Lecture 9 Electrical conductivity of electrolytes solutions Prepared


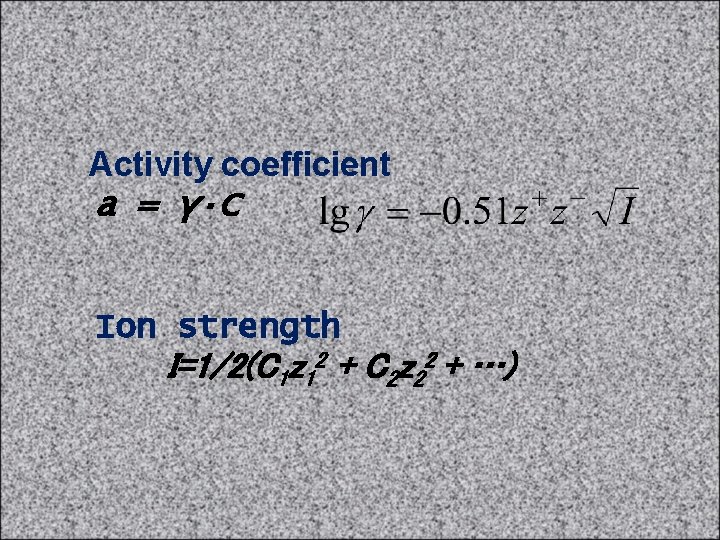


![Measurement of p. H (cont. ) • Ecell = E°cell - (0. 0591)log[H+] + Measurement of p. H (cont. ) • Ecell = E°cell - (0. 0591)log[H+] +](https://slidetodoc.com/presentation_image_h2/53bef5d85434bd051308d7cc6c8d2ff5/image-25.jpg)


![Concentration and Ecell (cont. ) • • If [Cu 2+] = 0. 3 M, Concentration and Ecell (cont. ) • • If [Cu 2+] = 0. 3 M,](https://slidetodoc.com/presentation_image_h2/53bef5d85434bd051308d7cc6c8d2ff5/image-33.jpg)

- Slides: 46

Lecture 9. Electrical conductivity of electrolyte’s solutions Prepared by Ph. D Halina Falfushynska

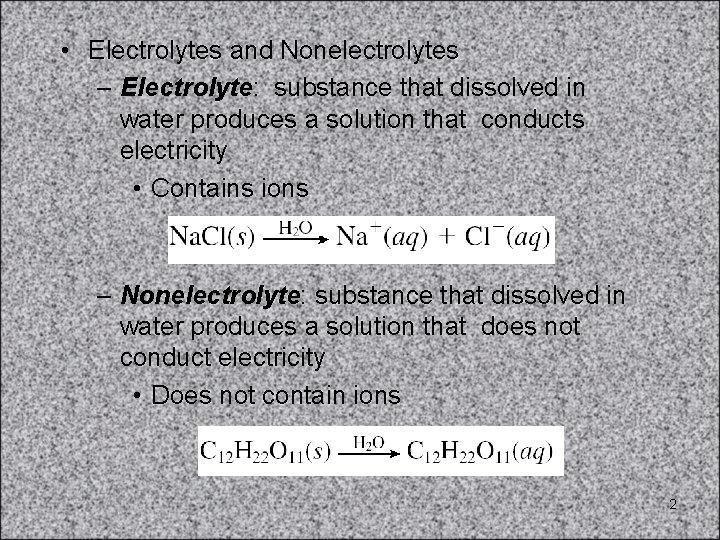
• Electrolytes and Nonelectrolytes – Electrolyte: substance that dissolved in water produces a solution that conducts electricity • Contains ions – Nonelectrolyte: substance that dissolved in water produces a solution that does not conduct electricity • Does not contain ions 2

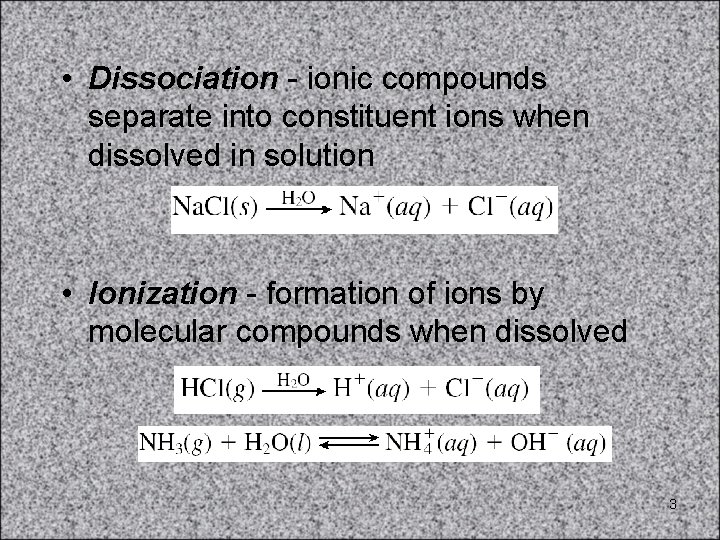
• Dissociation - ionic compounds separate into constituent ions when dissolved in solution • Ionization - formation of ions by molecular compounds when dissolved 3

• Strong and weak electrolytes – Strong Electrolyte: 100% dissociation • All water soluble ionic compounds, strong acids and strong bases – Weak electrolytes • Partially ionized in solution • Exist mostly as the molecular form in solution • Weak acids and weak bases 4

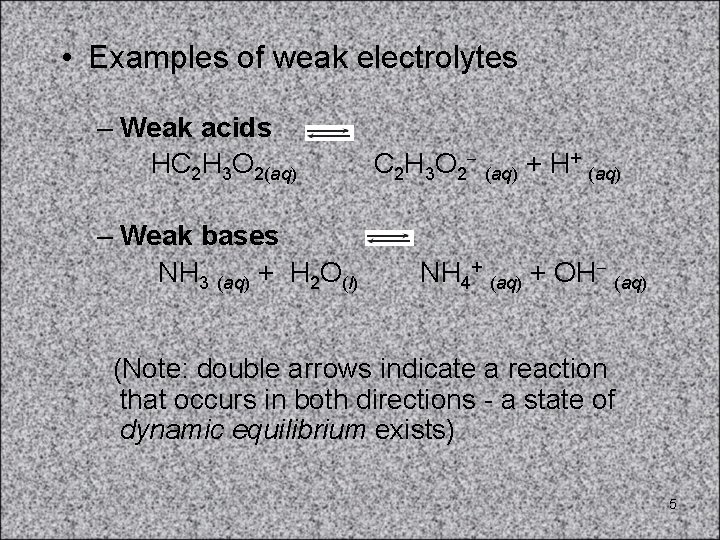
• Examples of weak electrolytes – Weak acids HC 2 H 3 O 2(aq) – Weak bases NH 3 (aq) + H 2 O(l) C 2 H 3 O 2 (aq) + H+ (aq) NH 4+ (aq) + OH (aq) (Note: double arrows indicate a reaction that occurs in both directions - a state of dynamic equilibrium exists) 5

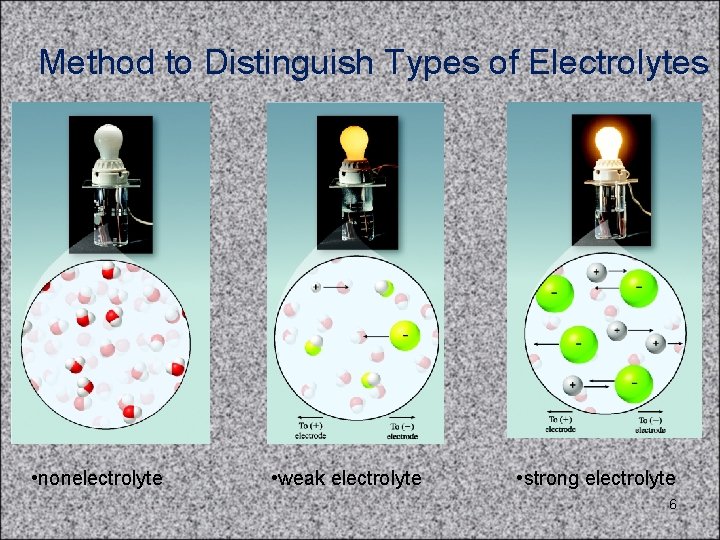
Method to Distinguish Types of Electrolytes • nonelectrolyte • weak electrolyte • strong electrolyte 6

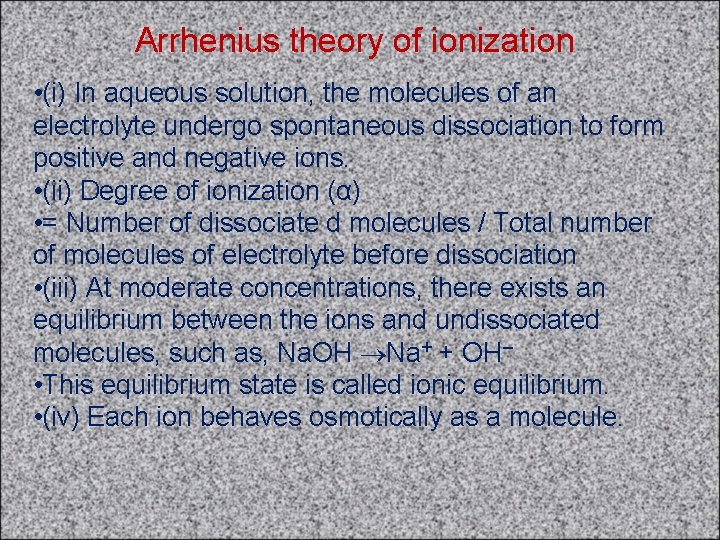
Arrhenius theory of ionization • (i) In aqueous solution, the molecules of an electrolyte undergo spontaneous dissociation to form positive and negative ions. • (ii) Degree of ionization (α) • = Number of dissociate d molecules / Total number of molecules of electrolyte before dissociation • (iii) At moderate concentrations, there exists an equilibrium between the ions and undissociated molecules, such as, Na. OH Na+ + OH– • This equilibrium state is called ionic equilibrium. • (iv) Each ion behaves osmotically as a molecule.

FACTORS AFFECTING DEGREE OF IONIZATION • (i) At normal dilution, value of α is nearly 1 for strong electrolytes, while it is very less than 1 for weak electrolytes. • (ii) Higher the dielectric constant of a solvent more is its ionising power. Water is the most powerful ionising solvent as its dielectric constant is highest. • (iii) α ∝ 1/Con. of solution ∝ 1/wt. of solution • ∝ Dilution of solution ∝ Amount of solvent • (iv) Degree of ionisation of an electrolyte in solution increases with rise in temperature. • (v) Presence of common ion: The degree of ionisation of an electrolyte decreases in the presence of a strong electrolyte having a common ion.

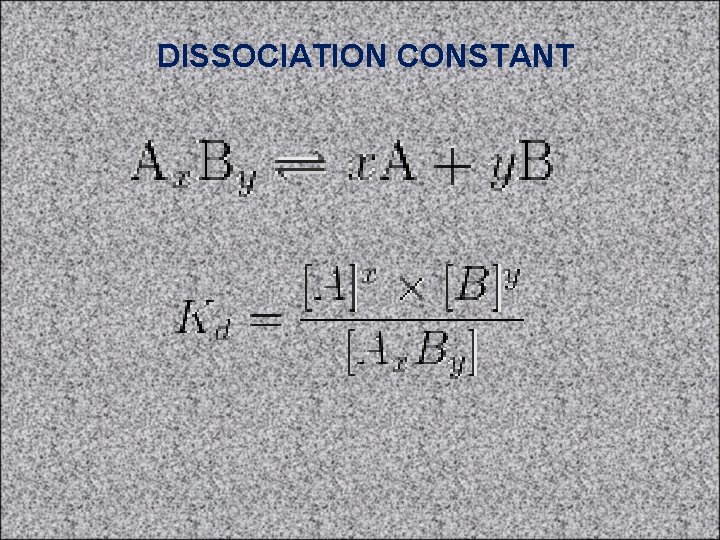
DISSOCIATION CONSTANT

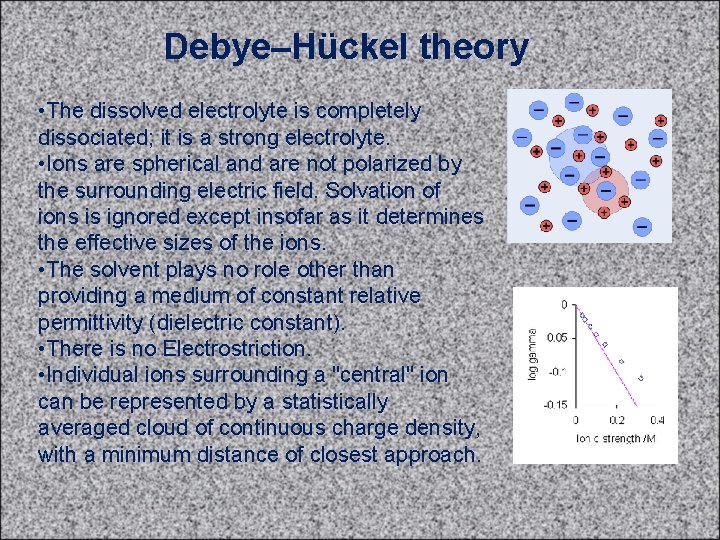
Debye–Hückel theory • The dissolved electrolyte is completely dissociated; it is a strong electrolyte. • Ions are spherical and are not polarized by the surrounding electric field. Solvation of ions is ignored except insofar as it determines the effective sizes of the ions. • The solvent plays no role other than providing a medium of constant relative permittivity (dielectric constant). • There is no Electrostriction. • Individual ions surrounding a "central" ion can be represented by a statistically averaged cloud of continuous charge density, with a minimum distance of closest approach.
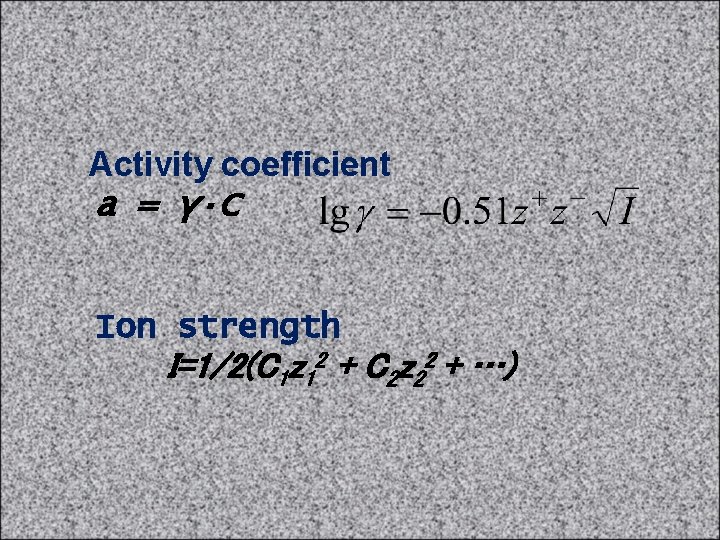
Activity coefficient а = γ·С Ion strength I=1/2(C 1 z 12 + C 2 z 22 + …)

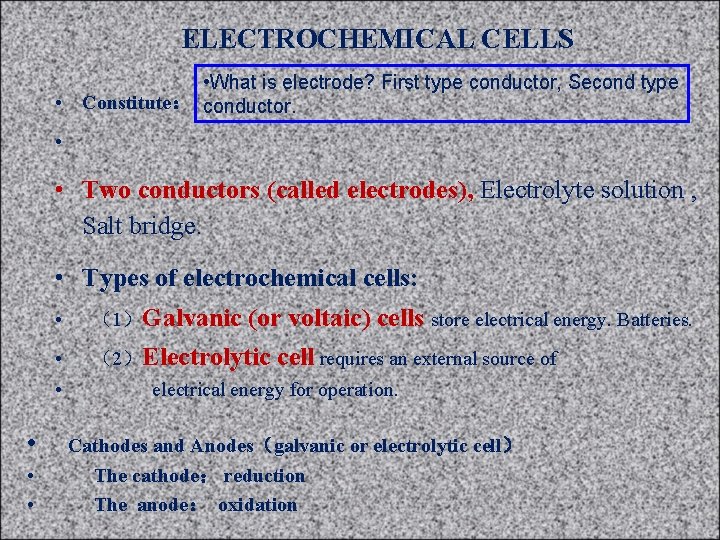
ELECTROCHEMICAL CELLS • What is electrode? First type conductor, Second type • Constitute: conductor. • • Two conductors (called electrodes), Electrolyte solution , Salt bridge. • Types of electrochemical cells: • • • (1)Galvanic (or voltaic) cells store electrical energy. Batteries. (2)Electrolytic cell requires an external source of electrical energy for operation. Cathodes and Anodes(galvanic or electrolytic cell) The cathode: reduction The anode: oxidation

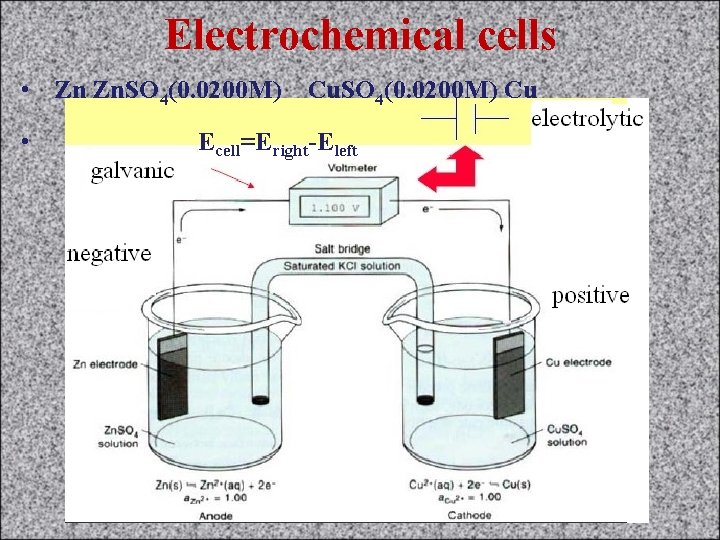
Electrochemical cells • Zn Zn. SO 4(0. 0200 M) Cu • Ecell=Eright-Eleft

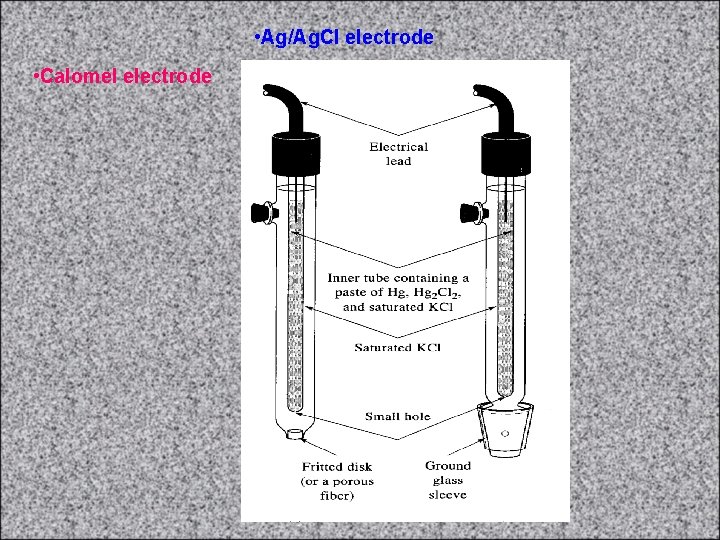
• Ag/Ag. Cl electrode • Calomel electrode

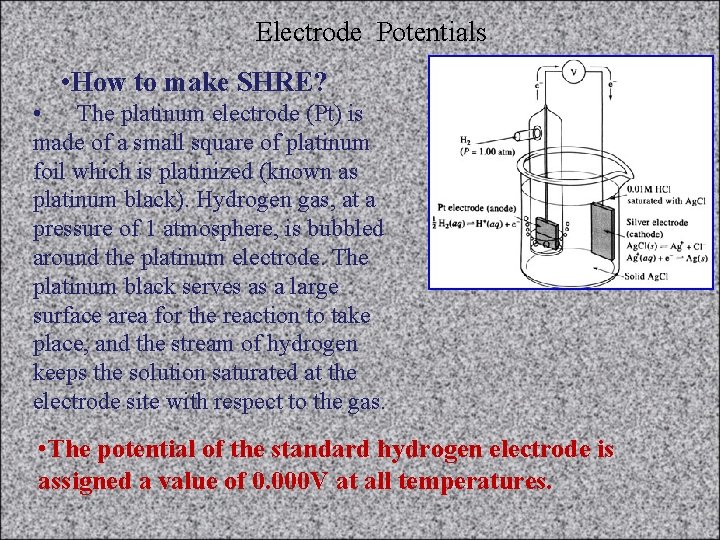
ELECTRODE POTENTIALS • The Standard Hydrogen Reference Electrode (SHE) The Standard Hydrogen Electrode (abbreviated SHE) is the universal reference for reporting relative half-cell potentials. It is a type of gas electrode and was widely used in early studies as a reference electrode. The SHE is also called the “ Normal Hydrogen Electrode ” • What is Reference Electrode ( RE)? • To provide the reference • standard for measuring • potential. • Figure 6 -3

Electrode Potentials • How to make SHRE? • The platinum electrode (Pt) is made of a small square of platinum foil which is platinized (known as platinum black). Hydrogen gas, at a pressure of 1 atmosphere, is bubbled around the platinum electrode. The platinum black serves as a large surface area for the reaction to take place, and the stream of hydrogen keeps the solution saturated at the electrode site with respect to the gas. • The potential of the standard hydrogen electrode is assigned a value of 0. 000 V at all temperatures.

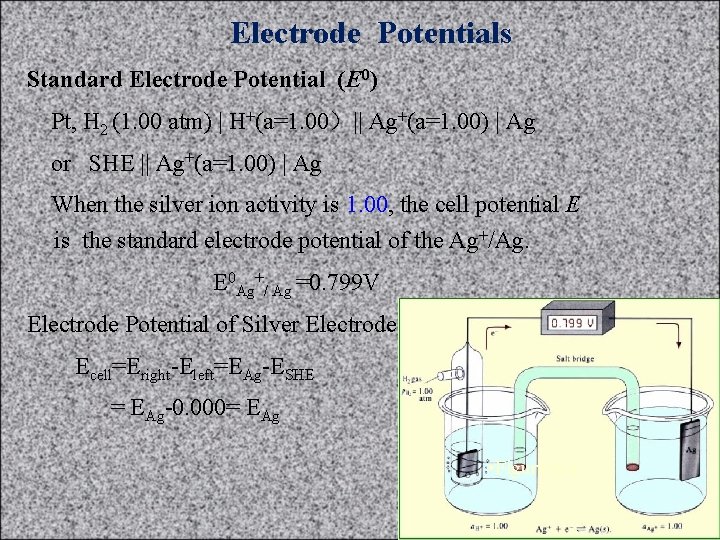
Electrode Potentials Standard Electrode Potential (E 0) Pt, H 2 (1. 00 atm) | H+(a=1. 00)|| Ag+(a=1. 00) | Ag or SHE || Ag+(a=1. 00) | Ag When the silver ion activity is 1. 00, the cell potential E is the standard electrode potential of the Ag+/Ag. E 0 Ag+/ Ag =0. 799 V Electrode Potential of Silver Electrode: Ecell=Eright-Eleft=EAg-ESHE = EAg-0. 000= EAg • Figure 6 -4

Electrochemical methods electrodes • Electrodes are conductors in contact with an electrolyte. It would be better to speak about half-cells, because they are “halves” of galvanic cells. We already know that certain (equilibrium) voltage arises on them. • Electrodes of the 1 st kind: exchange of ions and electrons between the solution and the electrode takes place. They can be cationic (metallic or gaseous hydrogen electrode) with equilibrium between neutral atoms and cations released into solution. Anionic electrodes are possible too. A typical electrode of the 1 st kind is the copper electrode immersed in a solution of Cu 2+ ions. • Electrodes of 2 nd kind consist of three parts. The metal is covered by a layer of its poorly soluble salt or hydroxide, and immersed into an electrolyte containing the same anion as the salt or hydroxide. Example: calomel electrode (Hg/Hg 2 Cl 2/KCl) and silver chloride electrode (Ag / Ag. Cl / KCl).

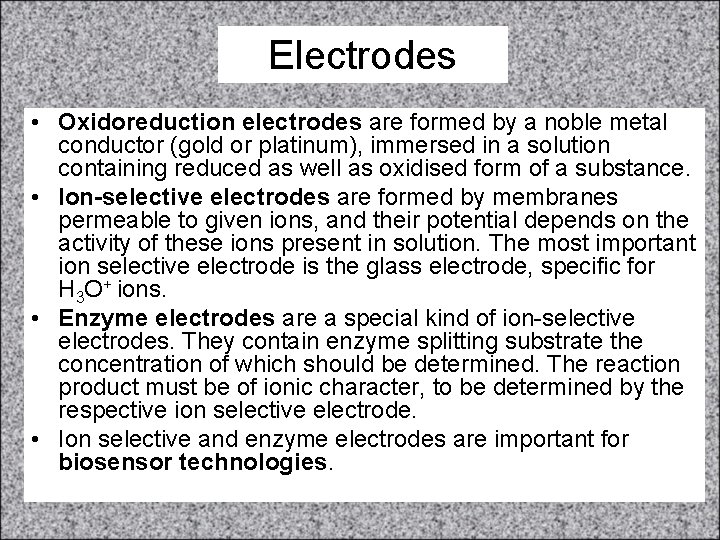
Electrodes • Oxidoreduction electrodes are formed by a noble metal conductor (gold or platinum), immersed in a solution containing reduced as well as oxidised form of a substance. • Ion-selective electrodes are formed by membranes permeable to given ions, and their potential depends on the activity of these ions present in solution. The most important ion selective electrode is the glass electrode, specific for H 3 O+ ions. • Enzyme electrodes are a special kind of ion-selective electrodes. They contain enzyme splitting substrate the concentration of which should be determined. The reaction product must be of ionic character, to be determined by the respective ion selective electrode. • Ion selective and enzyme electrodes are important for biosensor technologies.

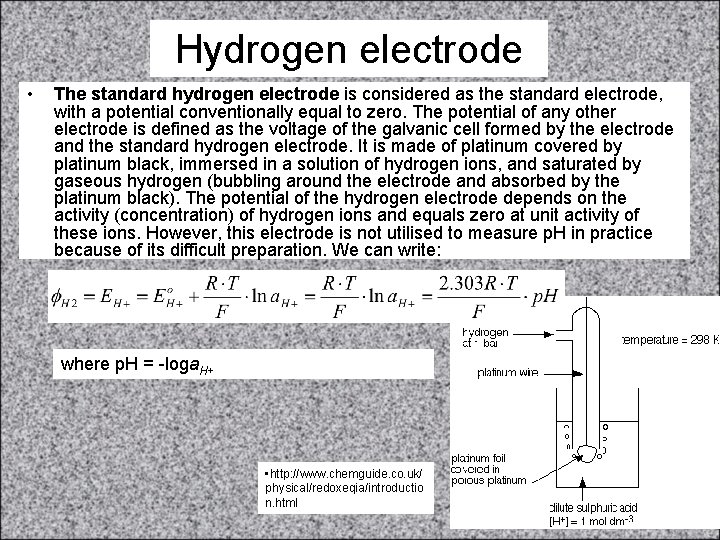
Hydrogen electrode • The standard hydrogen electrode is considered as the standard electrode, with a potential conventionally equal to zero. The potential of any other electrode is defined as the voltage of the galvanic cell formed by the electrode and the standard hydrogen electrode. It is made of platinum covered by platinum black, immersed in a solution of hydrogen ions, and saturated by gaseous hydrogen (bubbling around the electrode and absorbed by the platinum black). The potential of the hydrogen electrode depends on the activity (concentration) of hydrogen ions and equals zero at unit activity of these ions. However, this electrode is not utilised to measure p. H in practice because of its difficult preparation. We can write: where p. H = -loga. H+ • http: //www. chemguide. co. uk/ physical/redoxeqia/introductio n. html

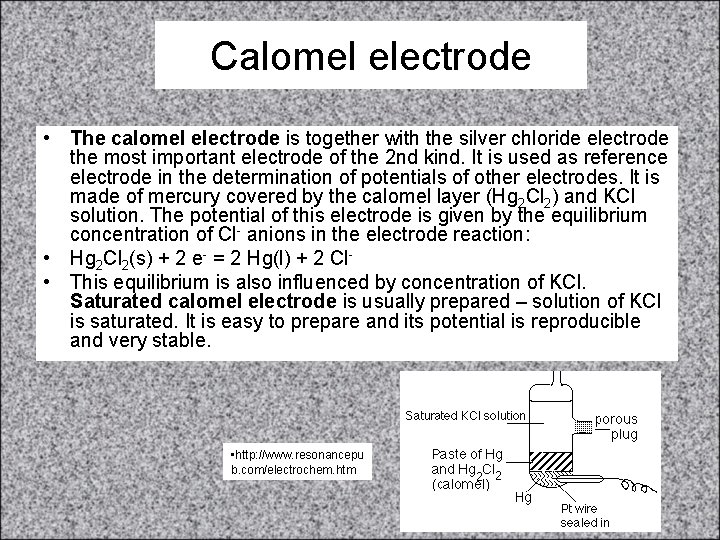
Calomel electrode • The calomel electrode is together with the silver chloride electrode the most important electrode of the 2 nd kind. It is used as reference electrode in the determination of potentials of other electrodes. It is made of mercury covered by the calomel layer (Hg 2 Cl 2) and KCl solution. The potential of this electrode is given by the equilibrium concentration of Cl- anions in the electrode reaction: • Hg 2 Cl 2(s) + 2 e- = 2 Hg(l) + 2 Cl • This equilibrium is also influenced by concentration of KCl. Saturated calomel electrode is usually prepared – solution of KCl is saturated. It is easy to prepare and its potential is reproducible and very stable. • http: //www. resonancepu b. com/electrochem. htm

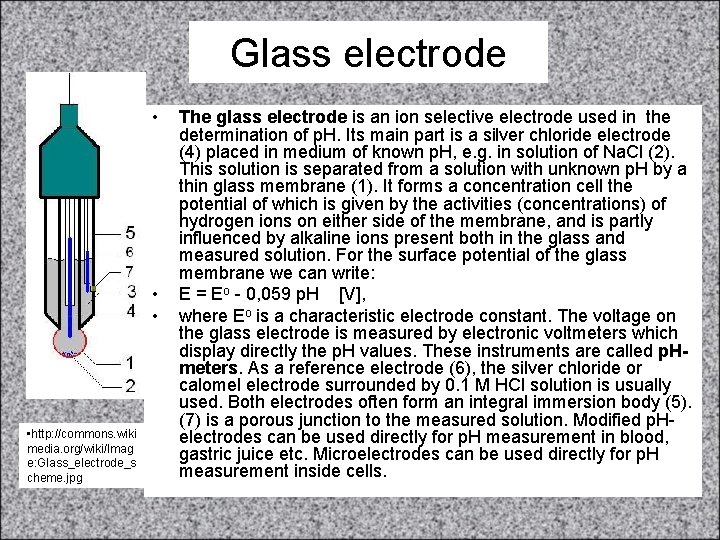
Glass electrode • • http: //commons. wiki media. org/wiki/Imag e: Glass_electrode_s cheme. jpg The glass electrode is an ion selective electrode used in the determination of p. H. Its main part is a silver chloride electrode (4) placed in medium of known p. H, e. g. in solution of Na. Cl (2). This solution is separated from a solution with unknown p. H by a thin glass membrane (1). It forms a concentration cell the potential of which is given by the activities (concentrations) of hydrogen ions on either side of the membrane, and is partly influenced by alkaline ions present both in the glass and measured solution. For the surface potential of the glass membrane we can write: E = Eo - 0, 059 p. H [V], where Eo is a characteristic electrode constant. The voltage on the glass electrode is measured by electronic voltmeters which display directly the p. H values. These instruments are called p. Hmeters. As a reference electrode (6), the silver chloride or calomel electrode surrounded by 0. 1 M HCl solution is usually used. Both electrodes often form an integral immersion body (5). (7) is a porous junction to the measured solution. Modified p. Helectrodes can be used directly for p. H measurement in blood, gastric juice etc. Microelectrodes can be used directly for p. H measurement inside cells.

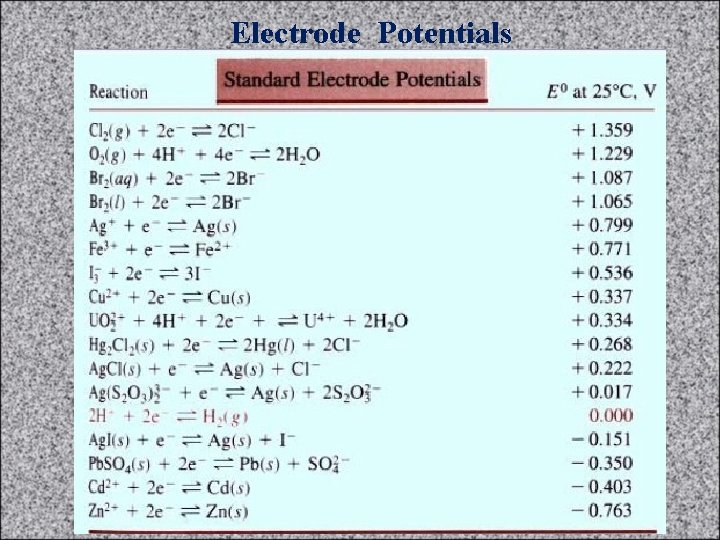
Electrode Potentials

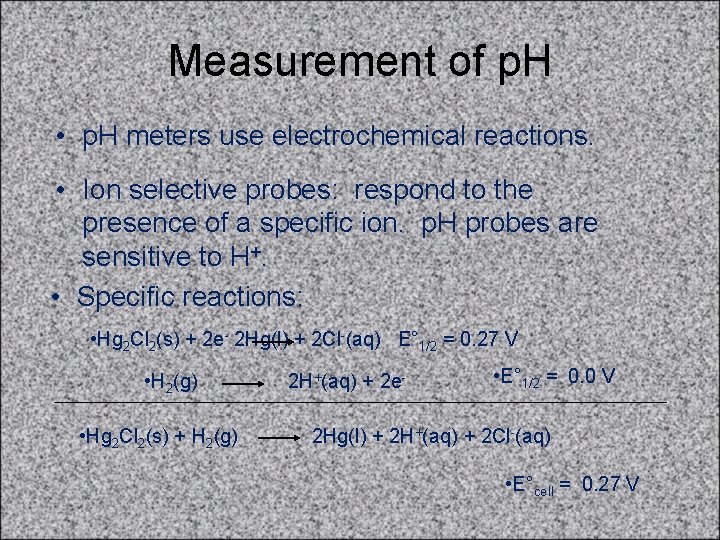
Measurement of p. H • p. H meters use electrochemical reactions. • Ion selective probes: respond to the presence of a specific ion. p. H probes are sensitive to H+. • Specific reactions: • Hg 2 Cl 2(s) + 2 e- 2 Hg(l) + 2 Cl-(aq) E° 1/2 = 0. 27 V • H 2(g) • Hg 2 Cl 2(s) + H 2(g) 2 H+(aq) + 2 e- • E° 1/2 = 0. 0 V 2 Hg(l) + 2 H+(aq) + 2 Cl-(aq) • E°cell = 0. 27 V
![Measurement of p H cont Ecell Ecell 0 0591logH Measurement of p. H (cont. ) • Ecell = E°cell - (0. 0591)log[H+] +](https://slidetodoc.com/presentation_image_h2/53bef5d85434bd051308d7cc6c8d2ff5/image-25.jpg)
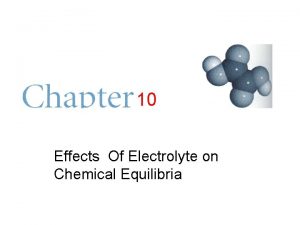
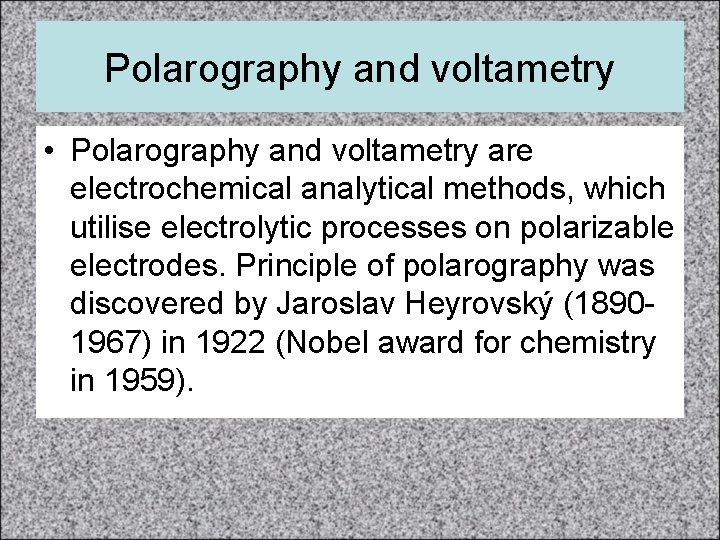
Measurement of p. H (cont. ) • Ecell = E°cell - (0. 0591)log[H+] + constant • • Ecell is directly proportional to log [H+] • electrode

The Nernst Equation • Walther Nernst(1864 -1941) received the 1920 Nobel Prize in chemistry for his numerous contributions to the field of chemical thermodynamics. Nernst (far left) is see here with Albert Einstein, Max Planck, Robert A. Millikan, and Max von Laue in 1982.

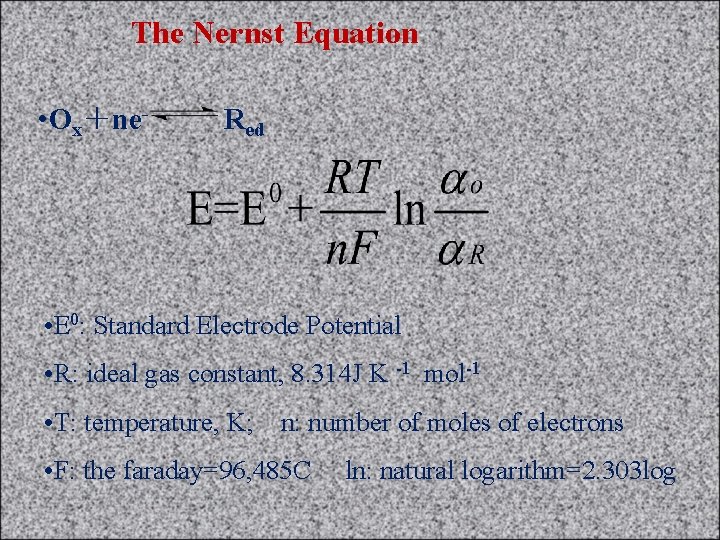
The Nernst Equation • Ox+ne- Red • E 0: Standard Electrode Potential • R: ideal gas constant, 8. 314 J K -1 mol-1 • T: temperature, K; n: number of moles of electrons • F: the faraday=96, 485 C ln: natural logarithm=2. 303 log

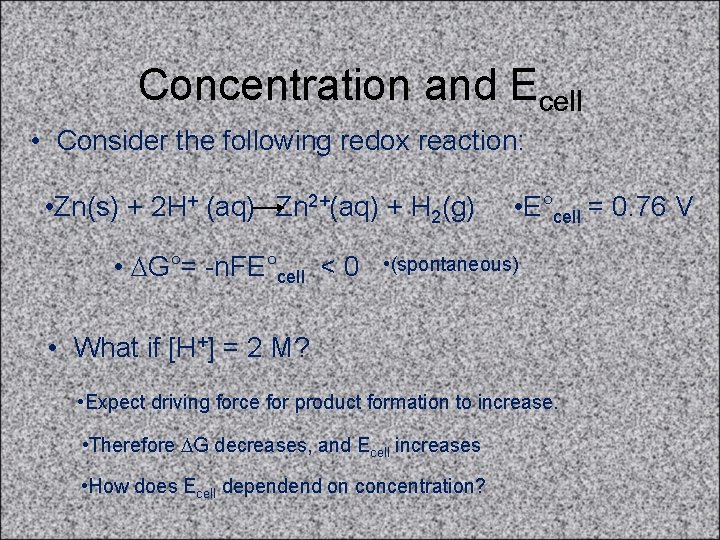
Concentration and Ecell • Consider the following redox reaction: • Zn(s) + 2 H+ (aq) Zn 2+(aq) + H 2(g) • DG°= -n. FE°cell < 0 • E°cell = 0. 76 V • (spontaneous) • What if [H+] = 2 M? • Expect driving force for product formation to increase. • Therefore DG decreases, and Ecell increases • How does Ecell dependend on concentration?

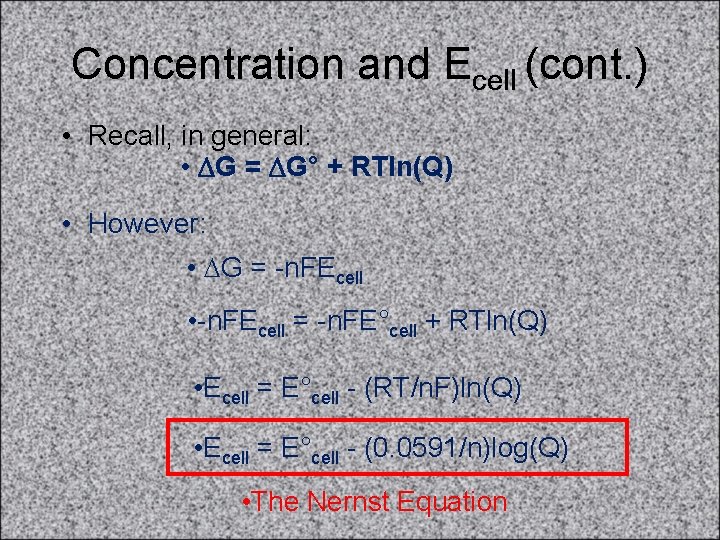
Concentration and Ecell (cont. ) • Recall, in general: • DG = DG° + RTln(Q) • However: • DG = -n. FEcell • -n. FEcell = -n. FE°cell + RTln(Q) • Ecell = E°cell - (RT/n. F)ln(Q) • Ecell = E°cell - (0. 0591/n)log(Q) • The Nernst Equation

Concentration and Ecell (cont. ) • With the Nernst Eq. , we can determine the effect of concentration on cell potentials. • Ecell = E°cell - (0. 0591/n)log(Q) • Example. Calculate the cell potential for the following: • Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) • Where [Cu 2+] = 0. 3 M and [Fe 2+] = 0. 1 M

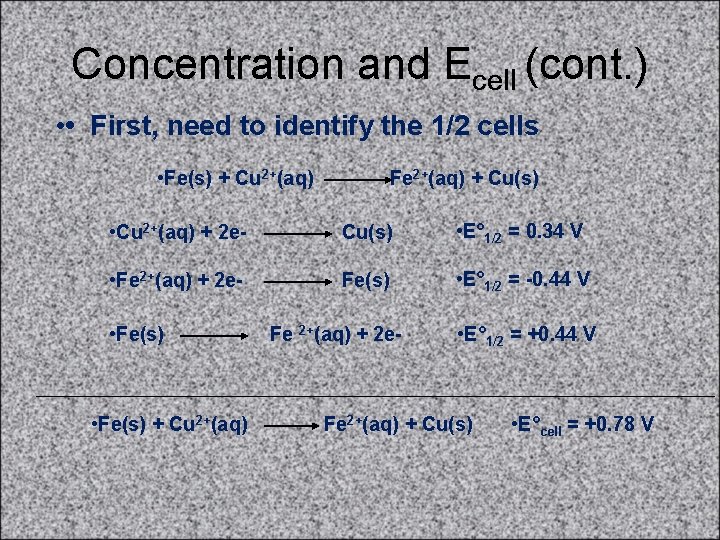
Concentration and Ecell (cont. ) • • First, need to identify the 1/2 cells • Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) • Cu 2+(aq) + 2 e- Cu(s) • E° 1/2 = 0. 34 V • Fe 2+(aq) + 2 e- Fe(s) • E° 1/2 = -0. 44 V • Fe(s) + Cu 2+(aq) Fe 2+(aq) + 2 e- • E° 1/2 = +0. 44 V Fe 2+(aq) + Cu(s) • E°cell = +0. 78 V

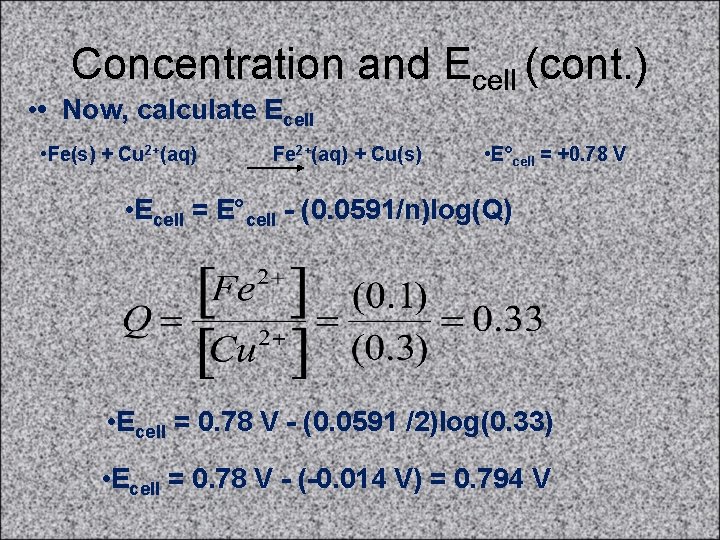
Concentration and Ecell (cont. ) • • Now, calculate Ecell • Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) • E°cell = +0. 78 V • Ecell = E°cell - (0. 0591/n)log(Q) • Ecell = 0. 78 V - (0. 0591 /2)log(0. 33) • Ecell = 0. 78 V - (-0. 014 V) = 0. 794 V
![Concentration and Ecell cont If Cu 2 0 3 M Concentration and Ecell (cont. ) • • If [Cu 2+] = 0. 3 M,](https://slidetodoc.com/presentation_image_h2/53bef5d85434bd051308d7cc6c8d2ff5/image-33.jpg)
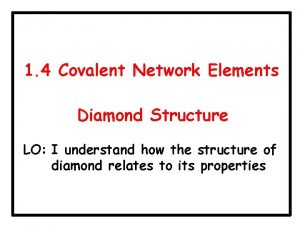
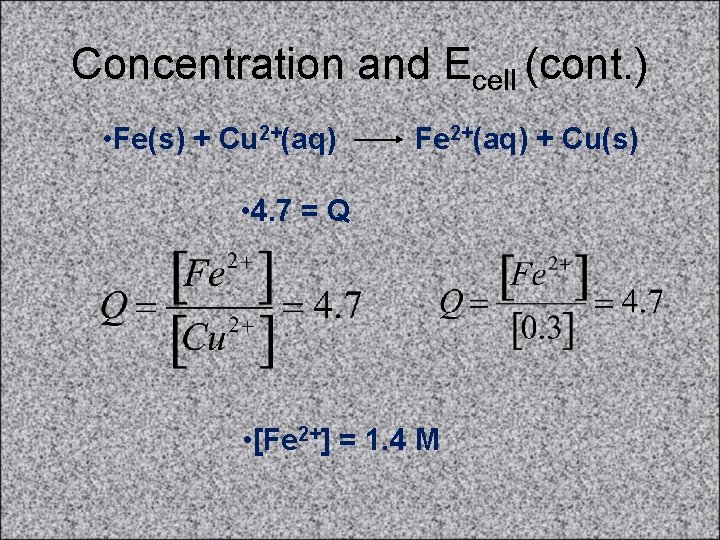
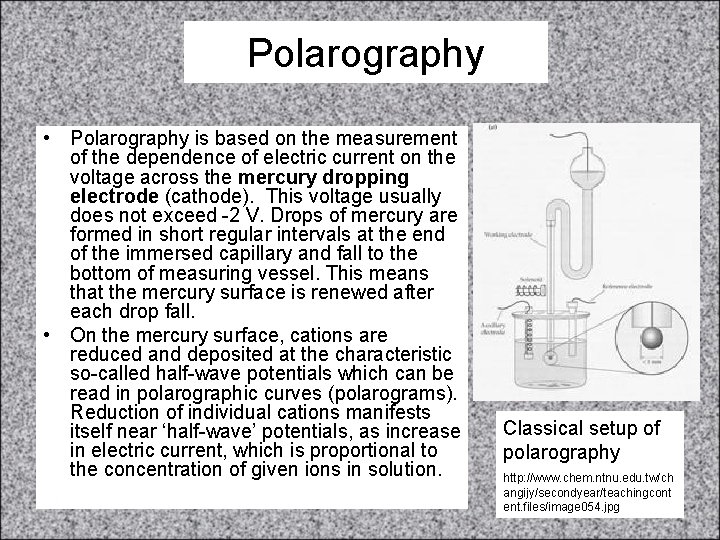
Concentration and Ecell (cont. ) • • If [Cu 2+] = 0. 3 M, what [Fe 2+] is needed so that • Ecell = 0. 76 V? • Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) • E°cell = +0. 78 V • Ecell = E°cell - (0. 0591/n)log(Q) • 0. 76 V = 0. 78 V - (0. 0591/2)log(Q) • 0. 02 V = (0. 0591/2)log(Q) • 0. 676 = log(Q) • 4. 7 = Q

Concentration and Ecell (cont. ) • Fe(s) + Cu 2+(aq) Fe 2+(aq) + Cu(s) • 4. 7 = Q • [Fe 2+] = 1. 4 M

Applications of Standard Electrode Potentials • Calculation redox-equilibrium constants • For example: biological redox systems The scheme Cytochrome c

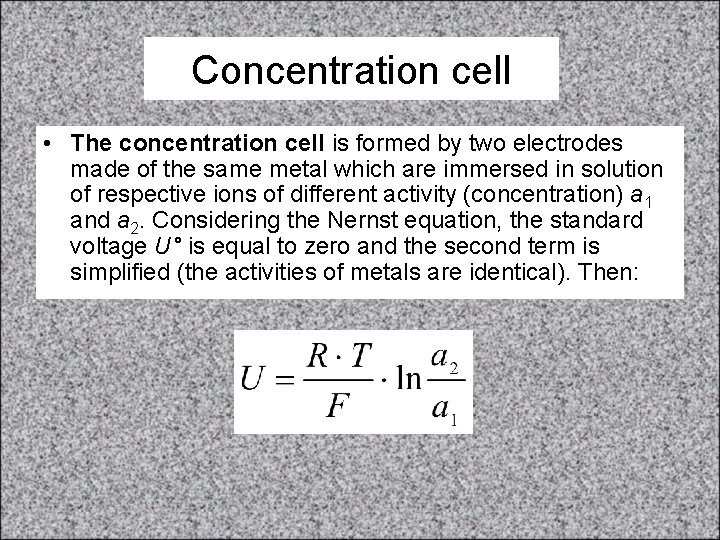
Concentration cell • The concentration cell is formed by two electrodes made of the same metal which are immersed in solution of respective ions of different activity (concentration) a 1 and a 2. Considering the Nernst equation, the standard voltage U° is equal to zero and the second term is simplified (the activities of metals are identical). Then:

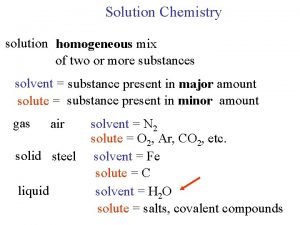
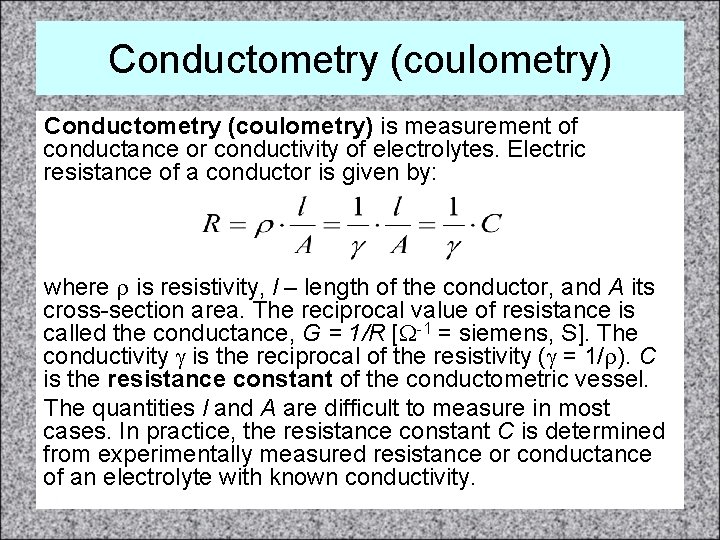
Conductometry (coulometry) is measurement of conductance or conductivity of electrolytes. Electric resistance of a conductor is given by: where r is resistivity, l – length of the conductor, and A its cross-section area. The reciprocal value of resistance is called the conductance, G = 1/R [W-1 = siemens, S]. The conductivity g is the reciprocal of the resistivity (g = 1/r). C is the resistance constant of the conductometric vessel. The quantities l and A are difficult to measure in most cases. In practice, the resistance constant C is determined from experimentally measured resistance or conductance of an electrolyte with known conductivity.

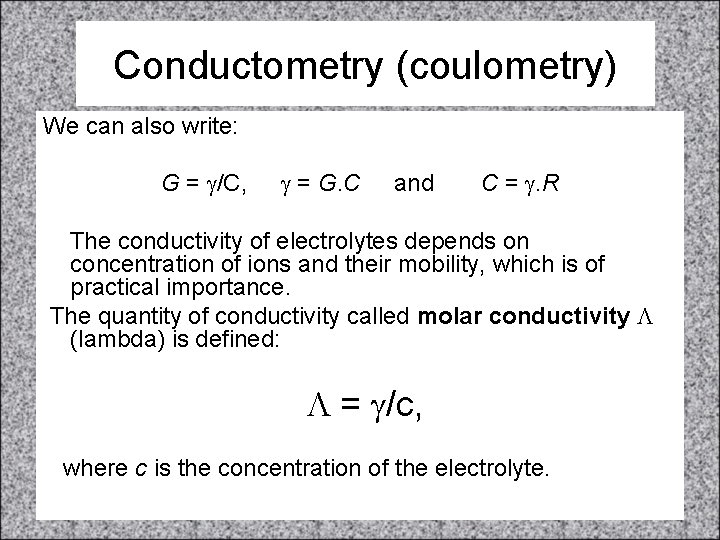
Conductometry (coulometry) We can also write: G = g/C, g = G. C and C = g. R The conductivity of electrolytes depends on concentration of ions and their mobility, which is of practical importance. The quantity of conductivity called molar conductivity L (lambda) is defined: L = g/c, where c is the concentration of the electrolyte.

Conductometers (coulometers) • Conductometers can consist of a common instrument for resistance measurement in a circuit of low-voltage alternating current with a frequency of e. g. 1 k. Hz. The direct current cannot be used, because it causes polarization of electrodes and electrolysis of the solution. The pair of measuring electrodes is made of platinum. The instrument scale is calibrated directly in units of conductance. • Conductometry is used to check purity of distilled water, to check for the quality of potable water, for the measurement of water content in food or soil, etc. Chemists use this method in conductometric titration (see practical exercises).

Polarography and voltametry • Polarography and voltametry are electrochemical analytical methods, which utilise electrolytic processes on polarizable electrodes. Principle of polarography was discovered by Jaroslav Heyrovský (18901967) in 1922 (Nobel award for chemistry in 1959).

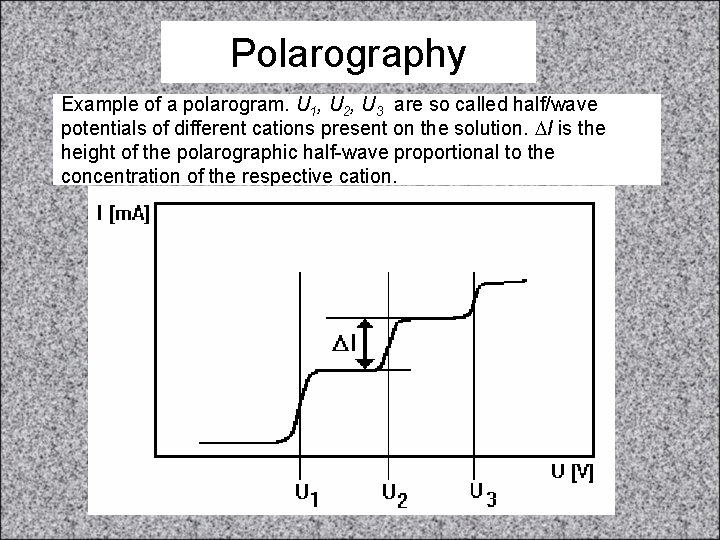
Polarography • Polarography is based on the measurement of the dependence of electric current on the voltage across the mercury dropping electrode (cathode). This voltage usually does not exceed -2 V. Drops of mercury are formed in short regular intervals at the end of the immersed capillary and fall to the bottom of measuring vessel. This means that the mercury surface is renewed after each drop fall. • On the mercury surface, cations are reduced and deposited at the characteristic so-called half-wave potentials which can be read in polarographic curves (polarograms). Reduction of individual cations manifests itself near ‘half-wave’ potentials, as increase in electric current, which is proportional to the concentration of given ions in solution. Classical setup of polarography http: //www. chem. ntnu. edu. tw/ch angijy/secondyear/teachingcont ent. files/image 054. jpg

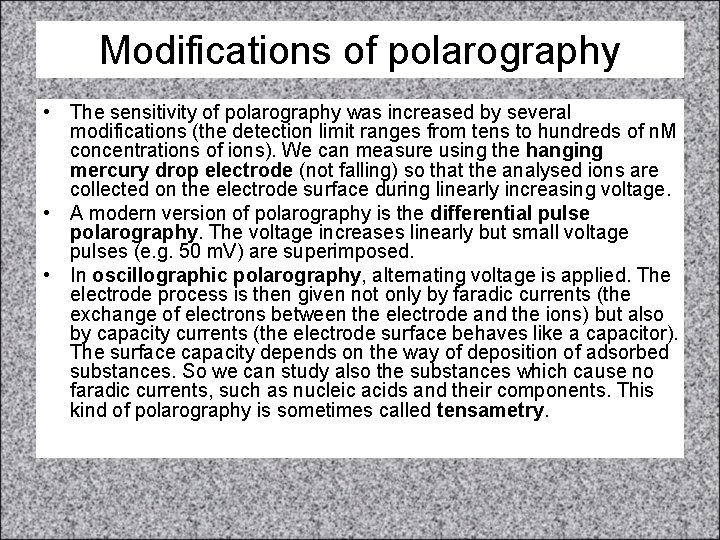
Polarography Example of a polarogram. U 1, U 2, U 3 are so called half/wave potentials of different cations present on the solution. DI is the height of the polarographic half-wave proportional to the concentration of the respective cation.

Modifications of polarography • The sensitivity of polarography was increased by several modifications (the detection limit ranges from tens to hundreds of n. M concentrations of ions). We can measure using the hanging mercury drop electrode (not falling) so that the analysed ions are collected on the electrode surface during linearly increasing voltage. • A modern version of polarography is the differential pulse polarography. The voltage increases linearly but small voltage pulses (e. g. 50 m. V) are superimposed. • In oscillographic polarography, alternating voltage is applied. The electrode process is then given not only by faradic currents (the exchange of electrons between the electrode and the ions) but also by capacity currents (the electrode surface behaves like a capacitor). The surface capacity depends on the way of deposition of adsorbed substances. So we can study also the substances which cause no faradic currents, such as nucleic acids and their components. This kind of polarography is sometimes called tensametry.

Potentiometry Devices • Electrochemical devices generally denoted as potentiometry devices, are used for the determination of ion concentrations based on measurement of potential of the respective electrodes. • The most important potentiometric measurement is the measurement of p. H. • Except of p. H-metry, we can often encounter potentiometric determination of potassium, sodium or calcium ions. • The measuring system always consists of a measuring electrode, reference electrode, and a sensitive voltmeter.

ELECTROLYTES • Na+: most abundant electrolyte in the body • K+: essential for normal membrane excitability for • • • nerve impulse Cl-: regulates osmotic pressure and assists in regulating acid-base balance Ca 2+: usually combined with phosphorus to form the mineral salts of bones and teeth, promotes nerve impulse and muscle contraction/relaxation Mg 2+: plays role in carbohydrate and protein metabolism, storage and use of intracellular energy and neural transmission. Important in the functioning of the heart, nerves, and muscles

MAJOR ELECTROLYTE IMBALANCES • • • Hyponatremia (sodium deficit < 130 m. Eq/L) Hypernatremia (sodium excess >145 m. Eq/L) Hypokalemia (potassium deficit <3. 5 m. Eq/L) Hyperkalemia (potassium excess >5. 1 m. Eq/L) Chloride imbalance (<98 m. Eq/L or >107 m. Eq/L) Magnesium imbalance (<1. 5 m. Eq/L or >2. 5 m. Eq/L)
 Electrical conductivity of aqueous solutions
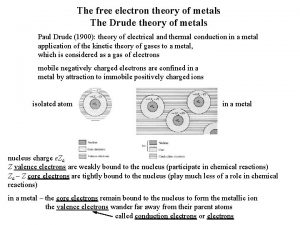
Electrical conductivity of aqueous solutions Drude theory of metals
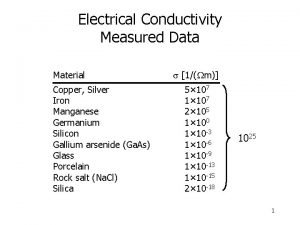
Drude theory of metals Electrical conductivity unit
Electrical conductivity unit Six strong acids
Six strong acids Electrical conductivity of solids
Electrical conductivity of solids Electrical conductivity soil definition
Electrical conductivity soil definition Electrical conductivity of soil
Electrical conductivity of soil Are thermal and electrical conductivity related
Are thermal and electrical conductivity related 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Major intra and extracellular electrolytes
Major intra and extracellular electrolytes Vacid meaning
Vacid meaning Strong vs weak electrolytes
Strong vs weak electrolytes Explain colloidal solution
Explain colloidal solution Weak electrolytes
Weak electrolytes Electrolytes and nonelectrolytes
Electrolytes and nonelectrolytes Solubility
Solubility Weak electrolytes
Weak electrolytes Congenital adrenal hyperplasia electrolytes
Congenital adrenal hyperplasia electrolytes Chapter 18 fluids and electrolytes
Chapter 18 fluids and electrolytes Hhs symptoms
Hhs symptoms Herbalife electrolytes
Herbalife electrolytes Refeeding syndrome electrolytes
Refeeding syndrome electrolytes Electrolytes ppt
Electrolytes ppt Normal electrolytes values
Normal electrolytes values Normal electrolytes values
Normal electrolytes values Vapor pressure lowering
Vapor pressure lowering Effect of electrolytes on chemical equilibria
Effect of electrolytes on chemical equilibria Medicare enteral qualification checklist
Medicare enteral qualification checklist Protective colloids
Protective colloids Strong vs weak electrolytes
Strong vs weak electrolytes Semiconductor conductivity vs temperature
Semiconductor conductivity vs temperature Calister
Calister Example
Example Viscosity units
Viscosity units Conduction formula
Conduction formula Covalent network conductivity
Covalent network conductivity Equivalence conductance
Equivalence conductance Hydraulic conductivity to permeability
Hydraulic conductivity to permeability Thermal conductivity of styrofoam
Thermal conductivity of styrofoam 26062003 color
26062003 color Excitability and conductivity
Excitability and conductivity Molar conductivity unit
Molar conductivity unit Darcy velocity units
Darcy velocity units Fundamentals of thermal-fluidsciences chapter 2 problem 24p
Fundamentals of thermal-fluidsciences chapter 2 problem 24p Transverse conductivity
Transverse conductivity Thermal conductivity detector
Thermal conductivity detector F(3)
F(3)