Separation methods 1 LiquidLiquid Extraction Focus on 2




![LLE One analyte component KP = [A]raffinate / [A]extractant KP = β * k LLE One analyte component KP = [A]raffinate / [A]extractant KP = β * k](https://slidetodoc.com/presentation_image/9bfcc157c10bbc7e4c638c50d0c61eed/image-23.jpg)

- Slides: 25

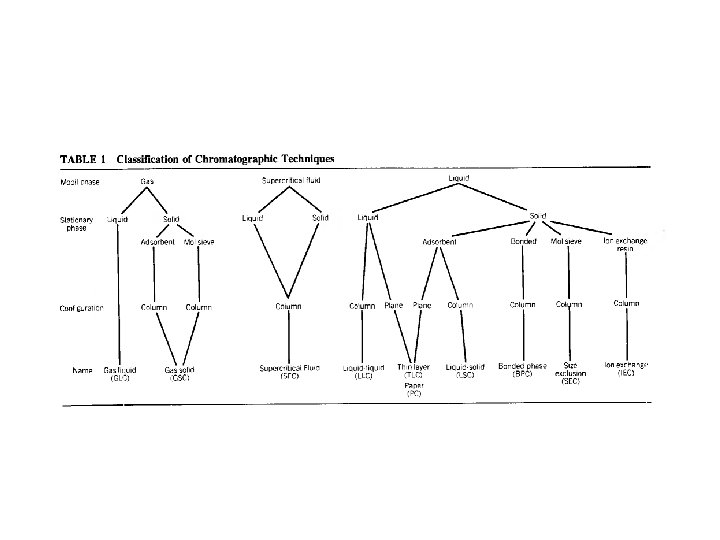
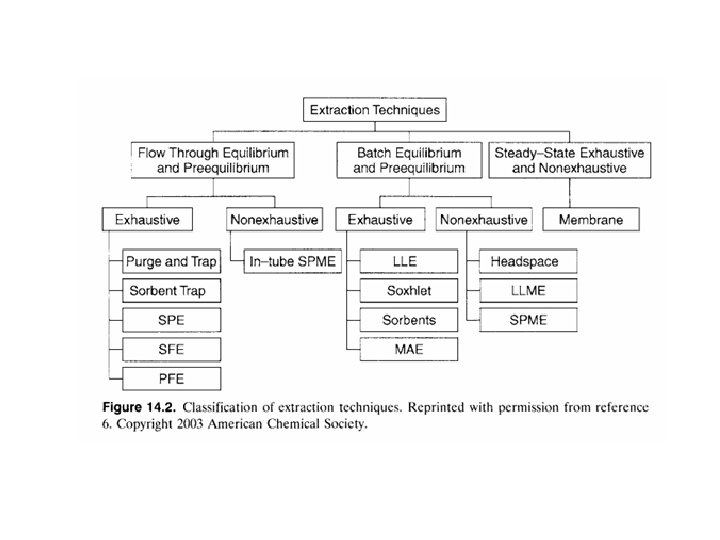
Separation methods 1 - Liquid-Liquid Extraction Focus on 2 - High-Performance Liquid Chromatography a) normal phase b) reversed phase Øion-pair c) ion exchange chromatography d) size exclusion chromatography (LLE) 3 - Supercritical fluid Extraction 4 - Solid Phase Extraction 5 - Capillary electrophoresis 6 - Capillary electrochromatography (SFE & SFC) (SPE & SPME) (CE, ZCE) (CEC) (HPLC) (NP) (RP) (IP-RP) (IEC) (SEC)

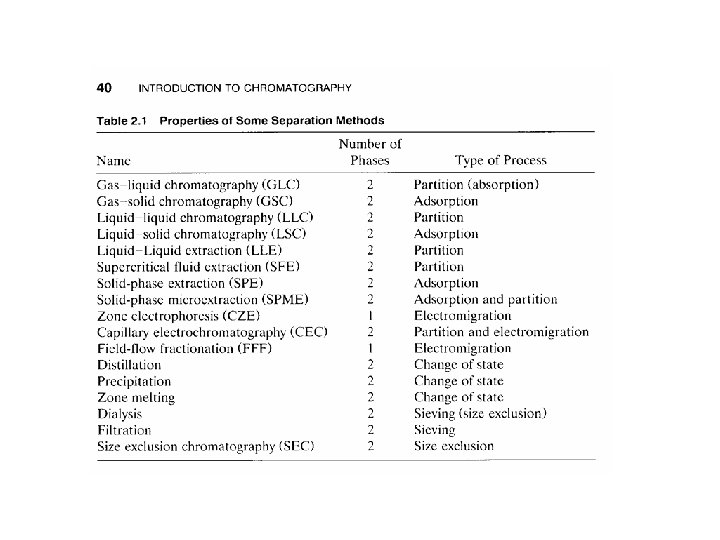
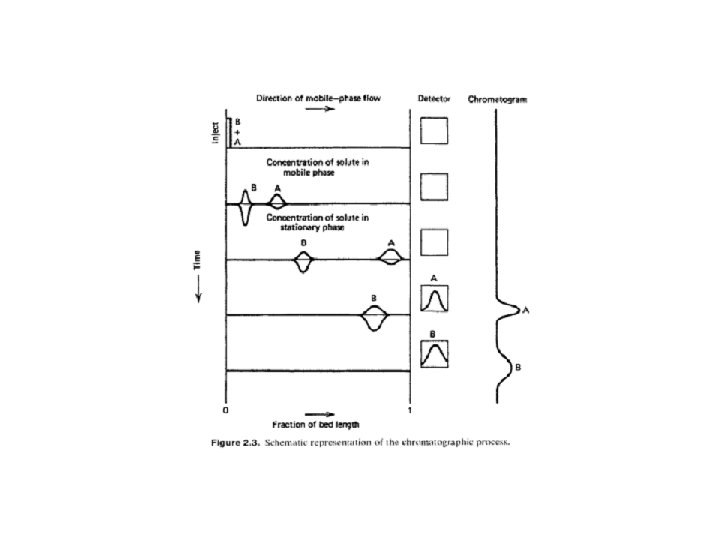
Ø Most separation methods employ two phases e. g. 1. Distillation vapor and liquid? ? ? 2. Liquid-liquid extraction extractant & raffinate 3. Chromatography mobile & stationary phase Ø The extractant in a multistage liquid-liquid extraction is similar to mobile phase in liquid chromatography Ø Chromatography mainly GC is thought of as similar to distillation Ø Applying the term theoretical plates to Gas Chromatography known for distillation

Ø Most chromatographic methods used to separate and quantify samples include additional steps in preparations of sample Ø These steps are referred to as preliminary treatments or sample clean up Ø More precisely are called preconcentration steps Ø These steps such as SPE & SPME are sometimes chromatographic in nature such as LSC Ø Some others such as LLE are separation steps similar to LLC. Ø Some stationary phases sometimes used for preconcentrations are made of material developed for GC or HPLC Ø Column chromatography, SPE and LLE are the most common methods of separations




Extraction Ø Classification of extraction techniques are as follows 1 - equilibrium versus preequibrium 2 - equilibrium versus steady state 3 - flow-through versus batch 4 - exhaustive versus non exhaustive Ø preequlibrium; contact between phases is broken before system reach equilibrium effectively nonequilibrium methods Ø steady state; refers to permeation technique such as membrane extraction operate by continuous steady state transport of analytes through membrane Ø flow-through; is a contact process as continuous (opposed to batch)


Comparison & Contrast of LLE vs. LLC



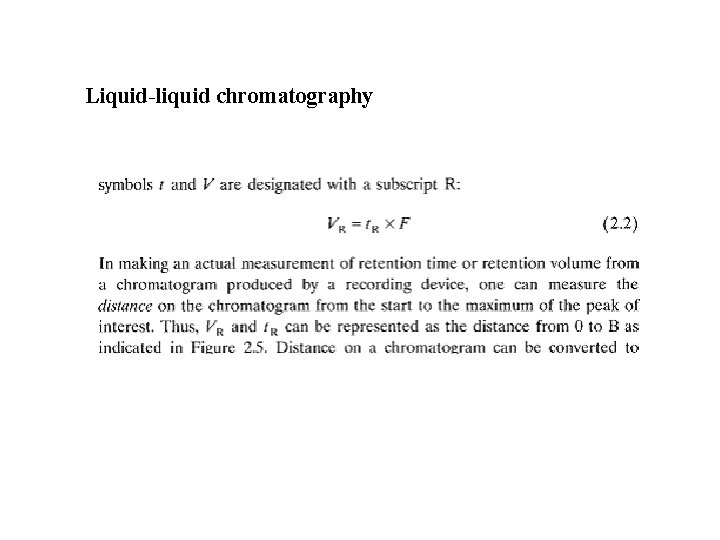
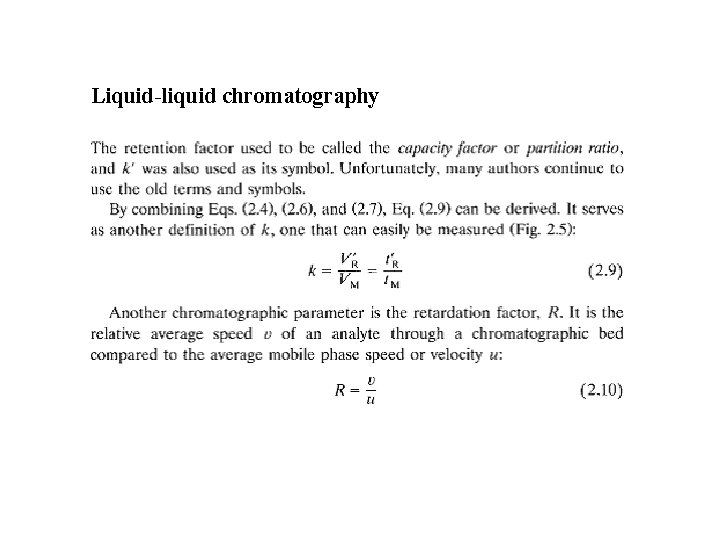
Liquid-liquid chromatography

Liquid-liquid chromatography

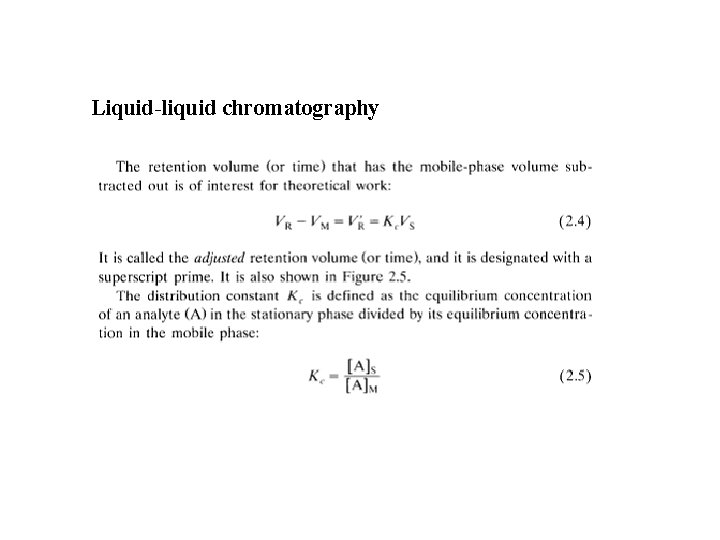
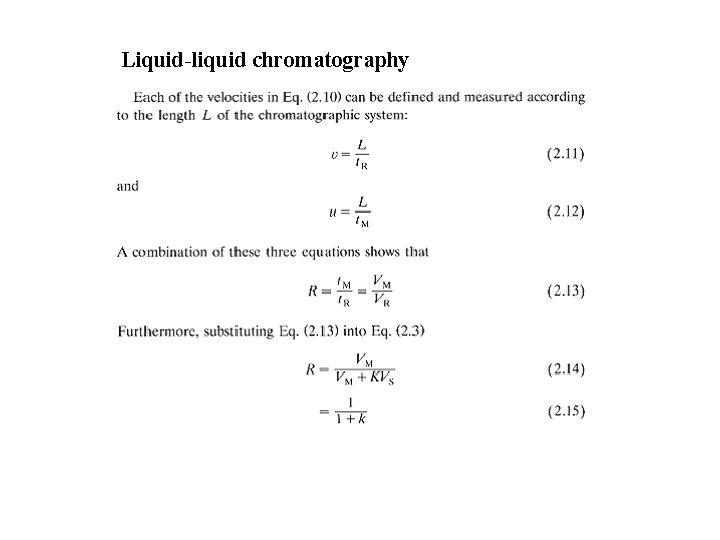
Liquid-liquid chromatography

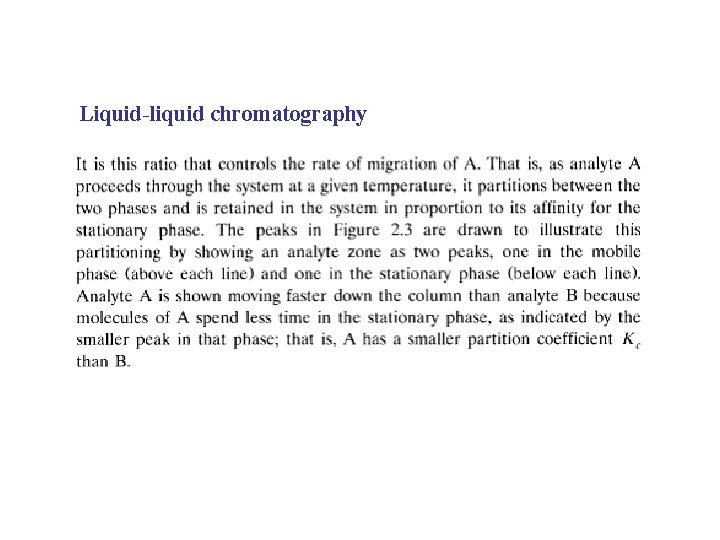
Liquid-liquid chromatography

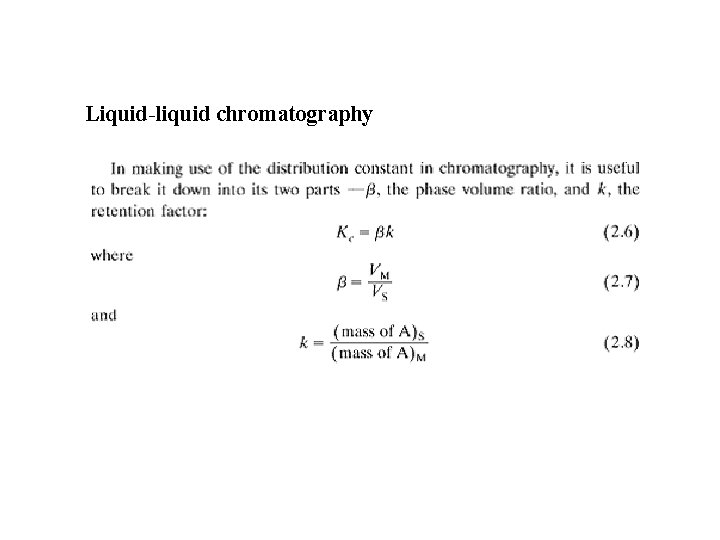
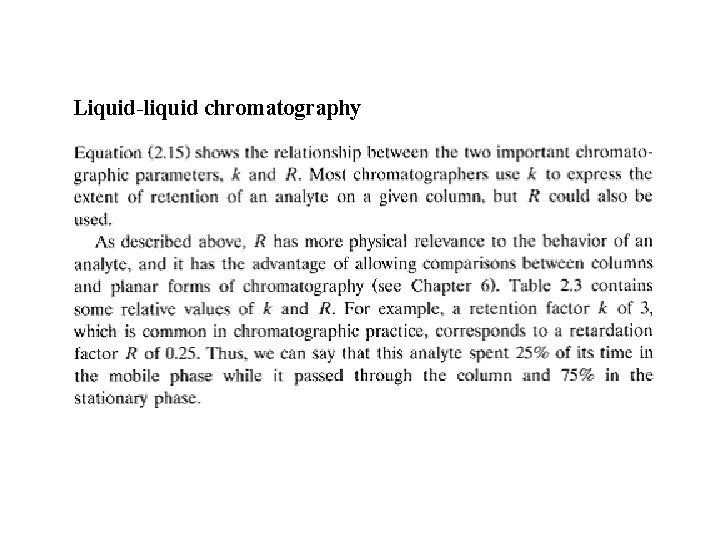
Liquid-liquid chromatography

Liquid-liquid chromatography

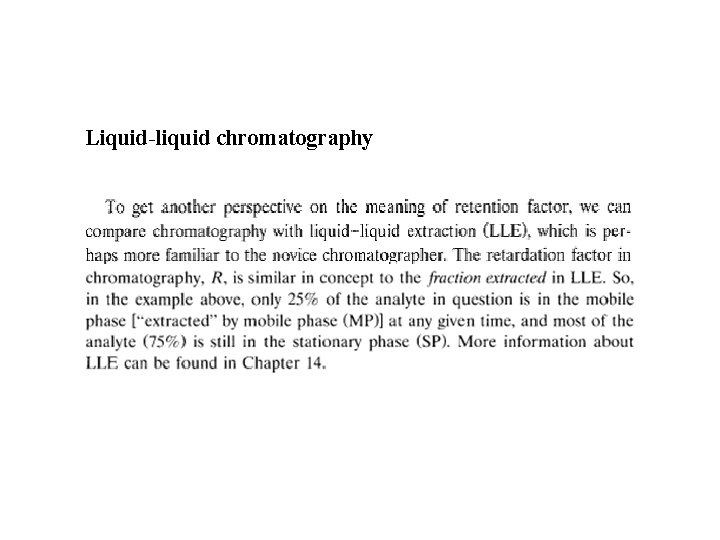
Liquid-liquid chromatography

Liquid-liquid chromatography

Liquid-liquid chromatography


![LLE One analyte component KP Araffinate Aextractant KP β k LLE One analyte component KP = [A]raffinate / [A]extractant KP = β * k](https://slidetodoc.com/presentation_image/9bfcc157c10bbc7e4c638c50d0c61eed/image-23.jpg)
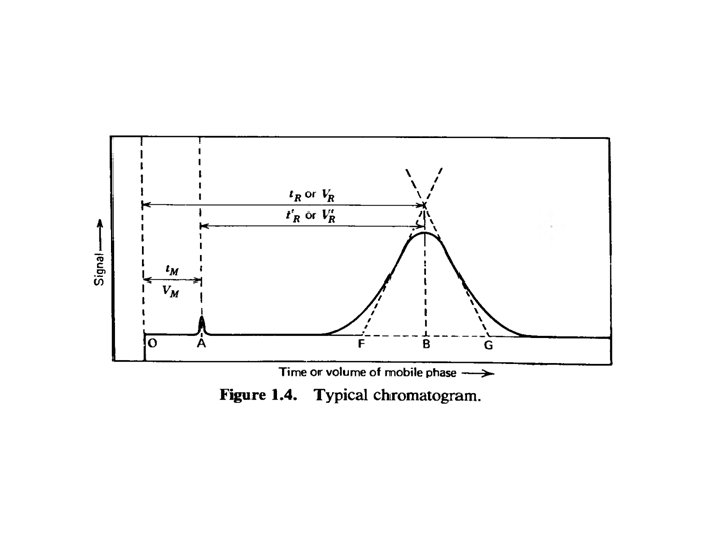
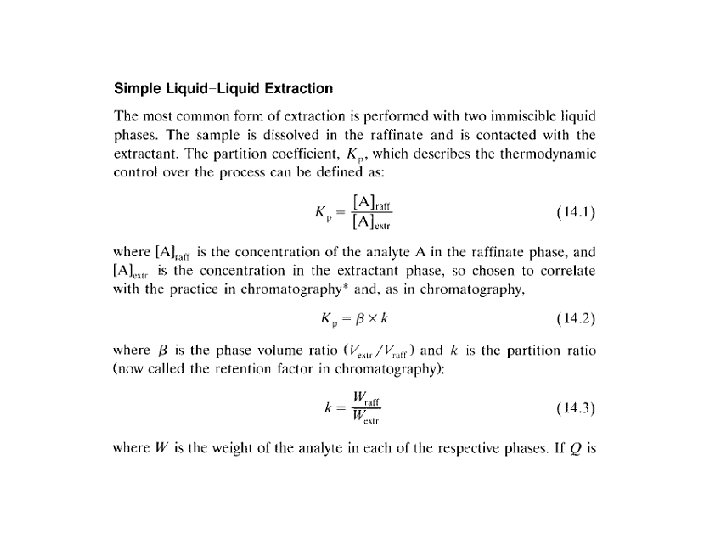
LLE One analyte component KP = [A]raffinate / [A]extractant KP = β * k vs. LLC KC = [A]S / [A]M KP = β * k (β phase volume ratio, k retention factor, capacity factor, partition factor) β = [Vextractant/Vraffinate] β = [VM/VS] k = (mass of A)raffinate / (mass of A)extractant k = (mass of A)S / (mass of A)M k = V’R / VM = t’R / t. M Q = fraction extracted = 1 / [1 + k] k = [1 -Q] / Q R = v / u (always ≤ 1) R Retardation factor, v average speed of analyte, u average speed of mobile phase v = L/t. R, u = L/t. M, R = t. M/ t. R = VM /VR R = VM / [VM + VR] = 1 / [1 + k]

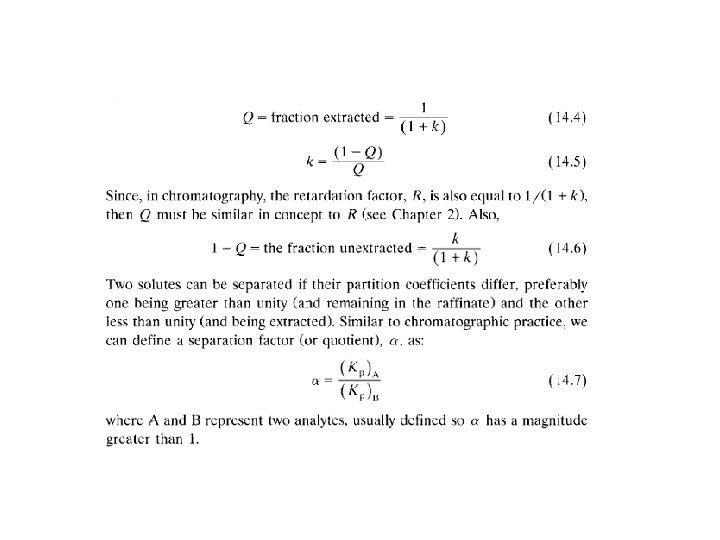
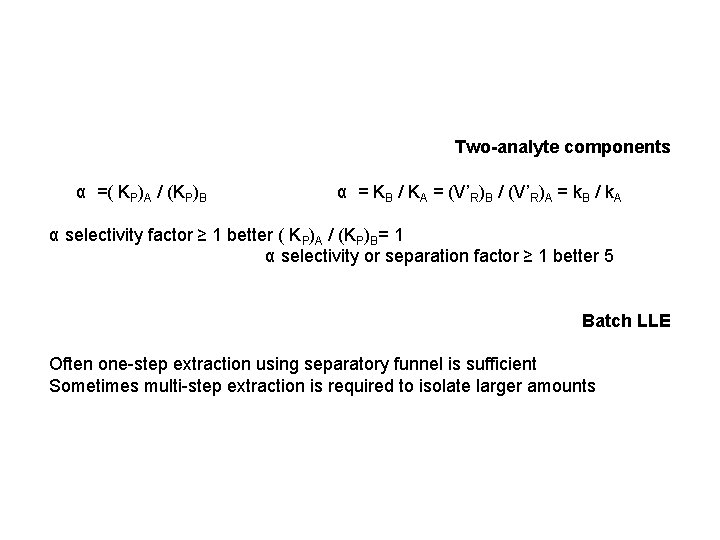
Two-analyte components α =( KP)A / (KP)B α = KB / KA = (V’R)B / (V’R)A = k. B / k. A α selectivity factor ≥ 1 better ( KP)A / (KP)B= 1 α selectivity or separation factor ≥ 1 better 5 Batch LLE Often one-step extraction using separatory funnel is sufficient Sometimes multi-step extraction is required to isolate larger amounts

Five consideration are involved in selecting the two liquid phases for extraction 1 - two phases must be immiscible 2 - phases should be saturated with each other before use to prevent change in volume 3 - the two phases must separate from each other quickly, forming no emulsion 4 - the recovery of the analyte from the extractant (or raffinate) 5 - the rate of the system to reach equilibrium