Type Plot YBE E 1 1. 0 0. 8 0. 6 F 1 E = 0. 3 XBF 0. 04 0. 03 0. 02 3 1. 0. 01 0. 008 0. 006 0. 004 0. 003 0. 002 20 E = Extraction Factor E = m (S 1/F 1) 0. 1 0. 08 0. 06 E= XBR R 1 0. 2 = YBS 0. 3 E S 1 XBR/XBF = Fraction Unextracted 0. 4 0. 001 0. 0008 0. 0006 0. 0005 1 2 3 4 5 6 7 8 10 15 20 Number of Ideal Stages

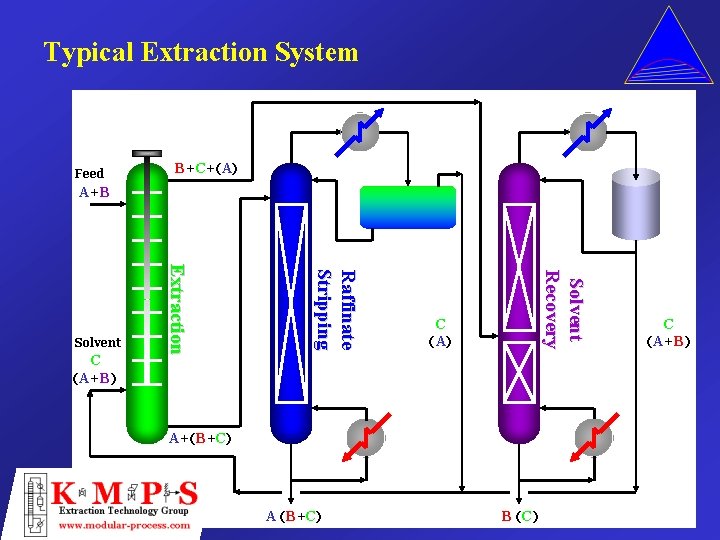
Typical Extraction System Feed B+C+(A) A+B Solvent Recovery Raffinate S tr i p p i n g C (A+B) Extraction Solvent C (A) A+(B+C) A (B+C) B (C) C (A+B)

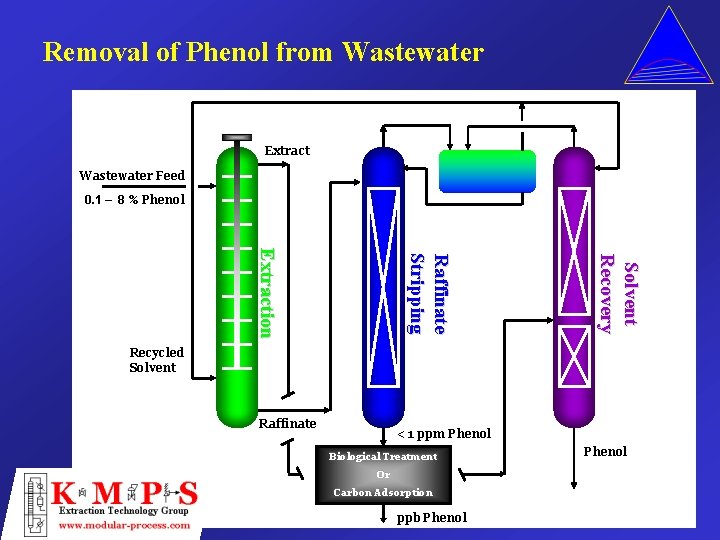
Removal of Phenol from Wastewater Extract Wastewater Feed 0. 1 – 8 % Phenol Solvent Recovery Raffinate S tr i p p i n g Extraction Recycled Solvent Raffinate < 1 ppm Phenol Biological Treatment Or Carbon Adsorption ppb Phenol

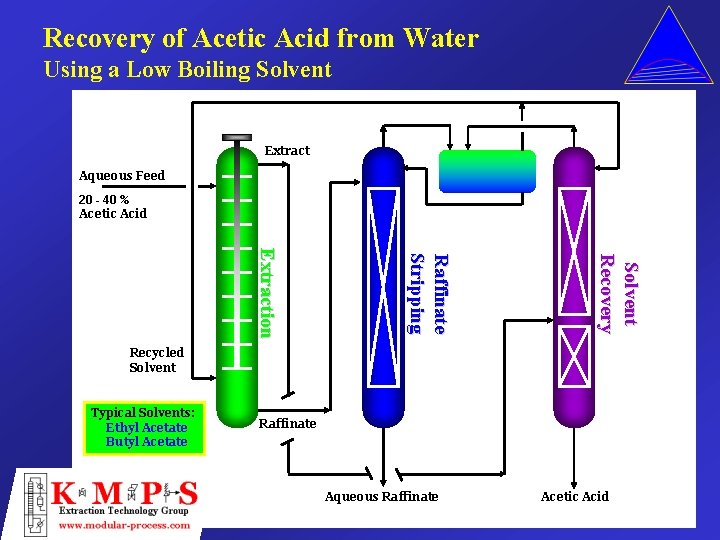
Recovery of Acetic Acid from Water Using a Low Boiling Solvent Extract Aqueous Feed 20 - 40 % Acetic Acid Solvent Recovery Raffinate S tr i p p i n g Extraction Recycled Solvent Typical Solvents: Ethyl Acetate Butyl Acetate Raffinate Aqueous Raffinate Acetic Acid

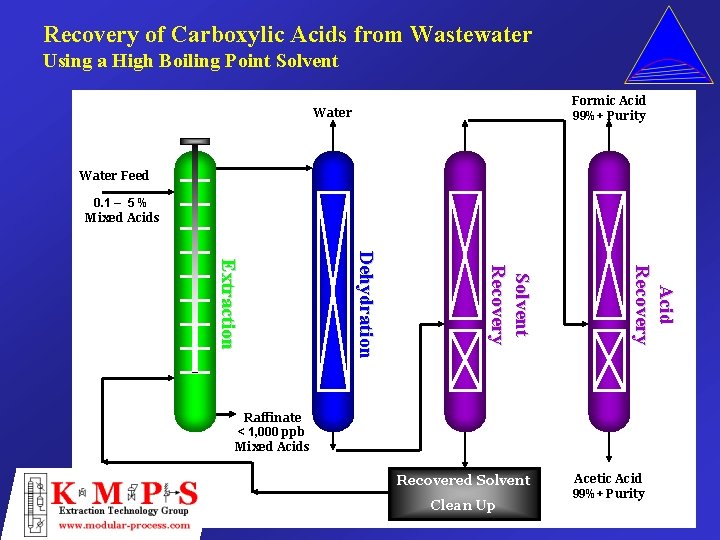
Recovery of Carboxylic Acids from Wastewater Using a High Boiling Point Solvent Formic Acid 99%+ Purity Water Feed 0. 1 – 5 % Mixed Acids Ac i d Recovery Solvent Recovery Dehydration Extraction Raffinate < 1, 000 ppb Mixed Acids Recovered Solvent Clean Up Acetic Acid 99%+ Purity

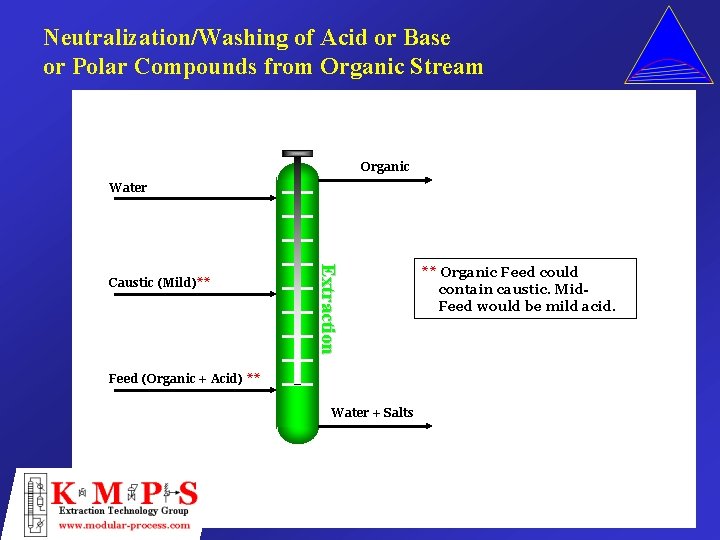
Neutralization/Washing of Acid or Base or Polar Compounds from Organic Stream Organic Water Extraction Caustic (Mild)** Feed (Organic + Acid) ** Water + Salts ** Organic Feed could contain caustic. Mid. Feed would be mild acid.

Series Extraction Feed Extract Solvent 1 B+C C Solvent 2 A+B D Extractor 1 & 2 May Differ By: - Temperature - p. H - Solvent Extractor #2 Extractor #1 Raffinate A Product B+D

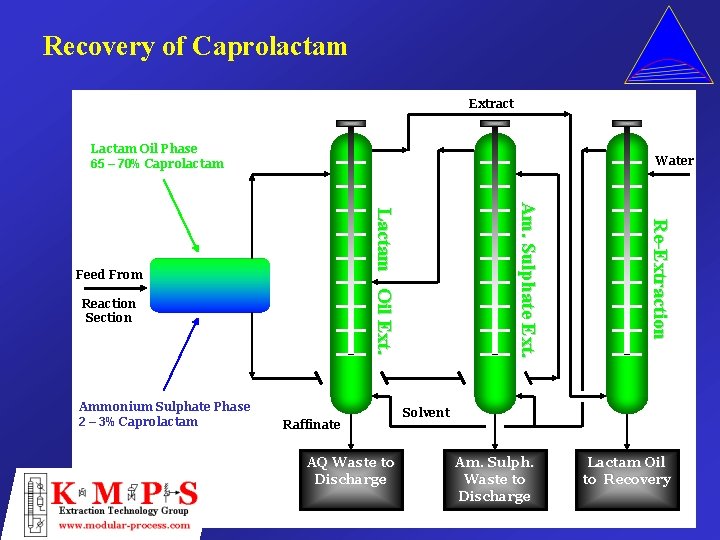
Recovery of Caprolactam Extract Lactam Oil Phase 65 – 70% Caprolactam Water Raffinate AQ Waste to Discharge Re-Extraction Reaction Section Ammonium Sulphate Phase 2 – 3% Caprolactam Am. Sulphate Ext. Lactam Oil Ext. Feed From Solvent Am. Sulph. Waste to Discharge Lactam Oil to Recovery

Phosphoric Acid Purification via Extraction Recycle Re-Extraction Scrub Extraction Feed Extraction HCL Phosphate Rock Digester Water Scrub Solv. Raffinate to Disposal Solvent Phosphoric Acid to Recovery

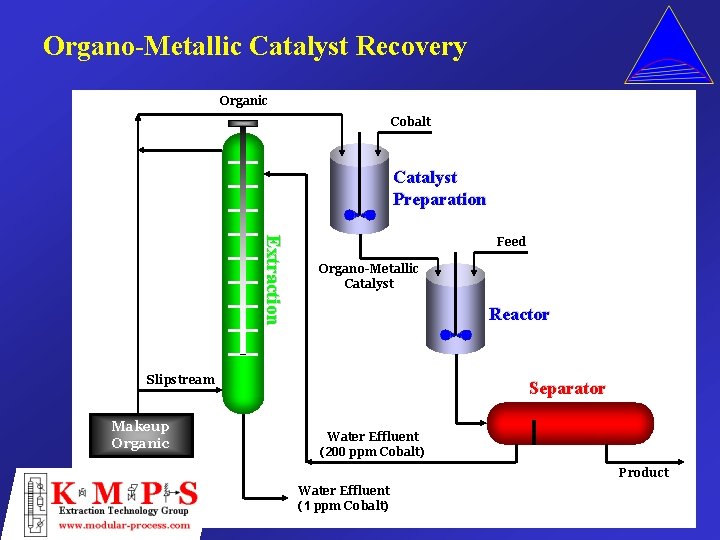
Organo-Metallic Catalyst Recovery Organic Cobalt Catalyst Preparation Extraction Feed Organo-Metallic Catalyst Reactor Slipstream Makeup Organic Separator Water Effluent (200 ppm Cobalt) Product Water Effluent (1 ppm Cobalt)

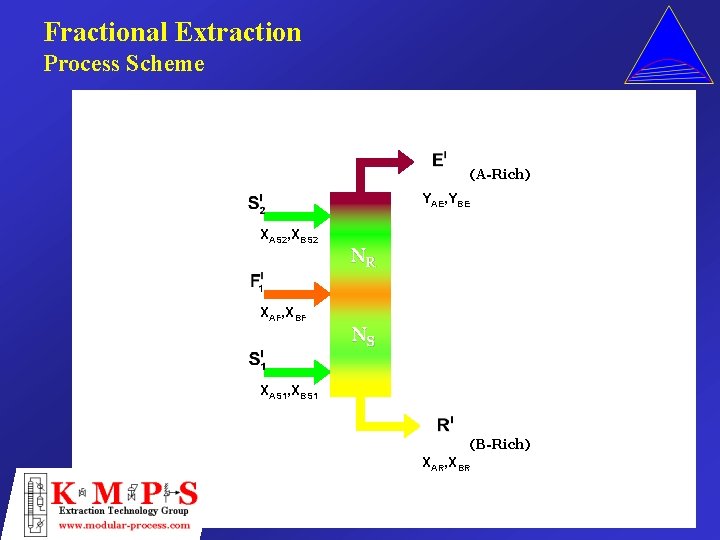
Fractional Extraction Process Scheme (A-Rich) YAE, YBE XAS 2, XBS 2 XAF, XBF NR NS XAS 1, XBS 1 (B-Rich) XAR, XBR

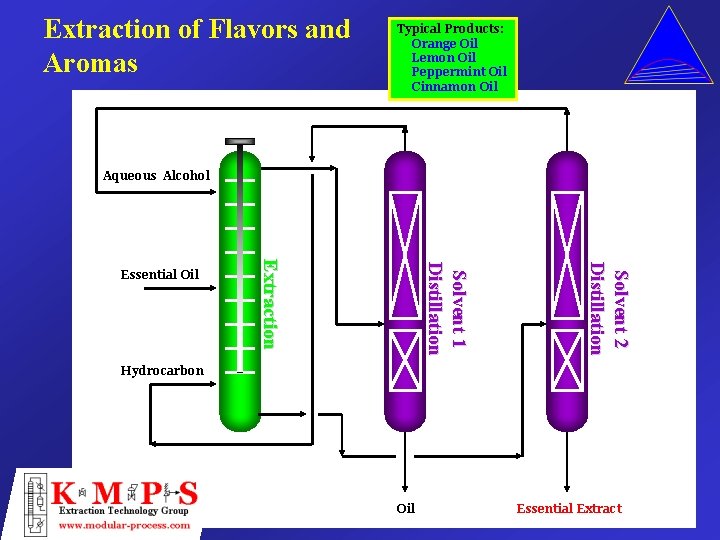
Extraction of Flavors and Aromas Typical Products: Orange Oil Lemon Oil Peppermint Oil Cinnamon Oil Aqueous Alcohol Solvent 2 Distillation Solvent 1 Distillation Extraction Essential Oil Hydrocarbon Oil Essential Extract

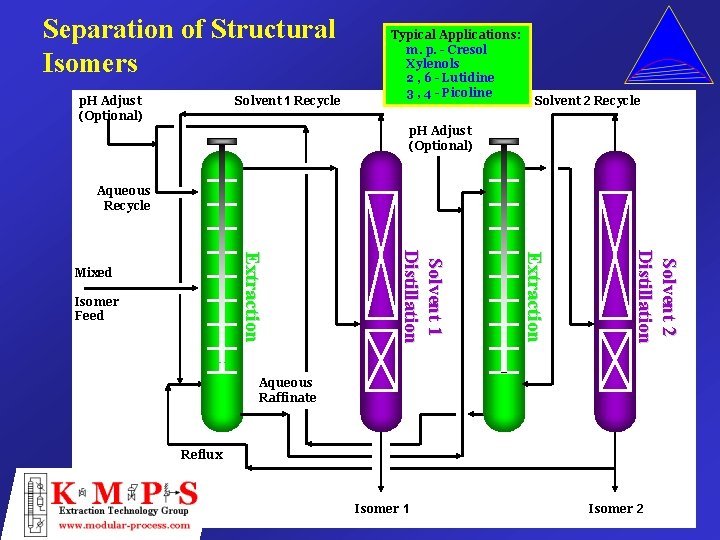
Separation of Structural Isomers p. H Adjust (Optional) Solvent 1 Recycle Typical Applications: m. p. - Cresol Xylenols 2 , 6 - Lutidine 3 , 4 - Picoline Solvent 2 Recycle p. H Adjust (Optional) Aqueous Recycle Solvent 2 Distillation Extraction Isomer Feed Solvent 1 Distillation Extraction Mixed Aqueous Raffinate Reflux Isomer 1 Isomer 2

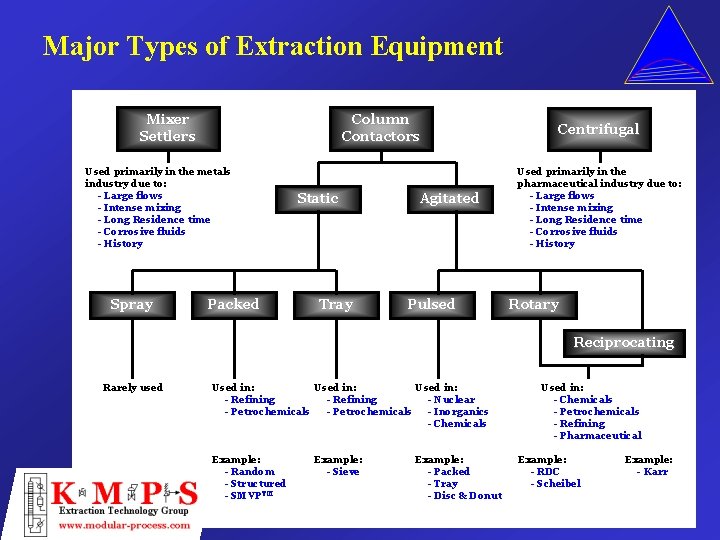
Major Types of Extraction Equipment Mixer Settlers Column Contactors Used primarily in the metals industry due to: - Large flows - Intense mixing - Long Residence time - Corrosive fluids - History Spray Packed Static Tray Agitated Pulsed Centrifugal Used primarily in the pharmaceutical industry due to: - Large flows - Intense mixing - Long Residence time - Corrosive fluids - History Rotary Reciprocating Rarely used Used in: - Refining - Nuclear - Petrochemicals - Inorganics - Chemicals Example: - Random - Structured - SMVPTM Example: - Sieve Example: - Packed - Tray - Disc & Donut Used in: - Chemicals - Petrochemicals - Refining - Pharmaceutical Example: - RDC - Scheibel Example: - Karr

Mix / Decant Tank Characteristics Feed Inlet • Mix – Settle – Phase separate in a single tank • Batch Processing only • Requires multiple solvent additions for more than one stage (crossflow operation) • Typically used for small capacity operations or intermittent processing Sight Glass Outlet

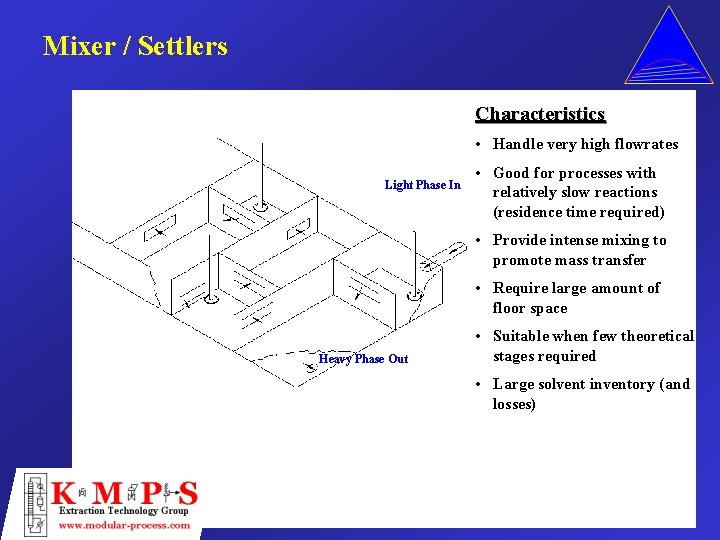
Mixer / Settlers Characteristics • Handle very high flowrates Light Phase In • Good for processes with relatively slow reactions (residence time required) • Provide intense mixing to promote mass transfer • Require large amount of floor space Heavy Phase Out • Suitable when few theoretical stages required • Large solvent inventory (and losses)

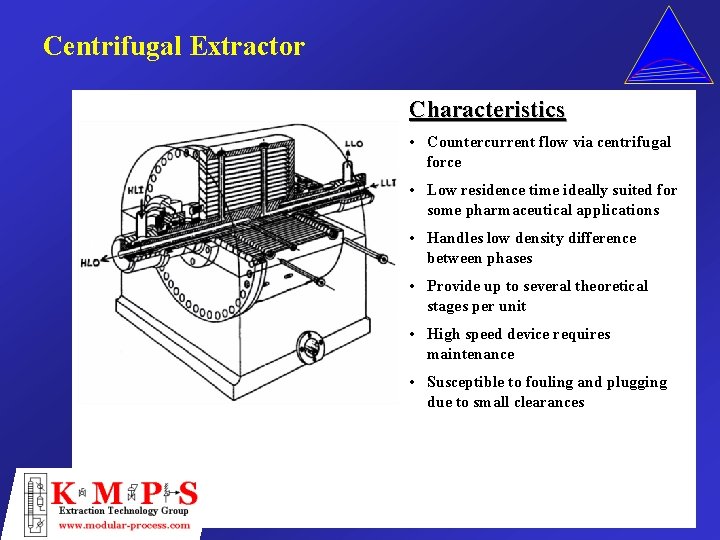
Centrifugal Extractor Characteristics • Countercurrent flow via centrifugal force • Low residence time ideally suited for some pharmaceutical applications • Handles low density difference between phases • Provide up to several theoretical stages per unit • High speed device requires maintenance • Susceptible to fouling and plugging due to small clearances

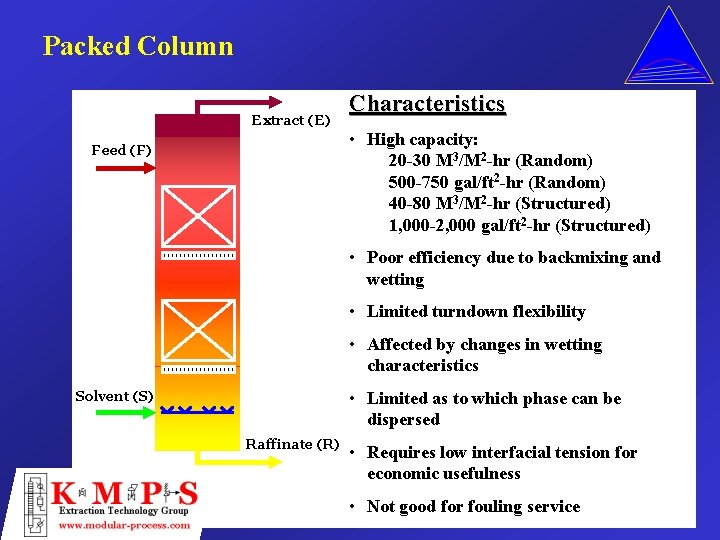
Packed Column Extract (E) Feed (F) Characteristics • High capacity: 20 -30 M 3/M 2 -hr (Random) 500 -750 gal/ft 2 -hr (Random) 40 -80 M 3/M 2 -hr (Structured) 1, 000 -2, 000 gal/ft 2 -hr (Structured) • Poor efficiency due to backmixing and wetting • Limited turndown flexibility • Affected by changes in wetting characteristics • Limited as to which phase can be dispersed Solvent (S) Raffinate (R) • Requires low interfacial tension for economic usefulness • Not good for fouling service

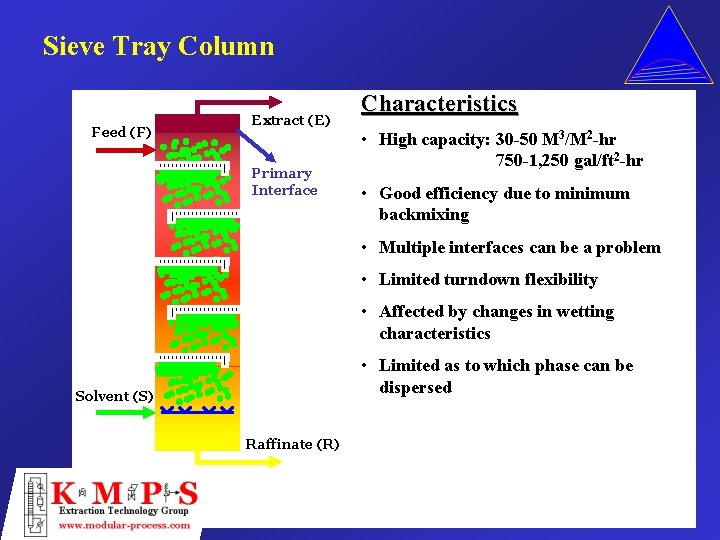
Sieve Tray Column Feed (F) Extract (E) Primary Interface Characteristics • High capacity: 30 -50 M 3/M 2 -hr 750 -1, 250 gal/ft 2 -hr • Good efficiency due to minimum backmixing • Multiple interfaces can be a problem • Limited turndown flexibility • Affected by changes in wetting characteristics • Limited as to which phase can be dispersed Solvent (S) Raffinate (R)

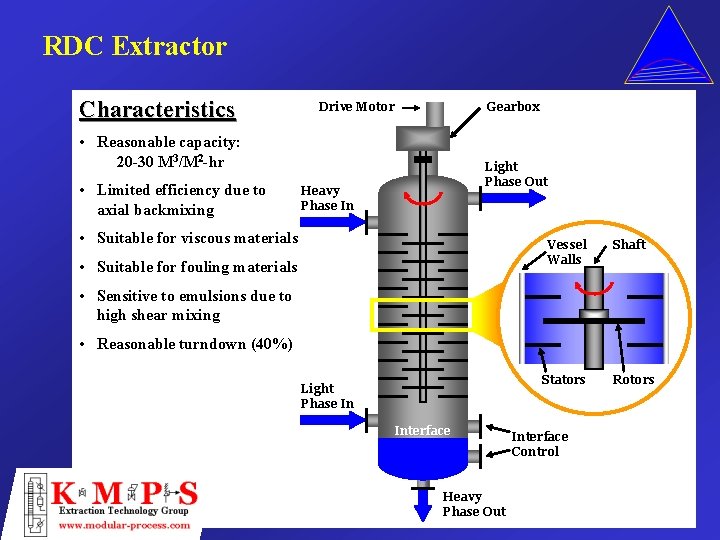
RDC Extractor Characteristics Drive Motor Gearbox • Reasonable capacity: 20 -30 M 3/M 2 -hr • Limited efficiency due to axial backmixing Light Phase Out Heavy Phase In • Suitable for viscous materials Vessel Walls • Suitable for fouling materials Shaft • Sensitive to emulsions due to high shear mixing • Reasonable turndown (40%) Stators Light Phase In Interface Heavy Phase Out Interface Control Rotors

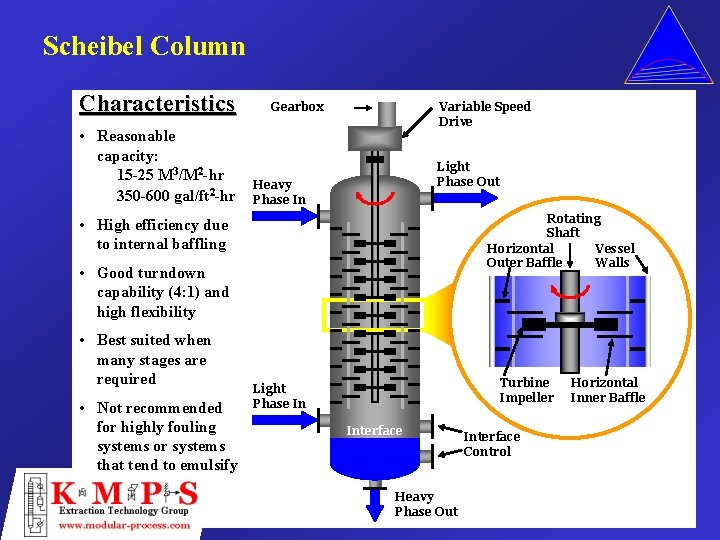
Scheibel Column Characteristics • Reasonable capacity: 15 -25 M 3/M 2 -hr 350 -600 gal/ft 2 -hr Variable Speed Drive Gearbox Light Phase Out Heavy Phase In Rotating Shaft Horizontal Vessel Outer Baffle Walls • High efficiency due to internal baffling • Good turndown capability (4: 1) and high flexibility • Best suited when many stages are required • Not recommended for highly fouling systems or systems that tend to emulsify Turbine Impeller Light Phase In Interface Heavy Phase Out Interface Control Horizontal Inner Baffle

Scheibel Column Internal Assembly

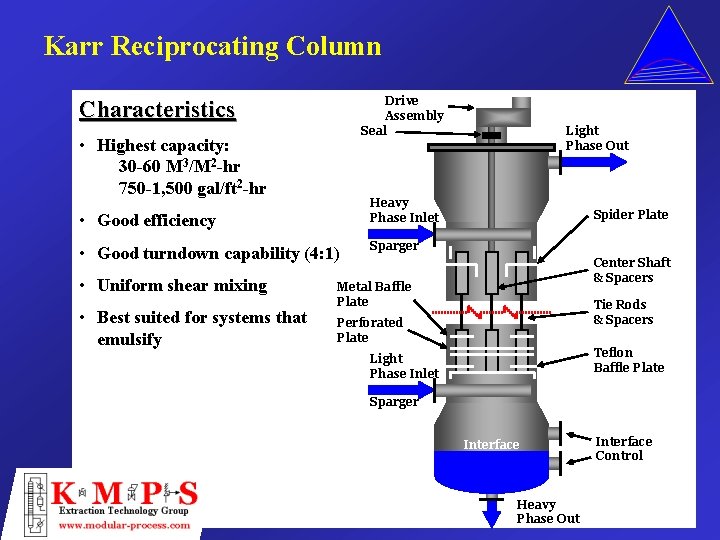
Karr Reciprocating Column Drive Assembly Seal Characteristics • Highest capacity: 30 -60 M 3/M 2 -hr 750 -1, 500 gal/ft 2 -hr Heavy Phase Inlet • Good efficiency • Good turndown capability (4: 1) • Uniform shear mixing • Best suited for systems that emulsify Light Phase Out Spider Plate Sparger Center Shaft & Spacers Metal Baffle Plate Tie Rods & Spacers Perforated Plate Teflon Baffle Plate Light Phase Inlet Sparger Interface Heavy Phase Out Interface Control

Karr Column Plate Stack Assembly

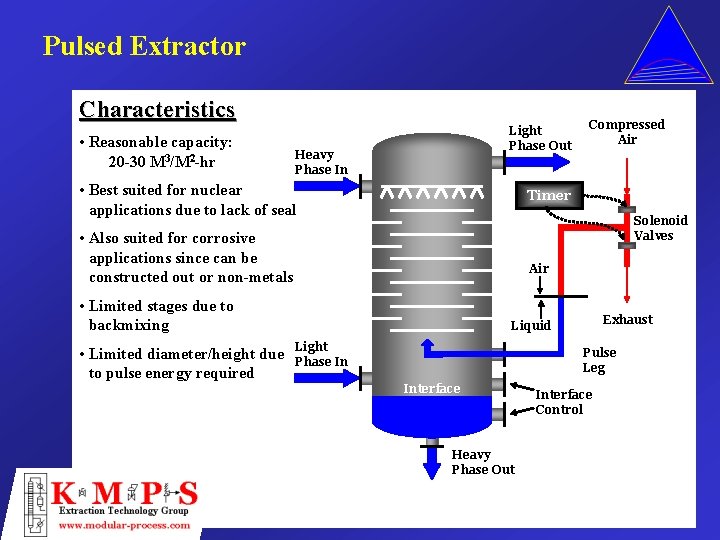
Pulsed Extractor Characteristics • Reasonable capacity: 20 -30 M 3/M 2 -hr Light Phase Out Heavy Phase In • Best suited for nuclear applications due to lack of seal Timer Solenoid Valves • Also suited for corrosive applications since can be constructed out or non-metals Air • Limited stages due to backmixing • Limited diameter/height due to pulse energy required Compressed Air Exhaust Liquid Light Phase In Pulse Leg Interface Heavy Phase Out Interface Control

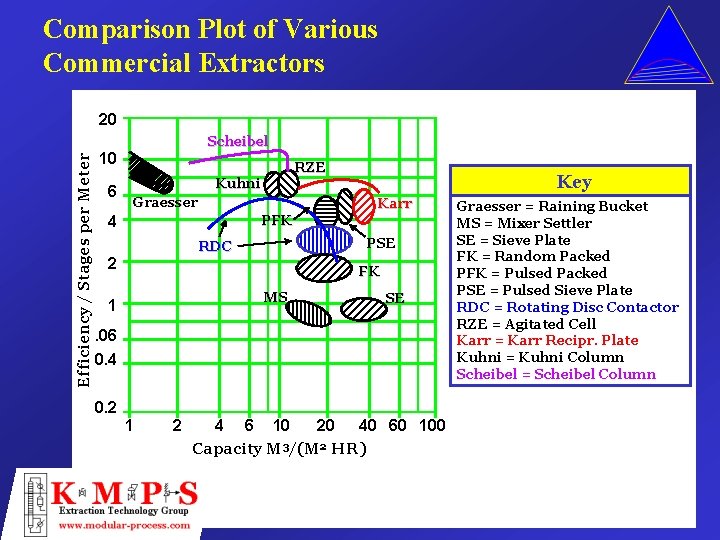
Comparison Plot of Various Commercial Extractors 20 Efficiency / Stages per Meter Scheibel 10 6 RZE Kuhni Graesser Key Karr 4 PFK PSE RDC 2 FK MS 1 SE . 06 0. 4 0. 2 1 2 4 6 10 20 40 60 100 Capacity M 3/(M 2 HR) Graesser = Raining Bucket MS = Mixer Settler SE = Sieve Plate FK = Random Packed PFK = Pulsed Packed PSE = Pulsed Sieve Plate RDC = Rotating Disc Contactor RZE = Agitated Cell Karr = Karr Recipr. Plate Kuhni = Kuhni Column Scheibel = Scheibel Column

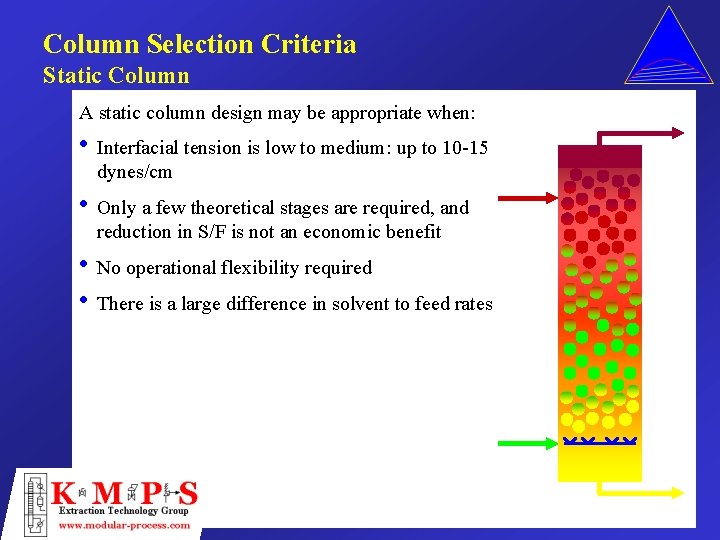
Column Selection Criteria Static Column A static column design may be appropriate when: • Interfacial tension is low to medium: up to 10 -15 dynes/cm • Only a few theoretical stages are required, and reduction in S/F is not an economic benefit • No operational flexibility required • There is a large difference in solvent to feed rates

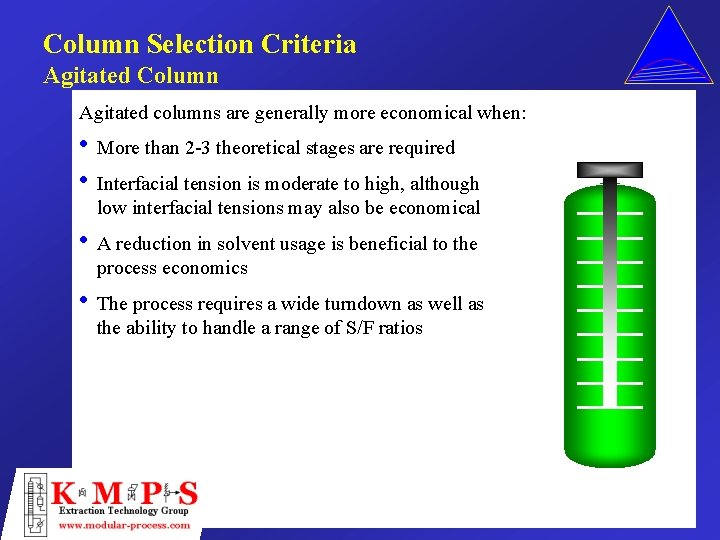
Column Selection Criteria Agitated Column Agitated columns are generally more economical when: • More than 2 -3 theoretical stages are required • Interfacial tension is moderate to high, although low interfacial tensions may also be economical • A reduction in solvent usage is beneficial to the process economics • The process requires a wide turndown as well as the ability to handle a range of S/F ratios

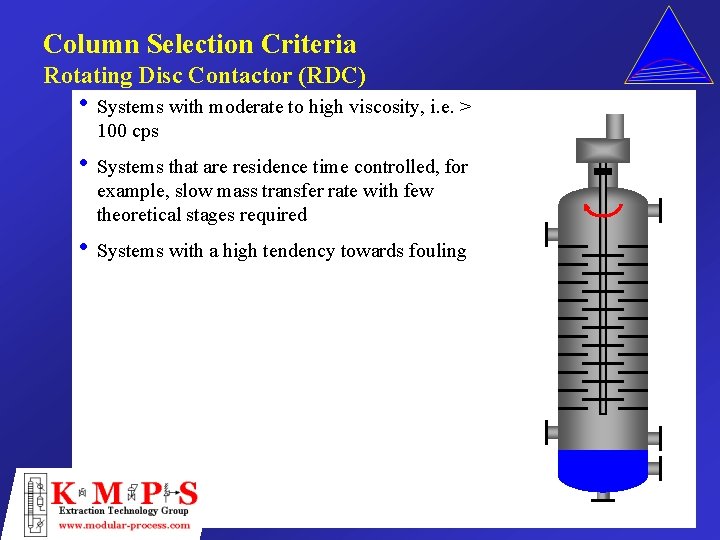
Column Selection Criteria Rotating Disc Contactor (RDC) • Systems with moderate to high viscosity, i. e. > 100 cps • Systems that are residence time controlled, for example, slow mass transfer rate with few theoretical stages required • Systems with a high tendency towards fouling

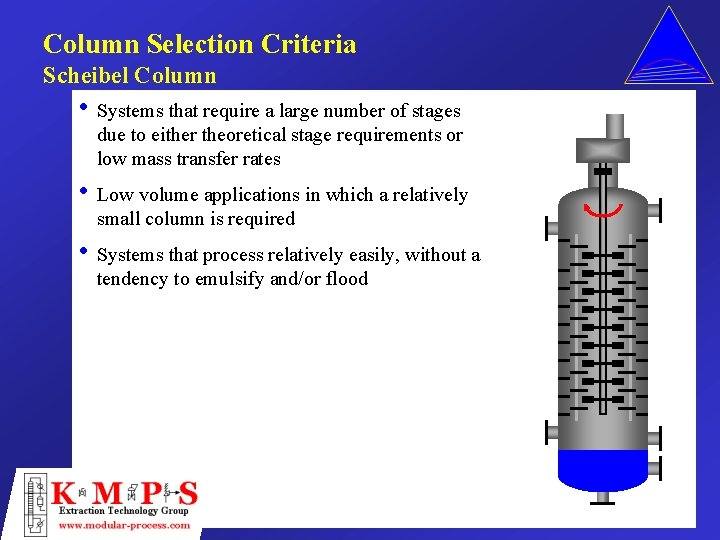
Column Selection Criteria Scheibel Column • Systems that require a large number of stages due to either theoretical stage requirements or low mass transfer rates • Low volume applications in which a relatively small column is required • Systems that process relatively easily, without a tendency to emulsify and/or flood

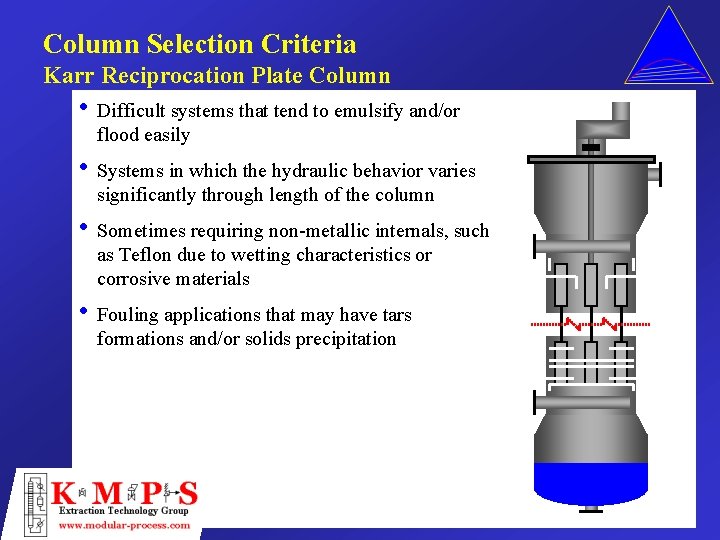
Column Selection Criteria Karr Reciprocation Plate Column • Difficult systems that tend to emulsify and/or flood easily • Systems in which the hydraulic behavior varies significantly through length of the column • Sometimes requiring non-metallic internals, such as Teflon due to wetting characteristics or corrosive materials • Fouling applications that may have tars formations and/or solids precipitation

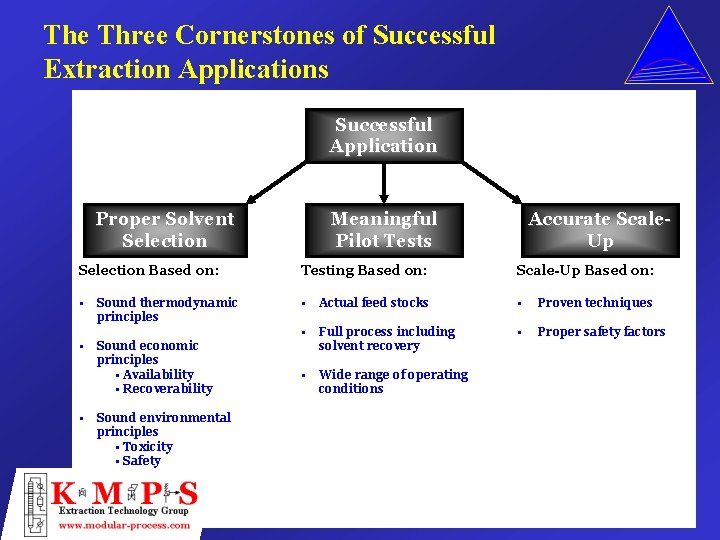
The Three Cornerstones of Successful Extraction Applications Successful Application Proper Solvent Selection Meaningful Pilot Tests Accurate Scale. Up Selection Based on: Testing Based on: Scale-Up Based on: • • Actual feed stocks • Proven techniques • Full process including solvent recovery • Proper safety factors • Wide range of operating conditions • • Sound thermodynamic principles Sound economic principles • Availability • Recoverability Sound environmental principles • Toxicity • Safety

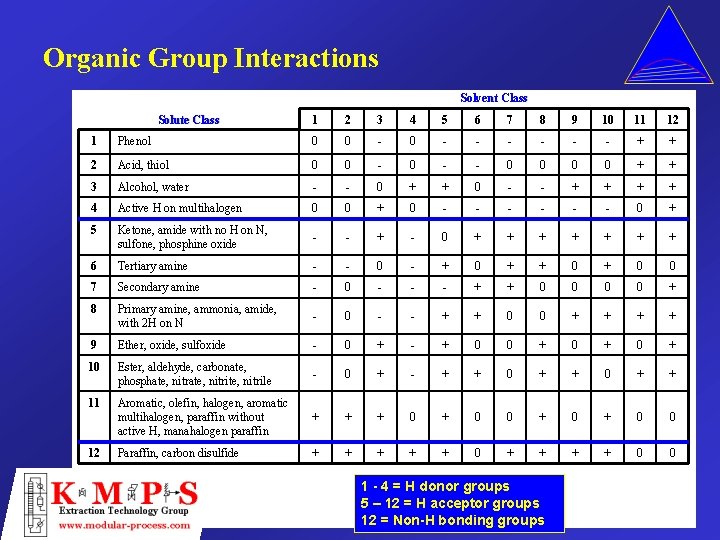
Organic Group Interactions Solvent Class Solute Class 1 2 3 4 5 6 7 8 9 10 11 12 1 Phenol 0 0 - - - - + + 2 Acid, thiol 0 0 - - 0 0 + + 3 Alcohol, water - - 0 + + 0 - - + + 4 Active H on multihalogen 0 0 + 0 - - - 0 + 5 Ketone, amide with no H on N, sulfone, phosphine oxide - - + - 0 + + + + 6 Tertiary amine - - 0 - + 0 + 0 0 7 Secondary amine - 0 - - - + + 0 0 + 8 Primary amine, ammonia, amide, with 2 H on N - 0 - - + + 0 0 + + 9 Ether, oxide, sulfoxide - 0 + - + 0 0 + 0 + 10 Ester, aldehyde, carbonate, phosphate, nitrite, nitrile - 0 + - + + 0 + + 11 Aromatic, olefin, halogen, aromatic multihalogen, paraffin without active H, manahalogen paraffin + + + 0 + 0 0 Paraffin, carbon disulfide + + + 0 0 12 1 - 4 = H donor groups 5 – 12 = H acceptor groups 12 = Non-H bonding groups

Liquid-Liquid Extraction Scale-Up • Theoretical scale-up is difficult • Complexity of processes taking place within an extractor Ø Droplet Breakup Ø Coalescence Ø Mass Transfer Ø Axial and radial mixing Ø Effects of impurities • Best method of design: Pilot testing followed by empirical scale-up

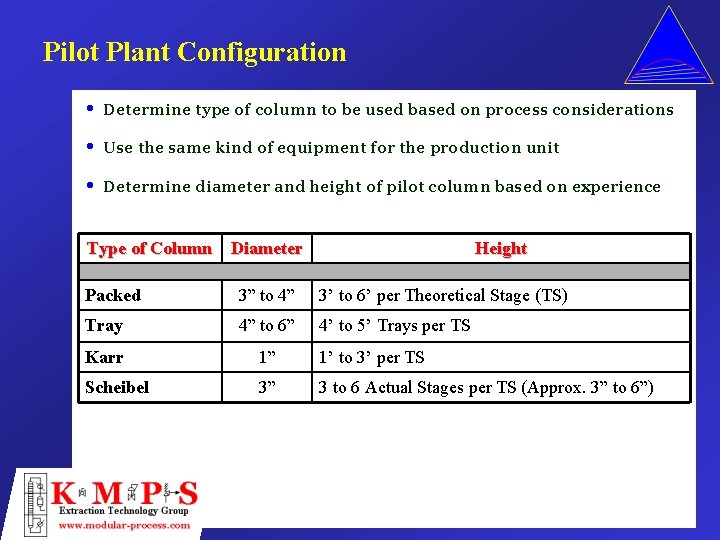
Pilot Plant Configuration • Determine type of column to be used based on process considerations • Use the same kind of equipment for the production unit • Determine diameter and height of pilot column based on experience Type of Column Diameter Height Packed 3” to 4” 3’ to 6’ per Theoretical Stage (TS) Tray 4” to 6” 4’ to 5’ Trays per TS Karr 1” 1’ to 3’ per TS Scheibel 3” 3 to 6 Actual Stages per TS (Approx. 3” to 6”)

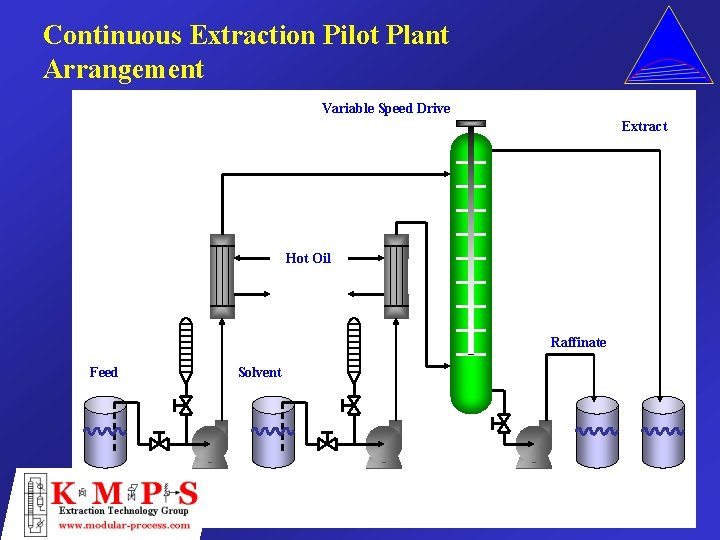
Continuous Extraction Pilot Plant Arrangement Variable Speed Drive Extract Hot Oil Raffinate Feed Solvent

KMPS Pilot Plant Services Group KMPS maintains a pilot plant dedicated to extraction R & D and applications testing

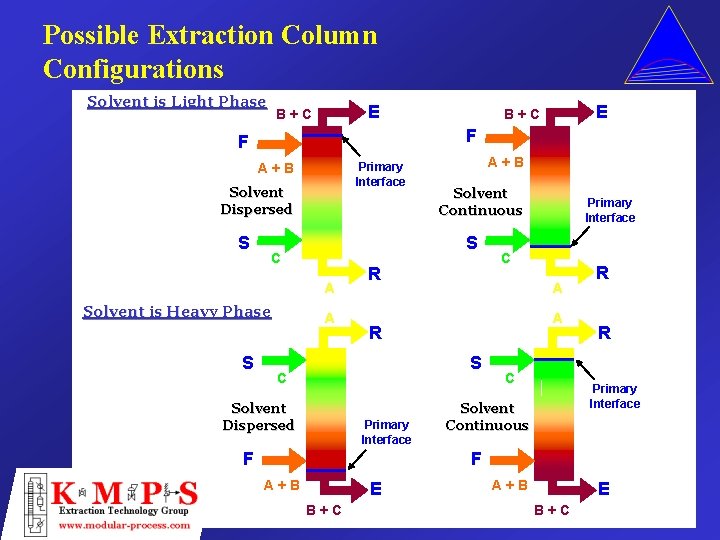
Possible Extraction Column Configurations Solvent is Light Phase E B+C F F Primary Interface A+B Solvent Dispersed S Solvent is Heavy Phase A+B Solvent Continuous S C A S E B+C A R A A S Solvent Dispersed C R C Primary Interface F Primary Interface C R R Primary Interface Solvent Continuous F A+B E B+C

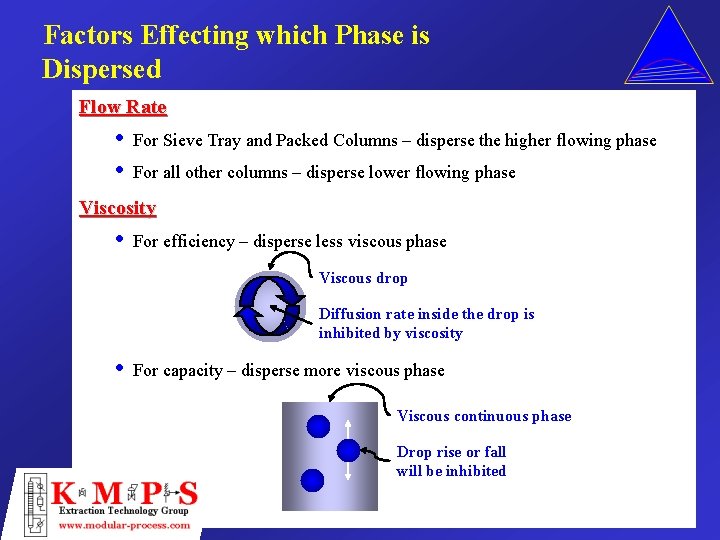
Factors Effecting which Phase is Dispersed Flow Rate • • For Sieve Tray and Packed Columns – disperse the higher flowing phase For all other columns – disperse lower flowing phase Viscosity • For efficiency – disperse less viscous phase Viscous drop Diffusion rate inside the drop is inhibited by viscosity • For capacity – disperse more viscous phase Viscous continuous phase Drop rise or fall will be inhibited

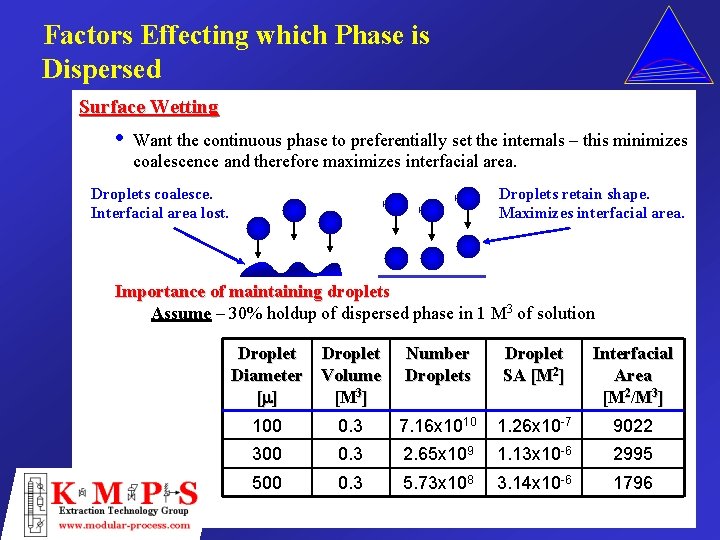
Factors Effecting which Phase is Dispersed Surface Wetting • Want the continuous phase to preferentially set the internals – this minimizes coalescence and therefore maximizes interfacial area. Droplets coalesce. Interfacial area lost. Droplets retain shape. Maximizes interfacial area. Importance of maintaining droplets Assume – 30% holdup of dispersed phase in 1 M 3 of solution Droplet Diameter Volume [m ] [M 3] Number Droplets Droplet SA [M 2] Interfacial Area [M 2/M 3] 100 0. 3 7. 16 x 1010 1. 26 x 10 -7 9022 300 0. 3 2. 65 x 109 1. 13 x 10 -6 2995 500 0. 3 5. 73 x 108 3. 14 x 10 -6 1796

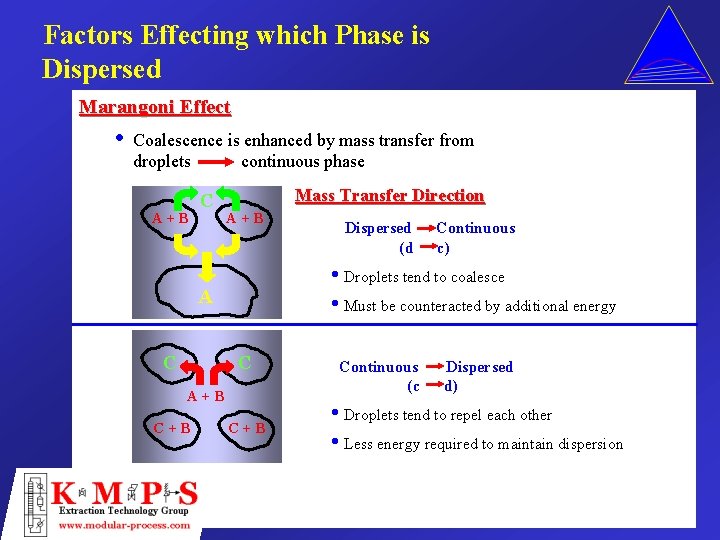
Factors Effecting which Phase is Dispersed Marangoni Effect • Coalescence is enhanced by mass transfer from droplets continuous phase A+B C Mass Transfer Direction A+B C+B Continuous c) • Droplets tend to coalesce • Must be counteracted by additional energy A C Dispersed (d C+B Continuous (c Dispersed d) • Droplets tend to repel each other • Less energy required to maintain dispersion

Interface Behavior Actions to control unstable interface As extraction proceeds, interface normally grows in thickness and forms a “rag” layer that stabilizes at some thickness Light Phase Dispersed Rag Layer If rag layer continues to grow, some action must be taken 1. Rag Draw Continuously withdraw a portion of the interface and pass through a filter to remove interfacial contamination 2. Reverse Phases Often a stable interface can be controlled by reversing which phase is dispersed Heavy Phase Dispersed Growing Uncontrolled Interface Filter 1 2

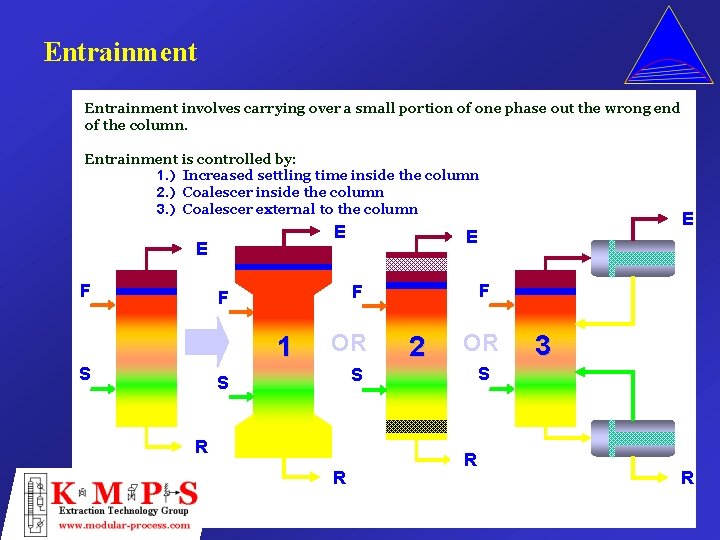
Entrainment involves carrying over a small portion of one phase out the wrong end of the column. Entrainment is controlled by: 1. ) Increased settling time inside the column 2. ) Coalescer inside the column 3. ) Coalescer external to the column E E F 1 S E F F F OR 2 OR R R 3 S S S E R R

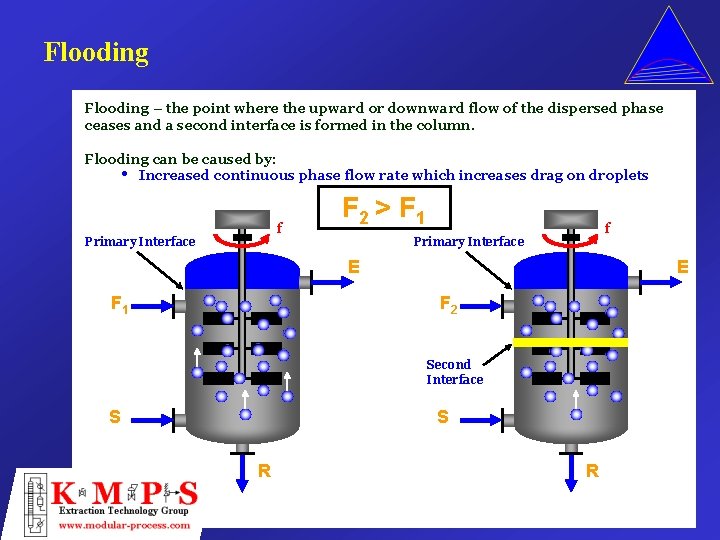
Flooding – the point where the upward or downward flow of the dispersed phase ceases and a second interface is formed in the column. Flooding can be caused by: • Increased continuous phase flow rate which increases drag on droplets f Primary Interface F 2 > F 1 f Primary Interface E F 1 E F 2 Second Interface S S R R

Flooding can be caused by: • Increased agitation speed which forms smaller droplets which cannot overcome flow of the continuous phase • Decreased interfacial tension – forms smaller drops – same effect as increased agitation f 1 Primary Interface f 2 > f 1 f 2 Primary Interface E F 1 E F 2 Second Interface S S R R

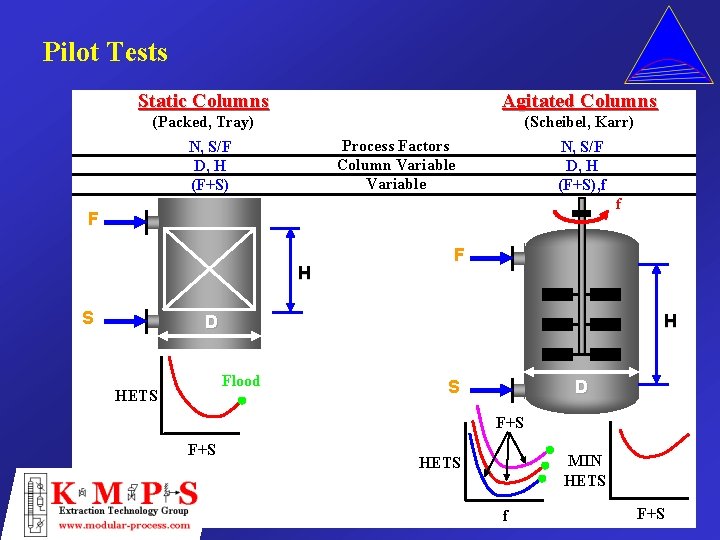
Pilot Tests Static Columns Agitated Columns (Packed, Tray) (Scheibel, Karr) Process Factors Column Variable N, S/F D, H (F+S), f f F H S F H D Flood HETS S D F+S MIN HETS f F+S

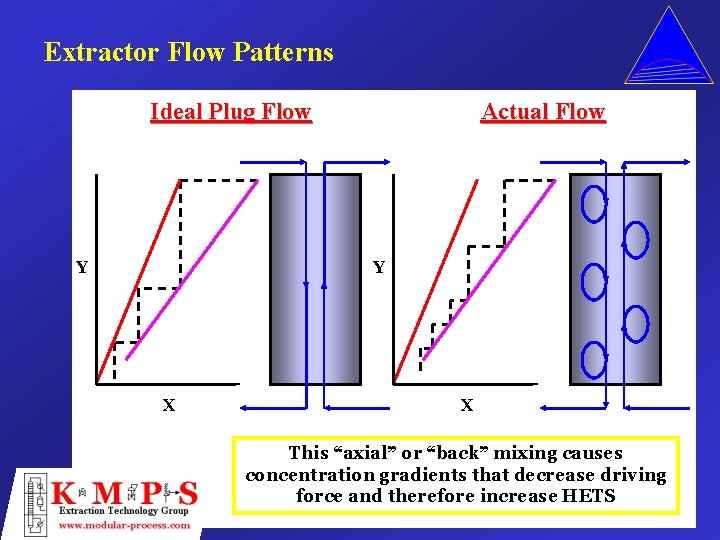
Extractor Flow Patterns Ideal Plug Flow Y Actual Flow Y X X This “axial” or “back” mixing causes concentration gradients that decrease driving force and therefore increase HETS

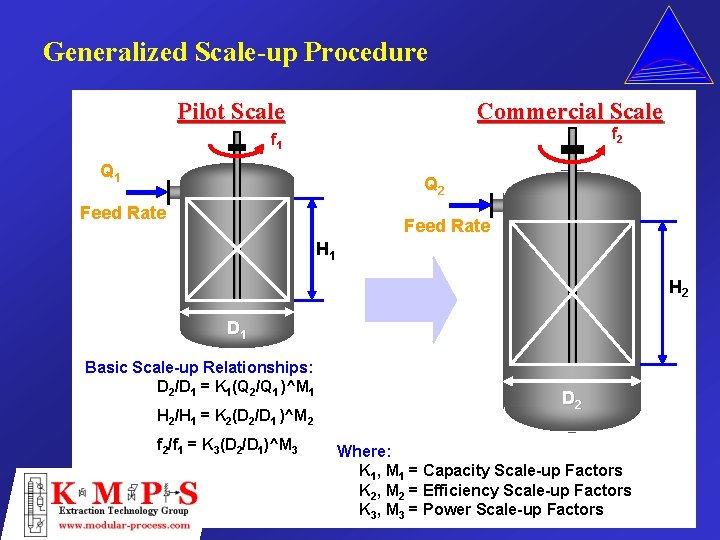
Generalized Scale-up Procedure Pilot Scale Commercial Scale f 2 f 1 Q 2 Feed Rate H 1 H 2 D 1 Basic Scale-up Relationships: D 2/D 1 = K 1(Q 2/Q 1 )^M 1 H 2/H 1 = K 2(D 2/D 1 )^M 2 f 2/f 1 = K 3(D 2/D 1)^M 3 D 2 Where: K 1, M 1 = Capacity Scale-up Factors K 2, M 2 = Efficiency Scale-up Factors K 3, M 3 = Power Scale-up Factors

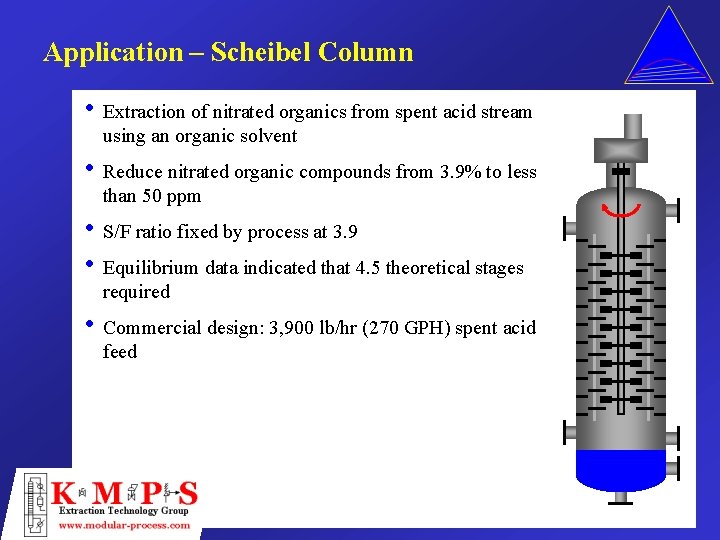
Application – Scheibel Column • Extraction of nitrated organics from spent acid stream using an organic solvent • Reduce nitrated organic compounds from 3. 9% to less than 50 ppm • S/F ratio fixed by process at 3. 9 • Equilibrium data indicated that 4. 5 theoretical stages required • Commercial design: 3, 900 lb/hr (270 GPH) spent acid feed

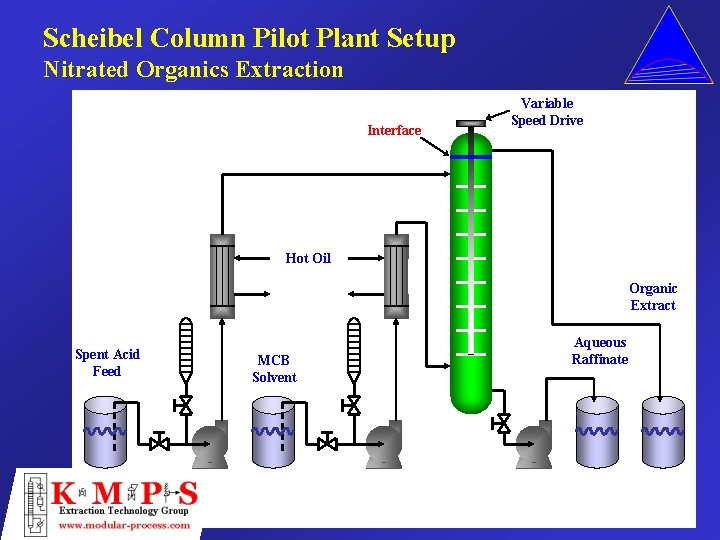
Scheibel Column Pilot Plant Setup Nitrated Organics Extraction Interface Variable Speed Drive Hot Oil Organic Extract Spent Acid Feed MCB Solvent Aqueous Raffinate

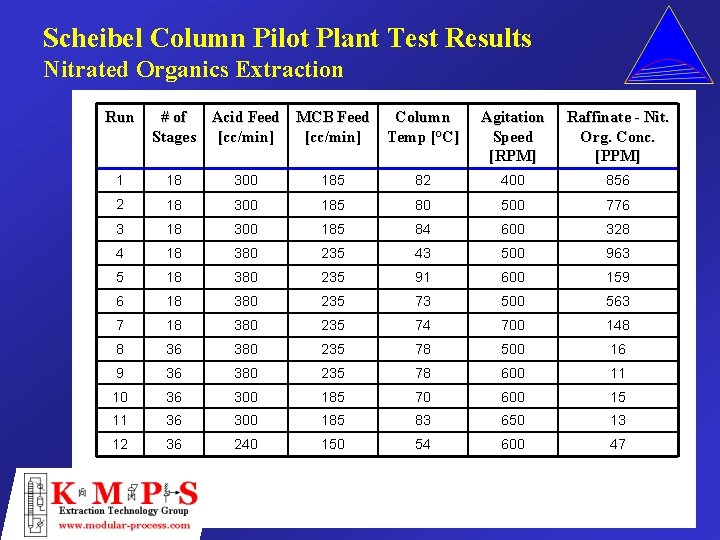
Scheibel Column Pilot Plant Test Results Nitrated Organics Extraction Run # of Acid Feed MCB Feed Stages [cc/min] Column Temp [°C] Agitation Speed [RPM] Raffinate - Nit. Org. Conc. [PPM] 1 18 300 185 82 400 856 2 18 300 185 80 500 776 3 18 300 185 84 600 328 4 18 380 235 43 500 963 5 18 380 235 91 600 159 6 18 380 235 73 500 563 7 18 380 235 74 700 148 8 36 380 235 78 500 16 9 36 380 235 78 600 11 10 36 300 185 70 600 15 11 36 300 185 83 650 13 12 36 240 150 54 600 47

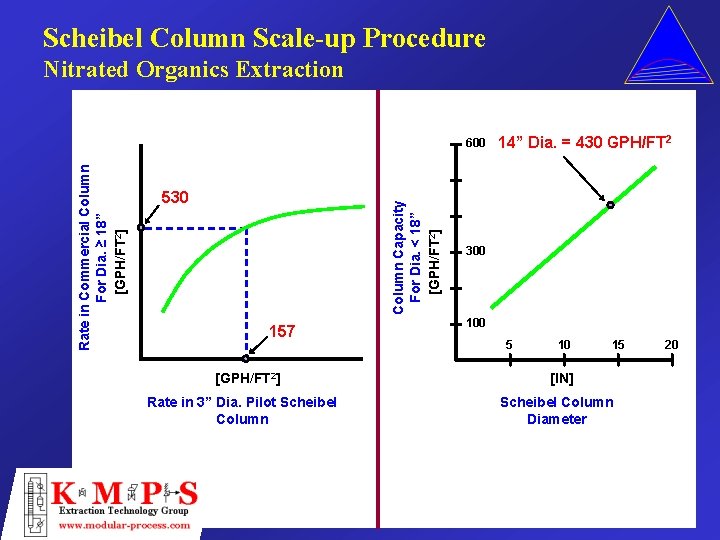
Scheibel Column Scale-up Procedure Nitrated Organics Extraction 530 Column Capacity For Dia. < 18” [GPH/FT 2] Rate in Commercial Column For Dia. ≥ 18” [GPH/FT 2] 600 157 [GPH/FT 2] Rate in 3” Dia. Pilot Scheibel Column 14” Dia. = 430 GPH/FT 2 300 100 5 10 15 [IN] Scheibel Column Diameter 20

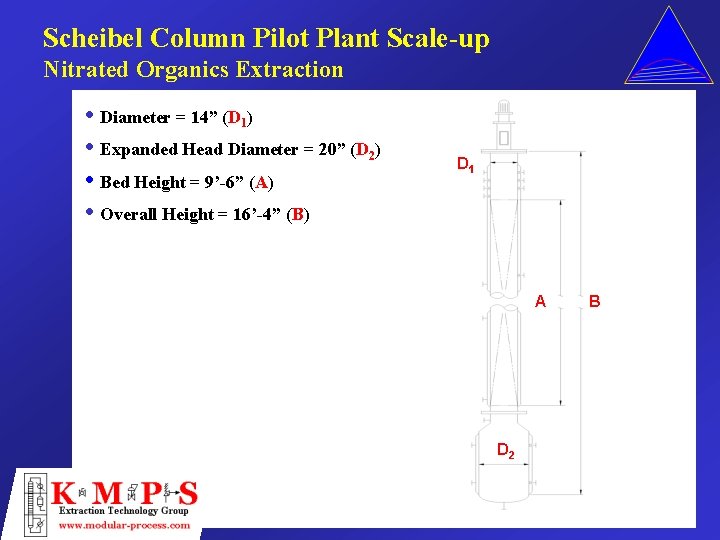
Scheibel Column Pilot Plant Scale-up Nitrated Organics Extraction • Diameter = 14” (D 1) • Expanded Head Diameter = 20” (D 2) • Bed Height = 9’-6” (A) • Overall Height = 16’-4” (B) D 1 A D 2 B

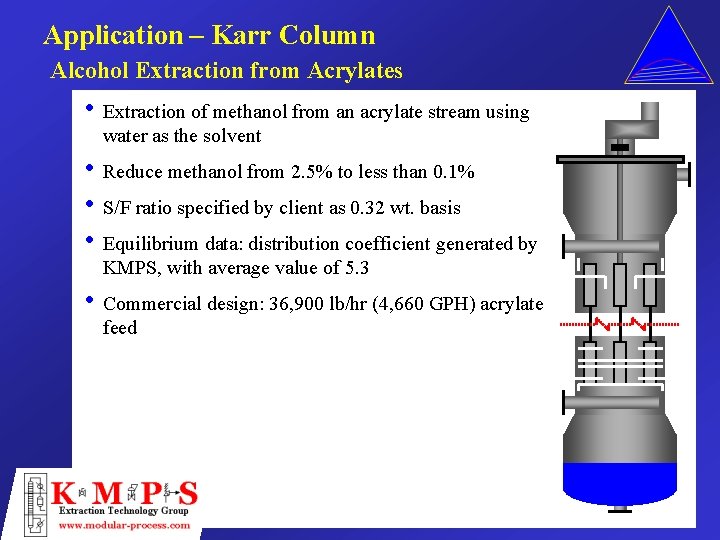
Application – Karr Column Alcohol Extraction from Acrylates • Extraction of methanol from an acrylate stream using water as the solvent • Reduce methanol from 2. 5% to less than 0. 1% • S/F ratio specified by client as 0. 32 wt. basis • Equilibrium data: distribution coefficient generated by KMPS, with average value of 5. 3 • Commercial design: 36, 900 lb/hr (4, 660 GPH) acrylate feed

Karr Column Pilot Plant Setup Alcohol Extraction from Acrylates Karr Column 1” Dia. x 8’ Plate Stack Plate Spacing from Top: 6’ of 2” 1’ of 4” 1’ of 6” 316 SS Shaft, Plates & Spacers Variable Speed Drive Hot Oil Raffinate (Acrylate Phase) Water Feed Extract (H 2 O + Alcohol) Acrylate Feed (methyl or ethyl) Interface

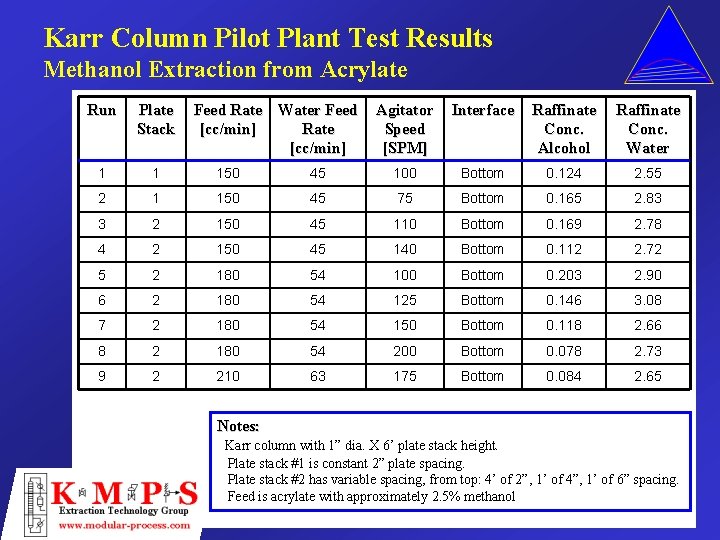
Karr Column Pilot Plant Test Results Methanol Extraction from Acrylate Run Plate Stack Feed Rate Water Feed [cc/min] Rate [cc/min] Agitator Speed [SPM] Interface Raffinate Conc. Alcohol Raffinate Conc. Water 1 1 150 45 100 Bottom 0. 124 2. 55 2 1 150 45 75 Bottom 0. 165 2. 83 3 2 150 45 110 Bottom 0. 169 2. 78 4 2 150 45 140 Bottom 0. 112 2. 72 5 2 180 54 100 Bottom 0. 203 2. 90 6 2 180 54 125 Bottom 0. 146 3. 08 7 2 180 54 150 Bottom 0. 118 2. 66 8 2 180 54 200 Bottom 0. 078 2. 73 9 2 210 63 175 Bottom 0. 084 2. 65 Notes: Karr column with 1” dia. X 6’ plate stack height. Plate stack #1 is constant 2” plate spacing. Plate stack #2 has variable spacing, from top: 4’ of 2”, 1’ of 4”, 1’ of 6” spacing. Feed is acrylate with approximately 2. 5% methanol

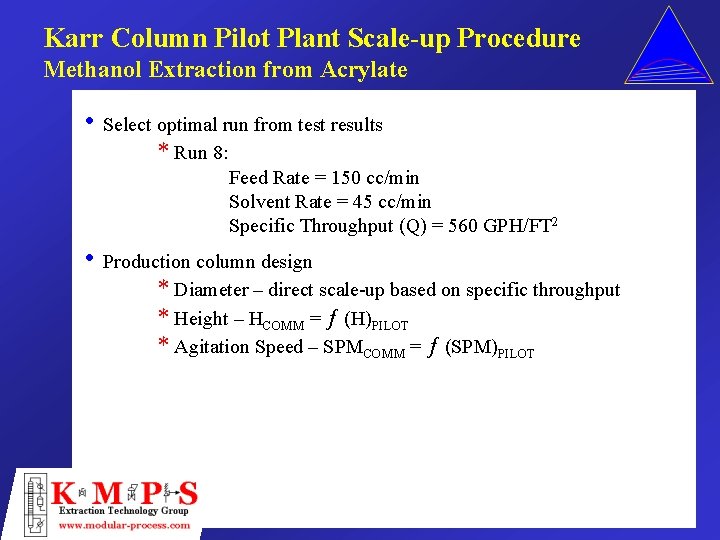
Karr Column Pilot Plant Scale-up Procedure Methanol Extraction from Acrylate • Select optimal run from test results * Run 8: Feed Rate = 150 cc/min Solvent Rate = 45 cc/min Specific Throughput (Q) = 560 GPH/FT 2 • Production column design * Diameter – direct scale-up based on specific throughput * Height – HCOMM = ƒ (H)PILOT * Agitation Speed – SPMCOMM = ƒ (SPM)PILOT

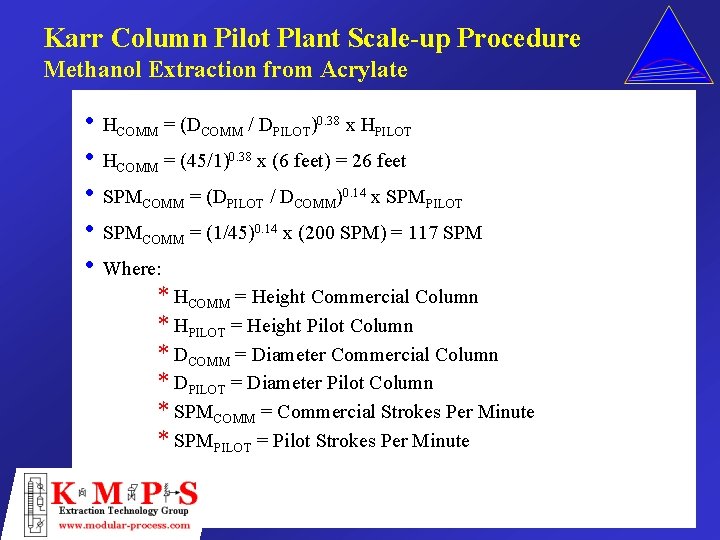
Karr Column Pilot Plant Scale-up Procedure Methanol Extraction from Acrylate • HCOMM = (DCOMM / DPILOT)0. 38 x HPILOT • HCOMM = (45/1)0. 38 x (6 feet) = 26 feet • SPMCOMM = (DPILOT / DCOMM)0. 14 x SPMPILOT • SPMCOMM = (1/45)0. 14 x (200 SPM) = 117 SPM • Where: * HCOMM = Height Commercial Column * HPILOT = Height Pilot Column * DCOMM = Diameter Commercial Column * DPILOT = Diameter Pilot Column * SPMCOMM = Commercial Strokes Per Minute * SPMPILOT = Pilot Strokes Per Minute

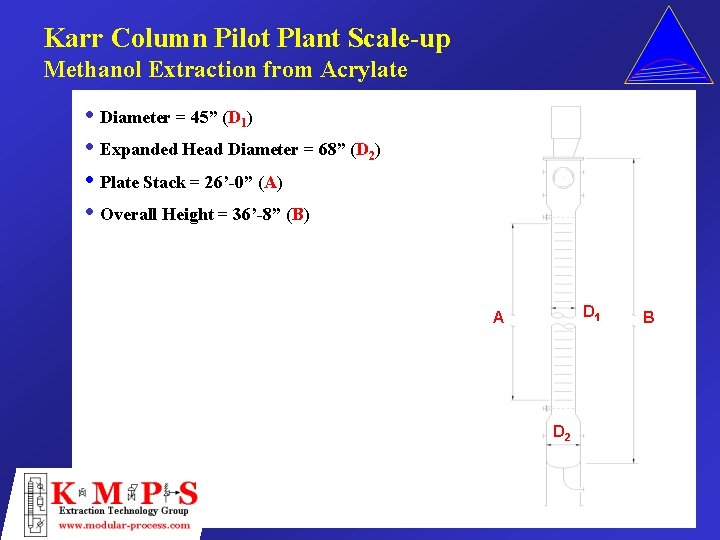
Karr Column Pilot Plant Scale-up Methanol Extraction from Acrylate • Diameter = 45” (D 1) • Expanded Head Diameter = 68” (D 2) • Plate Stack = 26’-0” (A) • Overall Height = 36’-8” (B) D 1 A D 2 B

Extraction Experience KMPS has supplied over 300 extraction columns.

Questions?