Separation Techniques Extraction and Chromatography Extraction Simple liquidliquid









- Slides: 25

Separation Techniques Extraction and Chromatography

Extraction Ø Simple liquid-liquid extraction in analytical work is generally done for one of two reasons: l l Purification, i. e. , separating the analyte from interferents, or Concentration, i. e. , getting the analyte into a smaller volume Ø If the analyte is a metal ion, the extracting medium will often include a complexing agent.

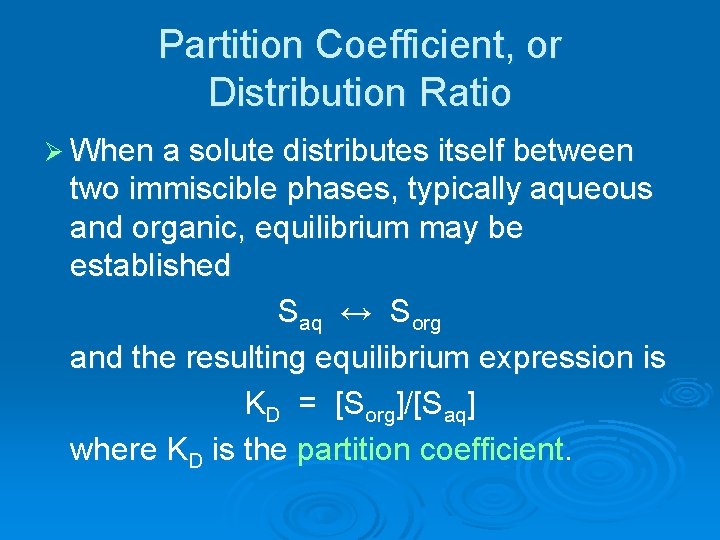
Partition Coefficient, or Distribution Ratio Ø When a solute distributes itself between two immiscible phases, typically aqueous and organic, equilibrium may be established Saq ↔ Sorg and the resulting equilibrium expression is KD = [Sorg]/[Saq] where KD is the partition coefficient

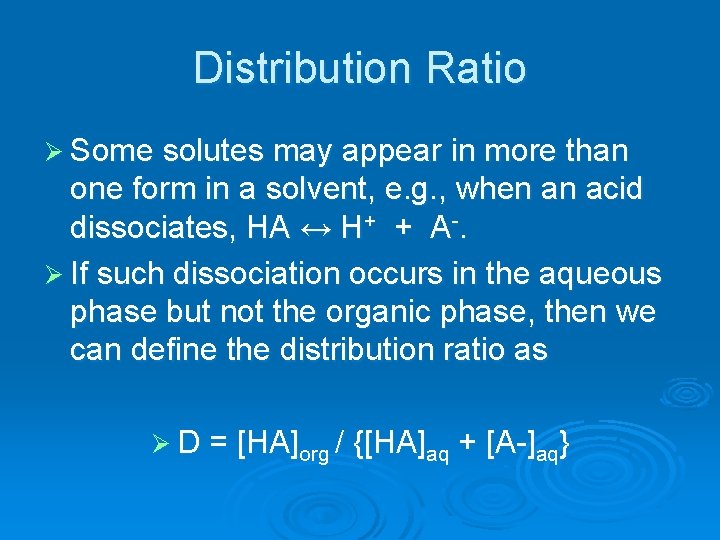
Distribution Ratio Ø Some solutes may appear in more than one form in a solvent, e. g. , when an acid dissociates, HA ↔ H+ + A-. Ø If such dissociation occurs in the aqueous phase but not the organic phase, then we can define the distribution ratio as Ø D = [HA]org / {[HA]aq + [A-]aq}

Extraction Efficiency Ø If we define the fraction of solute extracted in a single step extraction, Ei, and the fraction remaining behind unextracted as, Ui, it can be shown (see your handout) that E 1 = KD/ [(V 1/V 2)+KD], and U 1 = V 1/ [V 1 + V 2 KD] * Further, Ui = (U 1)i *(same as equation 7. 24 in your text with different symbols)

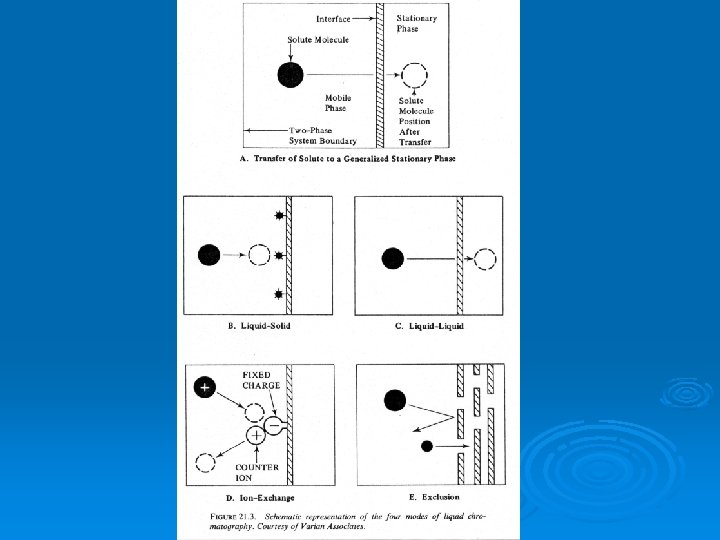
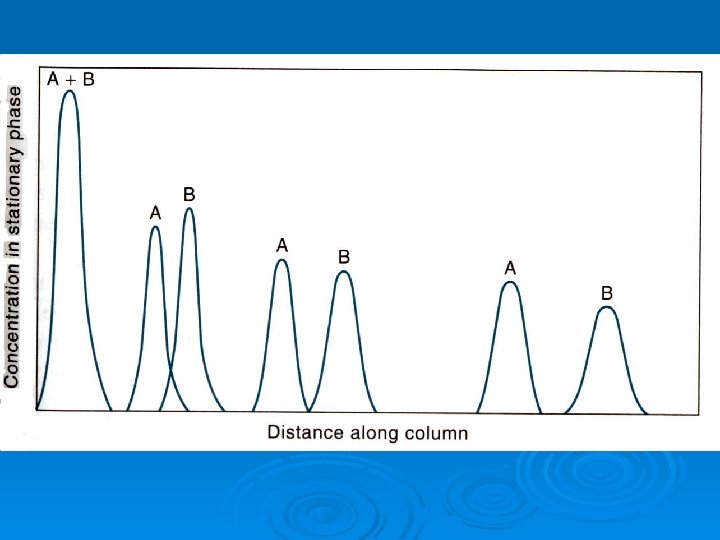
Chromatography can be thought of as a method involving continuous extraction in very small increments of clean extracting solvent. Ø Chromatography always has two phases, just as in extraction, but they are called the mobile phase and the stationary phase. Ø The movement of the solute through the column is called elution. Ø The phase distribution of the solute(s) in chromatography can occur by any of several equilibria, not just solubility, as in extraction. Ø

Types of Equilibria Applied to Chromatography Adsorption – the stationary phase is a solid on which the solutes adsorb. Ø Solubility – the stationary phase is a liquid into which the solutes dissolve. Ø Ion-exchange – the stationary phase is composed of ion exchange beads which attract cations or anions Ø Size-exclusion – the stationary phase is porous beads which temporarily trap solutes based on their particle size. Ø


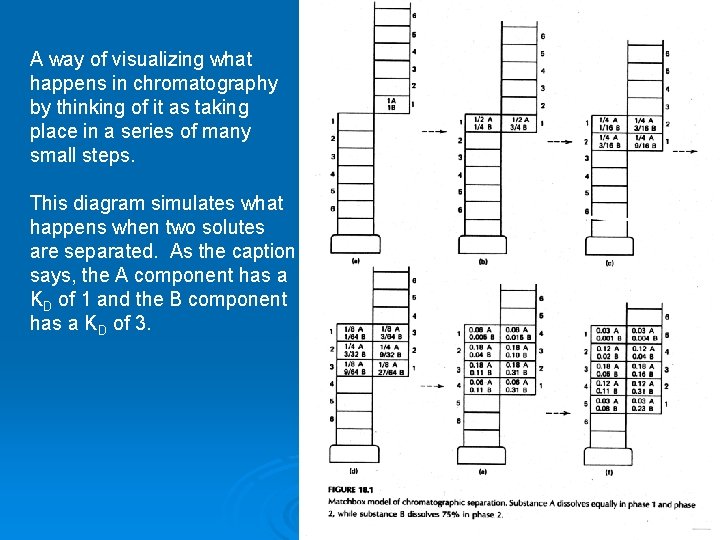
A way of visualizing what happens in chromatography by thinking of it as taking place in a series of many small steps. This diagram simulates what happens when two solutes are separated. As the caption says, the A component has a KD of 1 and the B component has a KD of 3.


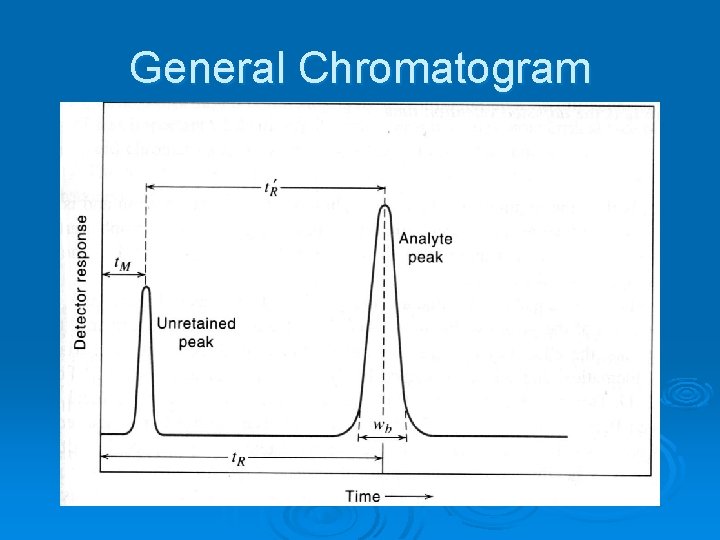
General Chromatogram

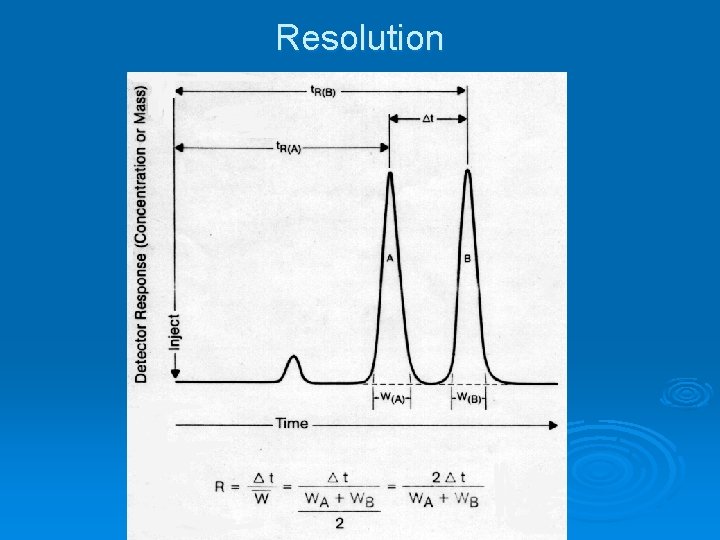
Chromatographic Terms Ø Retention time, tr, A, the time it takes com- ponent A to exit the column past the detector. Ø Void time, tm, the time it takes a nonretained component (air in GC) to exit. Ø Adjusted retention time, tr’ = tr – tm. Ø Baseline width, Wb, measured at intersection of tangents with sides and baseline.

Resolution

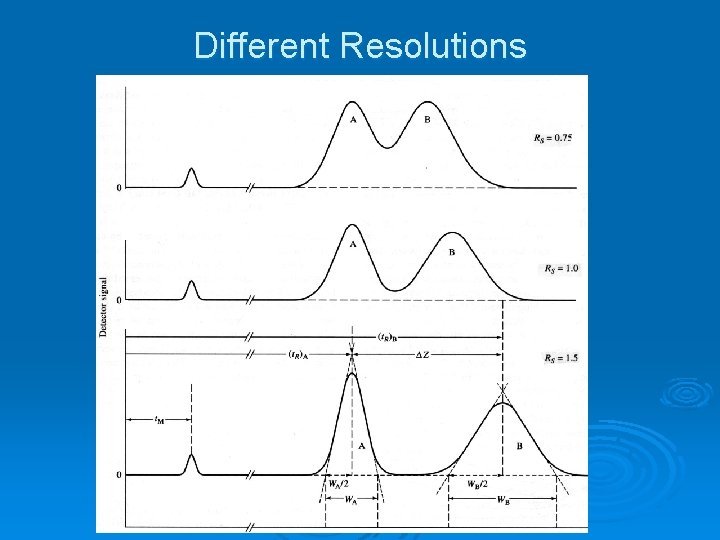
Different Resolutions

The Chromatographic Challenge Looking at the resolution curves, it can be seen that resolution can be increased by increasing the time between the peaks or by decreasing the width of the peaks. Ø The challenge for the chromatographer is to accomplish optimal resolution within a reasonable amount of time. Thus, simply increasing the time by some means is not necessarily the best way to go. Ø Let us consider what causes band broadening and see if we can figure ways to decrease it. Ø

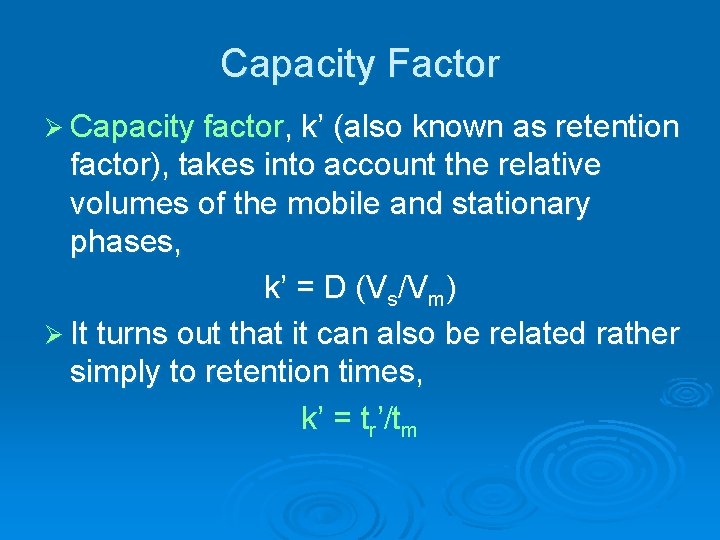
Capacity Factor Ø Capacity factor, k’ (also known as retention factor), takes into account the relative volumes of the mobile and stationary phases, k’ = D (Vs/Vm) Ø It turns out that it can also be related rather simply to retention times, k’ = tr’/tm

Column Selectivity Factor Ø The selectivity factor, α, relates to the ability of a column to separate two solutes, α = k. B’ / k. A’ Ø It will be equal to one when the two components have the same retention times.

Plate Theory of Band Broadening Early on, theorists drew parallels between the behavior of chromatographic columns and fractional distillation columns with plates separating temperature regions. Ø Chromatography can be thought of as behaving like a fractional distillation column with many plates. Ø Each time the solute enters the stationary phase essentially constitutes a ‘theoretical plate. ’ Ø

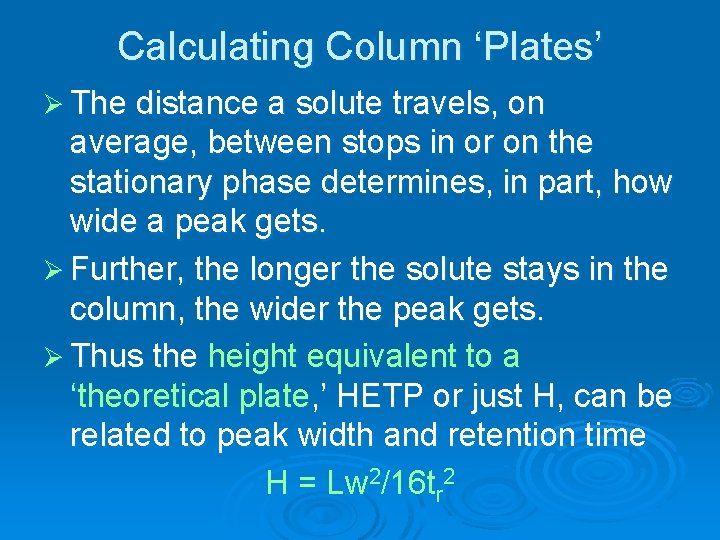
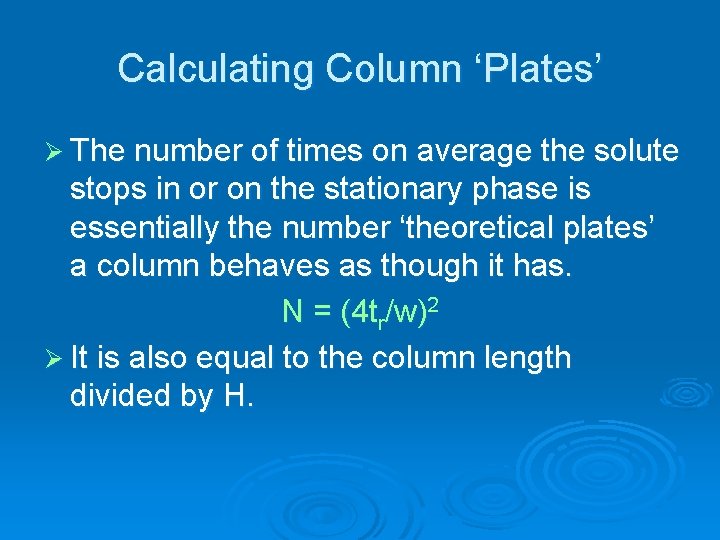
Calculating Column ‘Plates’ Ø The distance a solute travels, on average, between stops in or on the stationary phase determines, in part, how wide a peak gets. Ø Further, the longer the solute stays in the column, the wider the peak gets. Ø Thus the height equivalent to a ‘theoretical plate, ’ HETP or just H, can be related to peak width and retention time H = Lw 2/16 tr 2

Calculating Column ‘Plates’ Ø The number of times on average the solute stops in or on the stationary phase is essentially the number ‘theoretical plates’ a column behaves as though it has. N = (4 tr/w)2 Ø It is also equal to the column length divided by H.

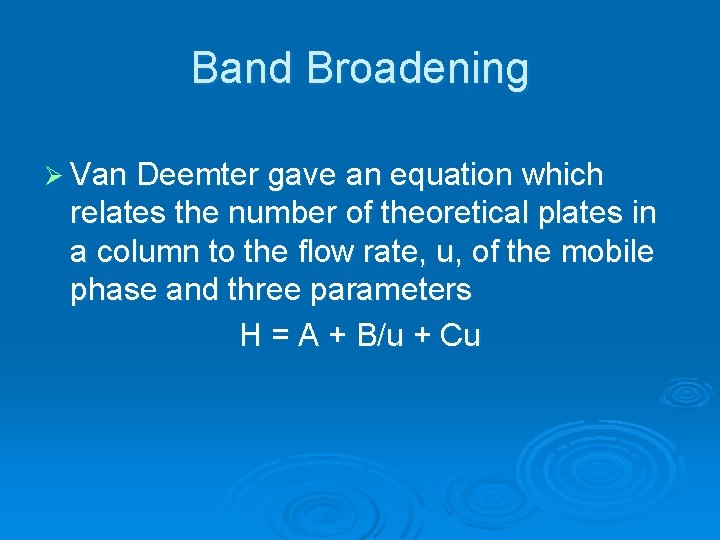
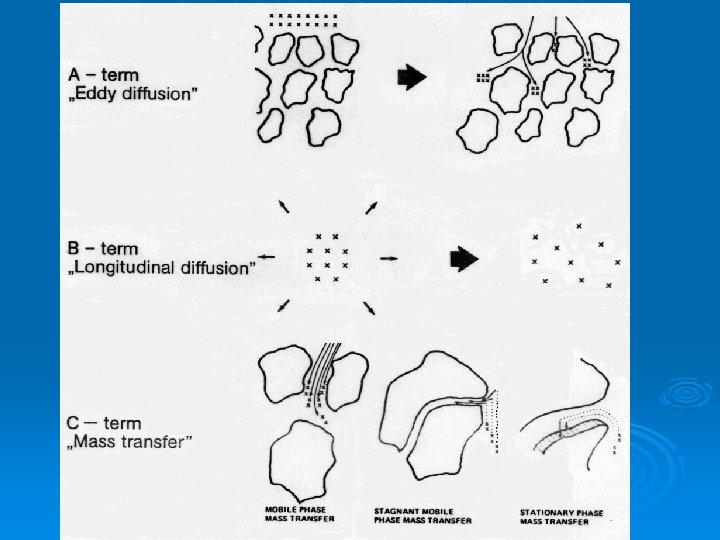
Band Broadening Ø Van Deemter gave an equation which relates the number of theoretical plates in a column to the flow rate, u, of the mobile phase and three parameters H = A + B/u + Cu

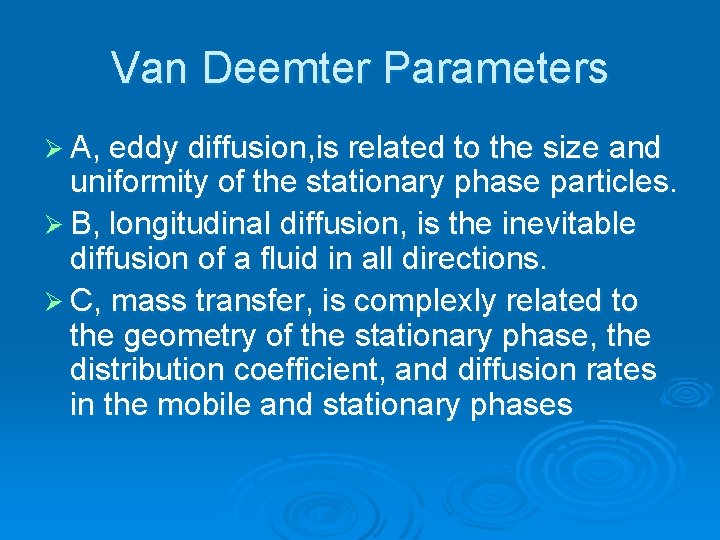
Van Deemter Parameters Ø A, eddy diffusion, is related to the size and uniformity of the stationary phase particles. Ø B, longitudinal diffusion, is the inevitable diffusion of a fluid in all directions. Ø C, mass transfer, is complexly related to the geometry of the stationary phase, the distribution coefficient, and diffusion rates in the mobile and stationary phases


So, with all that, how do I increase resolution? Ø Increase Δtr by l l l Increasing L Increase amount of stationary phase Get a better selectivity factor, α • • • Decrease temperature Get a better stationary phase Get a better liquid phase (in liquid chromatograpy) (more)

More resolution improvement Ø Decrease band width, w, by l l l l Using more uniform packing Using smaller packing Use no packing, use SCOT column (for GC) Optimize flow rate Reduce sample size Reduce dead space in column Decrease diameter of column