Chapter 23 An Introduction to Analytical Separations Partition













- Slides: 29

Chapter 23 An Introduction to Analytical Separations


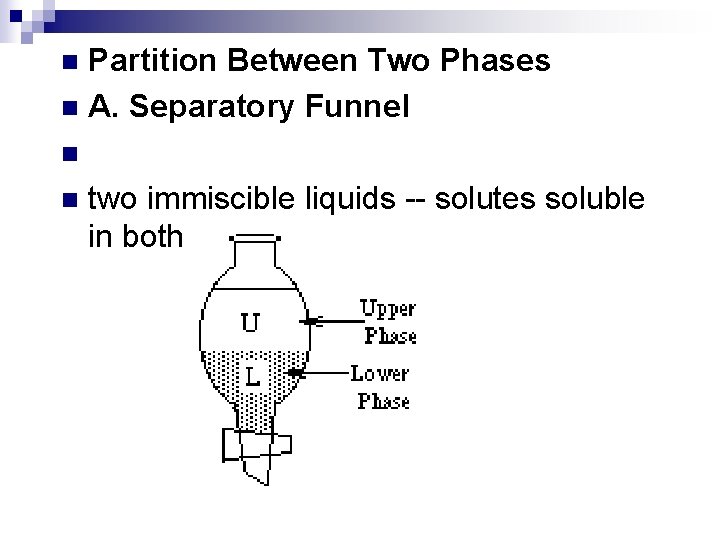
Partition Between Two Phases n A. Separatory Funnel n n n two immiscible liquids -- solutes soluble in both

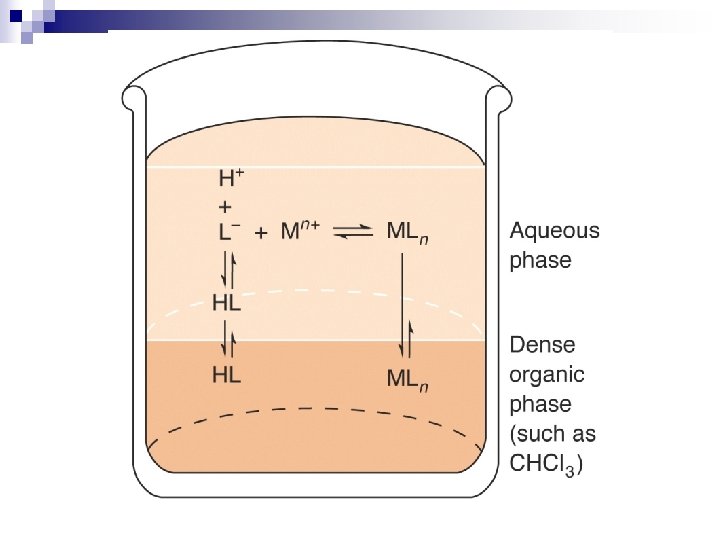
Partition Coefficient, K K = CU / CL after equilibration n K depends upon: ¨ solute -- polarity, ionization, etc. ¨ 2 liquid phases -- polarity etc. If K >> 1 or K << 1 for a particular solute ==> can purify the solute pretty well with a single extraction (or relatively few extractions). n But what if K is approximately equal to 1? n

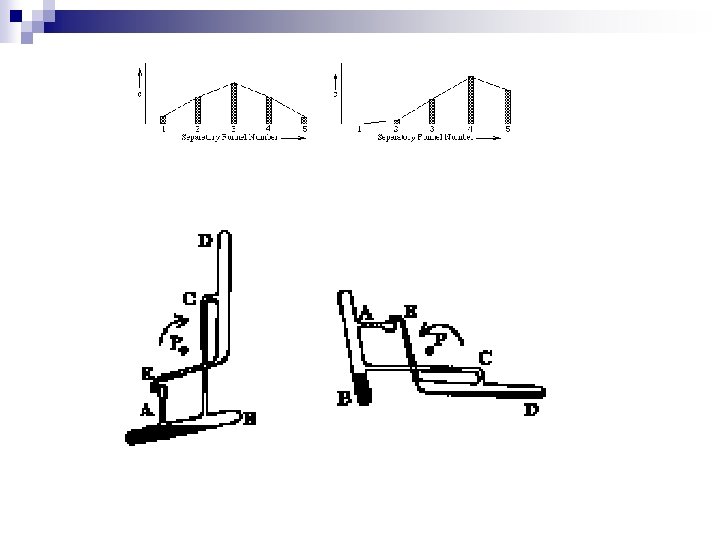
Let's look at two cases: (a) K = 1 (b) K = 3 n if K > 1 ==> more material in right most vessels --> peak moves to right quickly if K < 1 == > more material in leftmost vessels --> peak moves to right more slowly (In the next diagram, lower phase stays in place while the upper phase is transferred to the next vessel to the right with fresh lower phase. ) n

a. K=1 1/2 1/4 1/4 1/8 1/16 3/16 1/16 1/1 1/8 3/16 1/8 1/1

b. K=3 3/4 1/4 3/16 9/16 1/16 3/1 18/64 1/1 27/64 6/1 9/1 3/256 27/256 81/256 9/256 27/256 3/1024 36/1024 162/1024 324/1024 243/1024 12/1024 54/1024 108/1024 81/1024



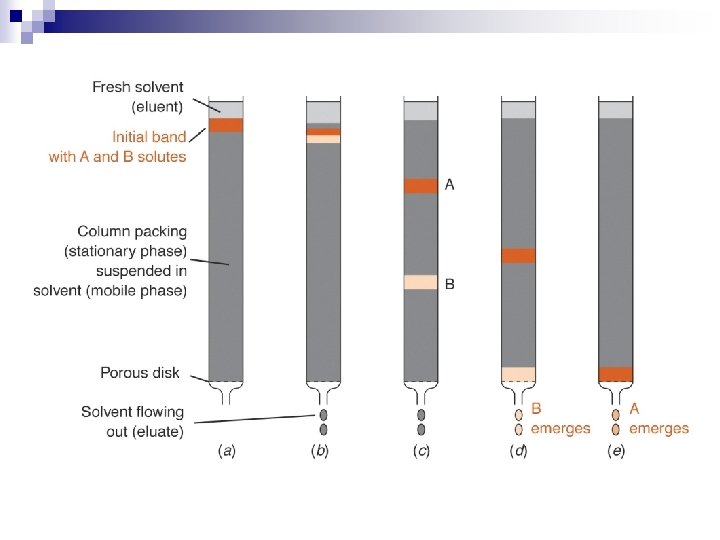
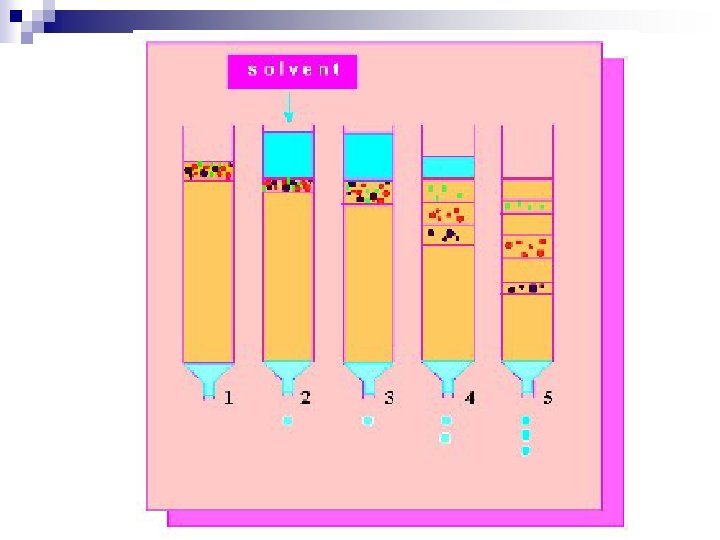
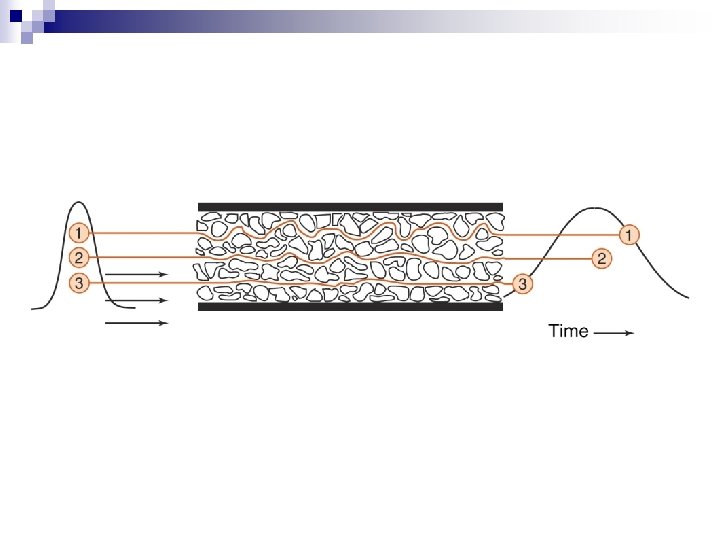
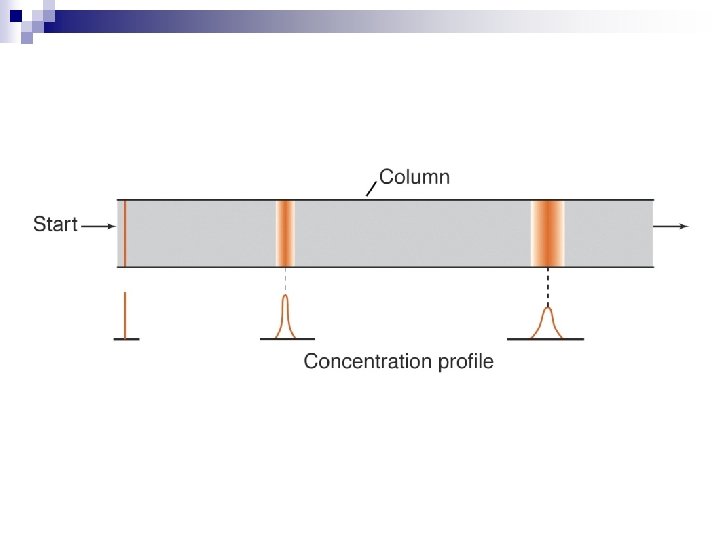
n General Principle - Differential Hold-up Method Column n Thin layer n Paper n Columns can be of various sizes on the benchtop, or smaller with smaller sized packing and used in an instrument. n Generally there is a MOVING PHASE and a STATIONARY PHASE n

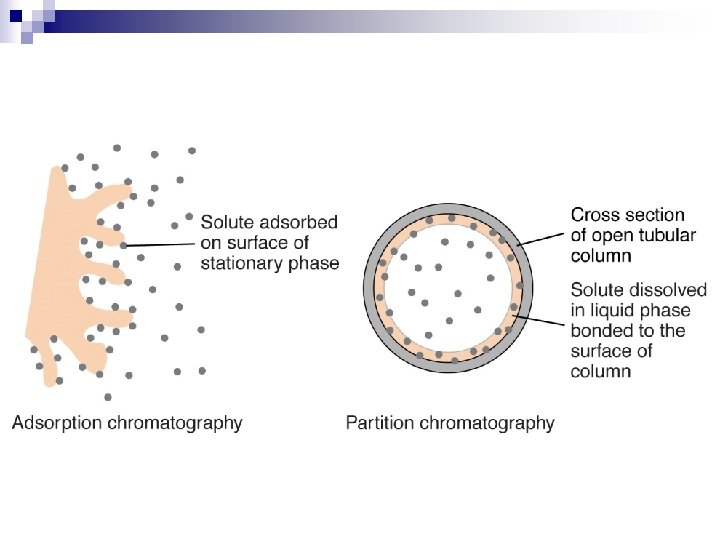
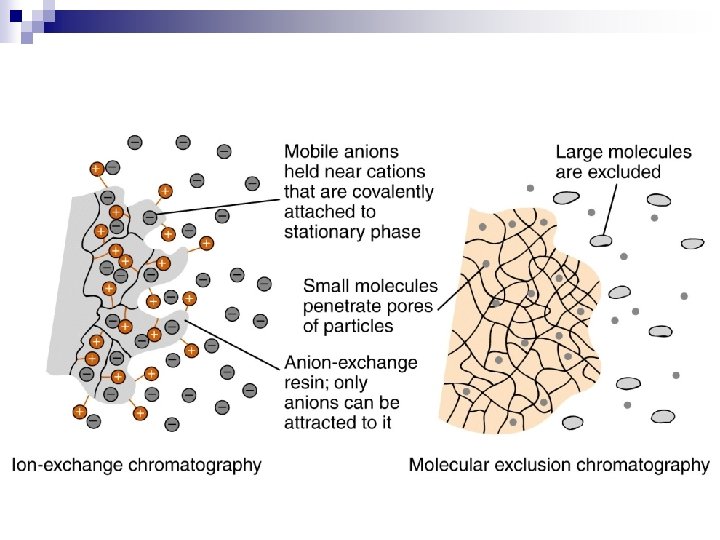
Principle (reason for hold-up) n n n Adsorption Partition Ion Exchange Size Exclusion Affinity




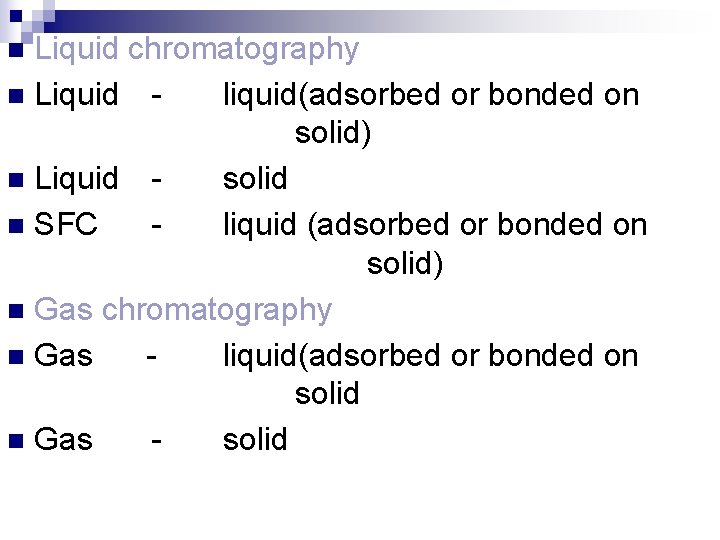
Liquid chromatography n Liquid liquid(adsorbed or bonded on solid) n Liquid solid n SFC liquid (adsorbed or bonded on solid) n Gas chromatography n Gas liquid(adsorbed or bonded on solid n Gas solid n

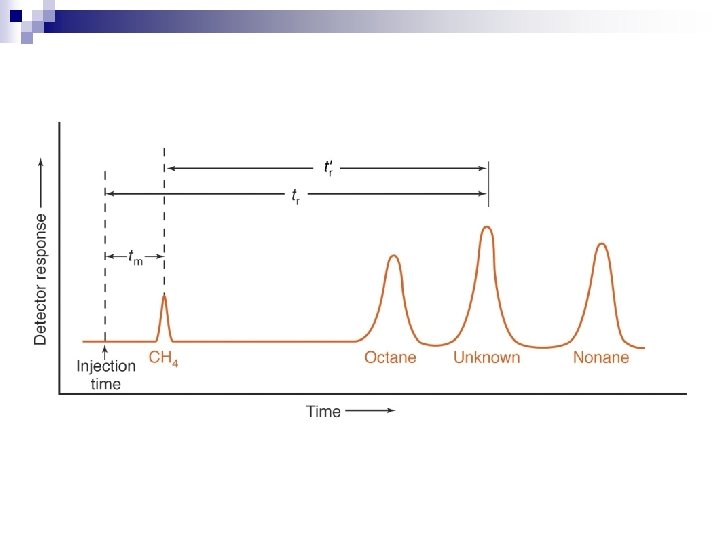
Chromatography is the separation - we must also have a detection method to follow the separation n Basic detectors - give a peak at a characteristic time for each component. n The most desirable, but more expensive detectors, in particular mass spectrometers, give a unique spectrum of each compound as it comes off, from which it can be identified. n

Ion Exchange Chromatography In ion exchange chromatography, charged substances are separated via column materials that carry an opposite charge. n The ionic groups of exchanger columns are covalently bound to the gel matrix and are compensated for by small concentrations of counter ions, which are present in the buffer. n When a sample is added to the column, an exchange with the weakly bound counter ions takes place. n

Resin Two basic types of ion exchangers: n those for binding positively charged ions or cations, which display on their surface negatively charged groups n and those for binding negatively charged ions or anions, which display on their surface positively charged groups.

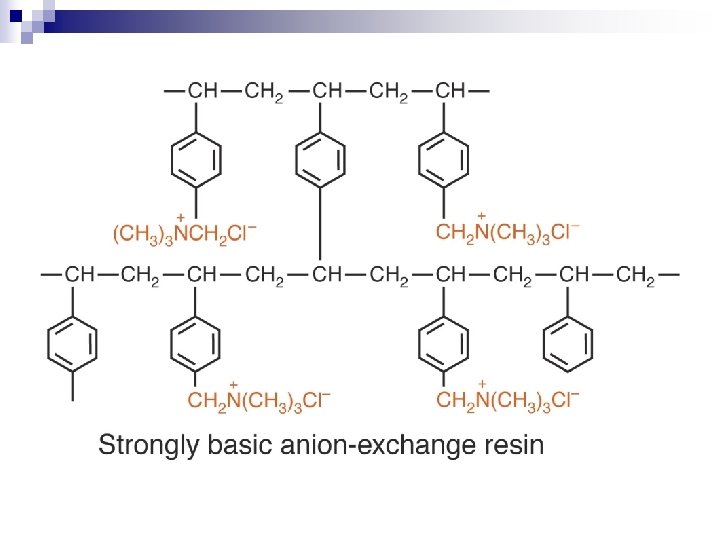
Polystyrene-divinylbenzene resin (PS-DVB) Most common polymer base for ion-exchange chromatography. Ionic groups are incorporated by chemical reactions. Porosity and mechanical stability are altered by varying the cross-linking through the variation of the DVB content. n Low crosslinking – resin swells a lot, changes size with different ions. n High crosslinking – solubility decreases - selectivity increases n

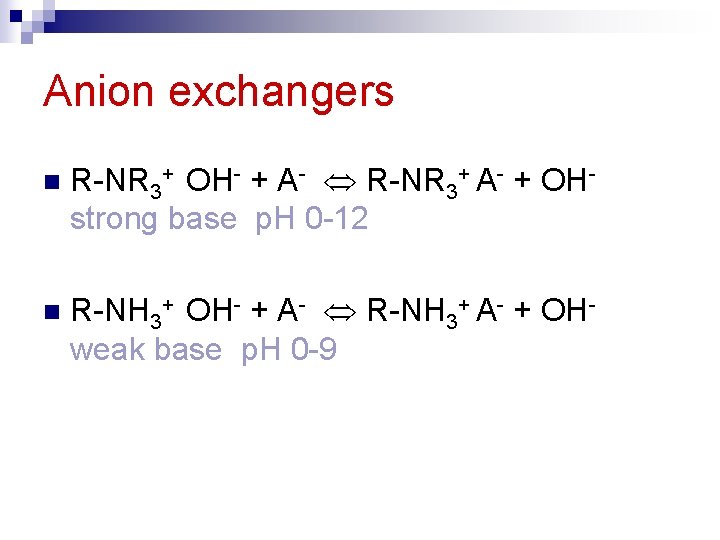
Anion exchangers n R-NR 3+ OH- + A- R-NR 3+ A- + OHstrong base p. H 0 -12 n R-NH 3+ OH- + A- R-NH 3+ A- + OHweak base p. H 0 -9


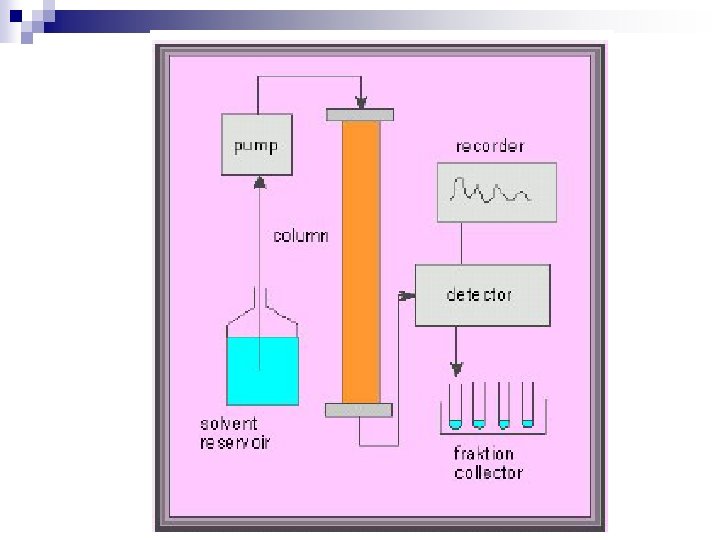
Apparatus column n support n stationary phase n mobile phase n detector n

Column copper tubing n stainless steel tubing n glass tubing n Polymer (plastic) n Support n finely divided solids ¨ ground firebrick ¨ alumina, specially treated n walls of column for capillary columns

Stationary Phase n stationary phase evenly dispersed on surface of support Mobile Phase n n n sample mixture carried through stationary phase by mobile phase non-reactive gas in glc (gas-liquid chromatography, gc) non-reactive liquid in llc (liquid-liquid chromatography, lc)




