Electrical Conductivity Protocol Draft 2005 Electrical Conductivity Protocol















- Slides: 16

Electrical Conductivity Protocol Draft 2005

Electrical Conductivity Protocol Goals for the Training Session • Provide an inquiry context for the data collection and science content • Provide accurate science content • Review procedures for instrument calibration • Review procedures for data collection • Review data entry and analysis • Discuss classroom implementation ideas • Collect feedback from participants Draft 2005

Electrical Conductivity Protocol Inquiry Context • How does the electrical conductivity of water at the study site vary across seasons? • How does a rain event or snow melt affect conductivity? • What human activities can impact the conductivity of water at the study site? • What local water quality standards govern the use and management of local water sources? Draft 2005

Electrical Conductivity Protocol Local Inquiry Example Draft 2005

Electrical Conductivity Protocol Why do GLOBE scientists use electrical conductivity measurements? • To help determine the usability of fresh water for different purposes • Drinking • Agriculture • Industrial • To determine the overall loading of salts and other compounds dissolved in fresh water • Snow melt • Industrial runoff Draft 2005

Electrical Conductivity Protocol Science Content: Why measure electrical conductivity? Electrical Conductivity is an indirect measure for finding the total dissolved solids in a water body. Total Dissolved Solids (TDS) is the amount of mineral and salt impurities in the water. Draft 2005

Electrical Conductivity Protocol Science Content: Sources of Dissolved Solids • Fresh water has many impurities, salts and minerals dissolved in water • Sources of dissolved solids: –Rocks and soils –Runoff –Wastewater releases • Drinking water should be < 500 ppm • Agriculture uses should be <1200 ppm Draft 2005

Electrical Conductivity Protocol The Measurements • Electrical Conductivity Meter Calibration • Water Temperature • Electrical Conductivity Draft 2005

Electrical Conductivity Protocol Instruments: Electrical Conductivity Meter measured in µS/cm or µmho/cm Draft 2005

Electrical Conductivity Protocol Collecting Data: Field/Lab Guides & Data Sheets Field/Lab Guides: • Water Temperature Protocol Field Guide • Electrical Conductivity Calibration Field Guide • Electrical Conductivity Protocol Field Guide Optional: • Collecting a Water Sample in a Bucket Field Guide • Bottling a Water Sample for Classroom Testing Field Guide Data Sheets: • Hydrology Investigation Data Sheet Draft 2005

Electrical Conductivity Protocol Collecting Data: Calibrating the Electrical Conductivity Meter • • Pour standard solution (at~25 °C) in two containers Rinse electrodes with distilled water, blot dry Place meter in first container, 2 -3 seconds Remove meter, shake gently, and place in second container (Do not rinse with distilled water) Stir gently and allow to stabilize Adjust meter until it reads the correct value for the standard Record value Draft 2005

Electrical Conductivity Protocol Collecting Data: Protocol • Record water temperature • Pour water sample into two containers (or measure in water body) • Rinse electrodes with distilled water, blot dry • Place meter in first container, 2 -3 seconds • Remove meter, shake gently, and place in second container, 1 minute (Do not rinse with distilled water) • Record value when stabilized • Repeat measurement with new sample water, twice • Average three measurements and check for accuracy Draft 2005

Electrical Conductivity Protocol Enter Data on the GLOBE Web Site Step 1: Confirm that the Hydrology Study Site definition is completed Step 2: Select “Hydrology Measurements” from the Hydrology data entry menu Step 3: Enter date/UT time/select site Step 4: Enter the “water state”, water temperature, and electrical conductivity measurements Step 5: Confirm data entries on verification page, especially the Average Electrical Conductivity Value Draft 2005

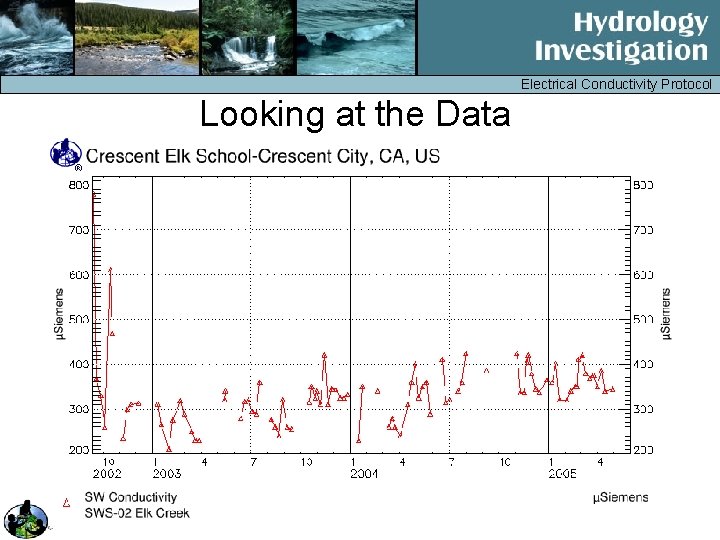
Electrical Conductivity Protocol Looking at the Data Draft 2005

Electrical Conductivity Protocol Classroom Implementation • • Inquiry Curriculum/Standards Alignment Assessment Classroom Management Draft 2005

Electrical Conductivity Protocol Getting Started • The GLOBE Web site has information http: //www. globe. gov • GLOBE Help Desk is available to support you as you get started. • Get your students involved doing real science! Draft 2005