Organic solvent extraction Liquidliquid extraction is a useful












- Slides: 19

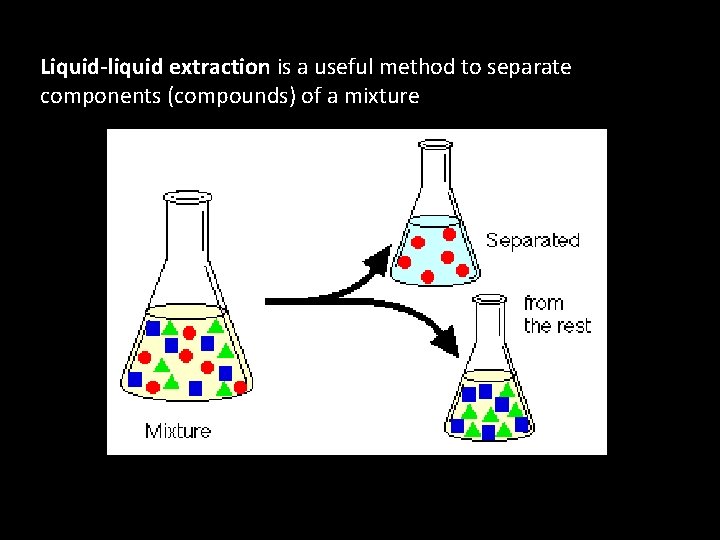
Organic solvent extraction

Liquid-liquid extraction is a useful method to separate components (compounds) of a mixture

Suppose that you have a mixture of sugar in vegetable oil (it tastes sweet!) and you want to separate the sugar from the oil. You observe that the sugar particles are too tiny to filter and you suspect that the sugar is partially dissolved in the vegetable oil.

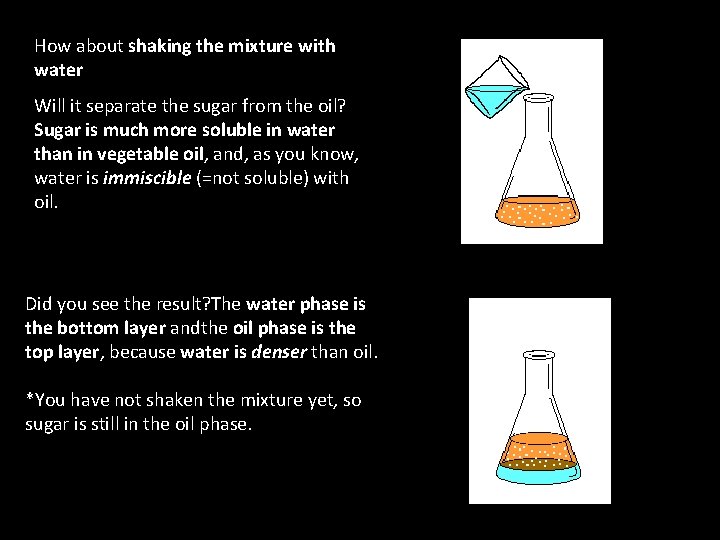
How about shaking the mixture with water Will it separate the sugar from the oil? Sugar is much more soluble in water than in vegetable oil, and, as you know, water is immiscible (=not soluble) with oil. Did you see the result? The water phase is the bottom layer andthe oil phase is the top layer, because water is denser than oil. *You have not shaken the mixture yet, so sugar is still in the oil phase.

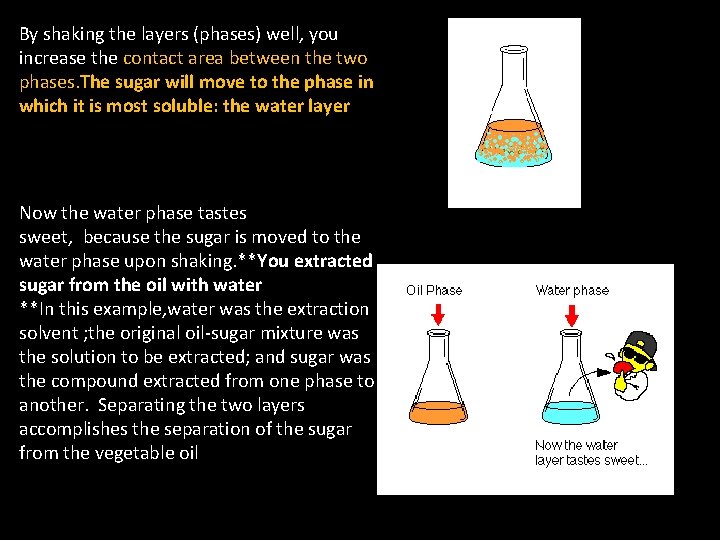
By shaking the layers (phases) well, you increase the contact area between the two phases. The sugar will move to the phase in which it is most soluble: the water layer Now the water phase tastes sweet, because the sugar is moved to the water phase upon shaking. **You extracted sugar from the oil with water **In this example, water was the extraction solvent ; the original oil-sugar mixture was the solution to be extracted; and sugar was the compound extracted from one phase to another. Separating the two layers accomplishes the separation of the sugar from the vegetable oil

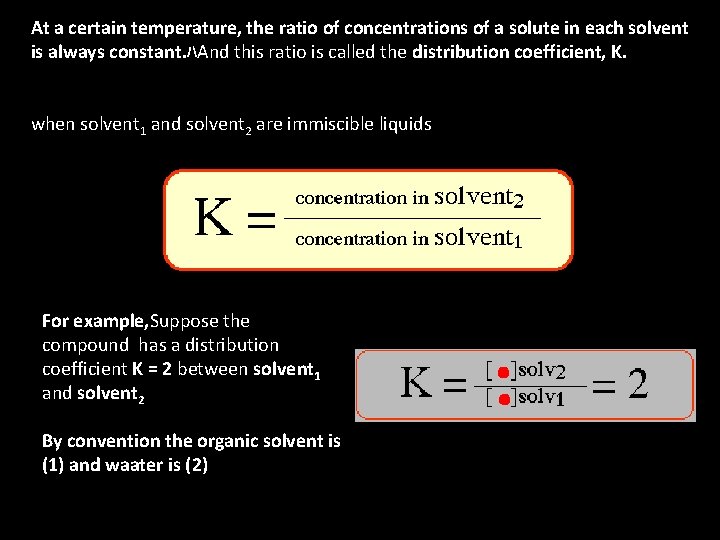
Liquid-liquid extraction is based on the transfer of a solute substance from one liquid phase into another liquid phase according to the solubility. Extraction becomes a very useful tool if you choose a suitable extraction solvent. You can use extraction to separate a substance selectively from a mixture, or to remove unwanted impurities from a solution. In the practical use, usually one phase is a water or water-based (aqueous) solution and the other an organic solvent which is immiscible with water. The success of this method depends upon the difference in solubility of a compound in various solvents. For a given compound, solubility differences between solvents is quantified as the "distribution coefficient"

At a certain temperature, the ratio of concentrations of a solute in each solvent is always constant. ハAnd this ratio is called the distribution coefficient, K. when solvent 1 and solvent 2 are immiscible liquids For example, Suppose the compound has a distribution coefficient K = 2 between solvent 1 and solvent 2 By convention the organic solvent is (1) and waater is (2)

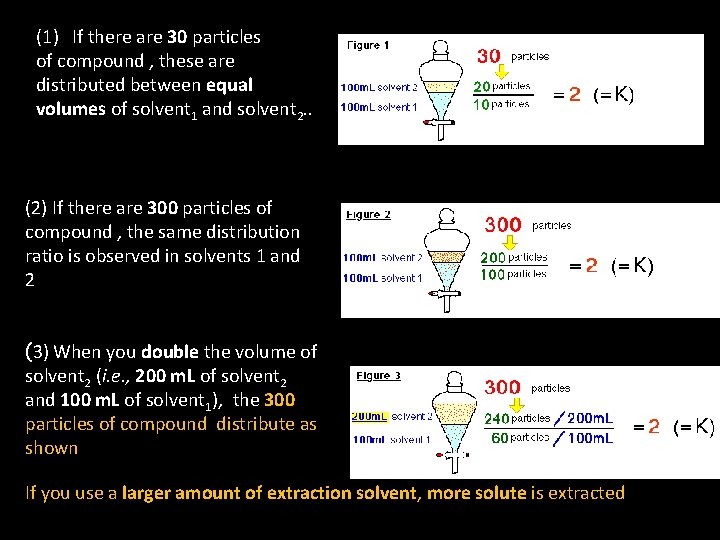
(1) If there are 30 particles of compound , these are distributed between equal volumes of solvent 1 and solvent 2. . (2) If there are 300 particles of compound , the same distribution ratio is observed in solvents 1 and 2 (3) When you double the volume of solvent 2 (i. e. , 200 m. L of solvent 2 and 100 m. L of solvent 1), the 300 particles of compound distribute as shown If you use a larger amount of extraction solvent, more solute is extracted

Liquid-Liquid Extraction • LLE is a separation technique based upon transfer of a solute between two immiscible liquids. • The key principle underlying LLE is often that the solubility of the solute differs significantly between the liquids, providing a thermodynamic driving force for transfer from one phase to the other. • Any two immiscible liquids may be used, but it is common to use water and an organic solvent. • In a small-scale chemistry laboratory, batch LLE is often performed using a separatory funnel:

The funnel is shaken vigorously, while taking care to vent any gases or vapors formed. The shaking enhances the surface area of contact between the phases, while the agitation enhances mass transfer from one phase to the other by providing convection. The shaken mixture is allowed to stand until the two phases separate into layers (hopefully without forming an emulsion!)

Care is taken to recover the extract phase, which is thought to contain a high concentration of the solute. The extract phase may be either the top or the bottom phase! The extraction may be repeated by shaking the remaining raffinate phase with more solvent two or three additional times until most of the solute is extracted. The raffinate phase is usually discarded at this point. After possible additional washing or drying steps, the solute is generally recovered by distillation of the solvent, or by crystallization from solution followed by filtration, to yield a concentrated solute.

An example of a lab-scale LLE procedure Suppose we wish to extract caffeine (a solid) from a cup of tea (mostly water) to determine the caffeine content of the beverage. We might follow a procedure like this: 1) The (cooled) cup of tea is shaken with methylene chloride (CH 2 Cl 2), an organic solvent, in a separatory funnel. 2) Much of the caffeine goes into the CH 2 Cl 2 phase, but other organic compounds (e. g. proteins) tend to remain in the water phase. 3) The CH 2 Cl 2 phase (extract) is recovered and saved. Needle-like caffeine crystals

4) The extraction of the raffinate (water) phase is done two more times, and the CH 2 Cl 2 phase is recovered and saved each time. 5) The CH 2 Cl 2 phase is dried over anhydrous sodium sulfate three times to remove residual H 2 O. 6) The solvent is removed by rotary evaporation, and the caffeine crystals (and possibly other compounds) are recovered and dried in an oven.

Acetone precipitation of proteins • A strategy for removing undesirable substances is to add a compound that causes protein to precipitate. • After centrifugation the pellet contains the precipitated protein, the supernatant containing the interfering substance is removed and the protein pellet is re-dissolved in buffer

• Precipitation has an advantage over dialysis or desalting methods in that it enables concentration of the protein sample as well as purification from undesirable substances. • One disadvantage of protein precipitation is that proteins may be denatured

Materials Required • Cold (-20°C) acetone, a volume four times that of the protein samples to be precipitated • Centrifuge tube, made of acetone-compatible material such as polypropylene and able to hold five times the sample volume • Centrifuge and rotor for the tubes used, minimum 13, 000 x g required

Protocol • 1. Cool the required volume of acetone to -20°C. • 2. Place protein sample in acetone-compatible tube. • 3. Add four times the sample volume of cold (-20°C) acetone to the tube. • 4. Vortex tube and incubate for 60 minutes at -20°C. • 5. Centrifuge 10 minutes at 13, 000 -15, 000 x g. • 6. Decant and properly dispose of the supernatant, being careful to not dislodge the protein pellet.


Precipitation of protein using TCA/acetone Precipitation with a concentrated acid such as Trichloroacetic acid (TCA) causes extremely low p. H resulting in precipitation. To minimize the risk of acid hydrolysis of peptide bonds the experiment should be performed on ice and care should be taken to remove residual acid by washing the protein pellet with acetone.