METALS AND NON METALS Electrical conductivity thermal conductivity






- Slides: 15

METALS AND NON METALS Electrical conductivity, thermal conductivity, melting point, density, tensile strength

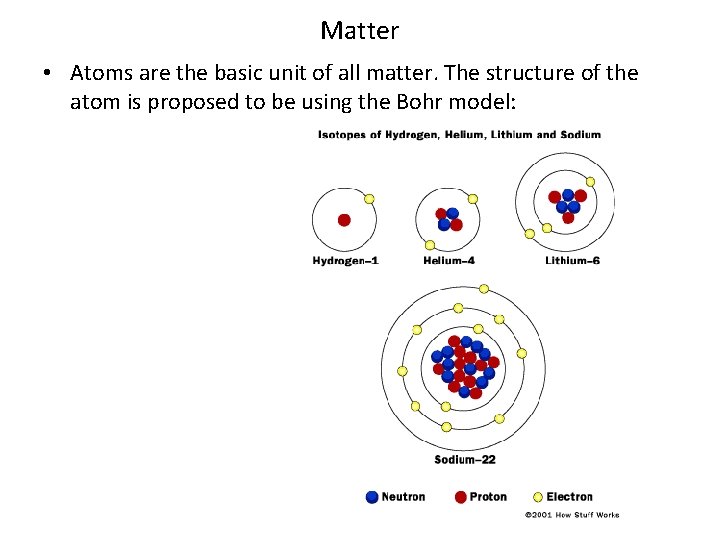
Matter • Atoms are the basic unit of all matter. The structure of the atom is proposed to be using the Bohr model:

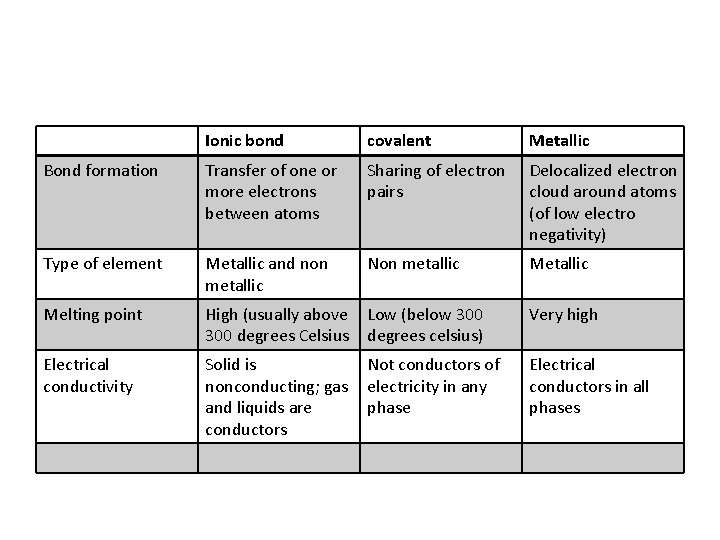
Ionic bond covalent Metallic Bond formation Transfer of one or more electrons between atoms Sharing of electron pairs Delocalized electron cloud around atoms (of low electro negativity) Type of element Metallic and non metallic Non metallic Melting point High (usually above 300 degrees Celsius Low (below 300 degrees celsius) Very high Electrical conductivity Solid is nonconducting; gas and liquids are conductors Not conductors of electricity in any phase Electrical conductors in all phases

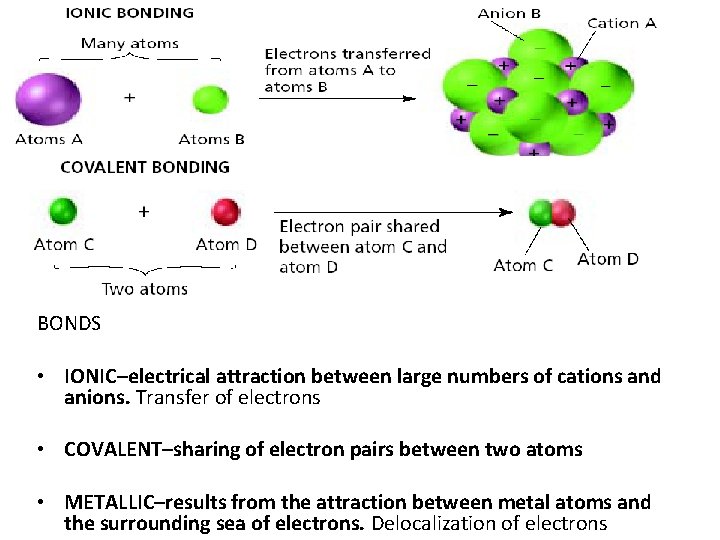
BONDS • IONIC–electrical attraction between large numbers of cations and anions. Transfer of electrons • COVALENT–sharing of electron pairs between two atoms • METALLIC–results from the attraction between metal atoms and the surrounding sea of electrons. Delocalization of electrons

• Ionic Compounds Held by strong electrostatic force of attraction between + and –ions High melting and boiling points • Covalent or Molecular Compounds Force of attraction between individual molecules much weaker than ionic bond Molecular compounds boil and melt at low temperatures Usually gaseous and vaporize at room temperature

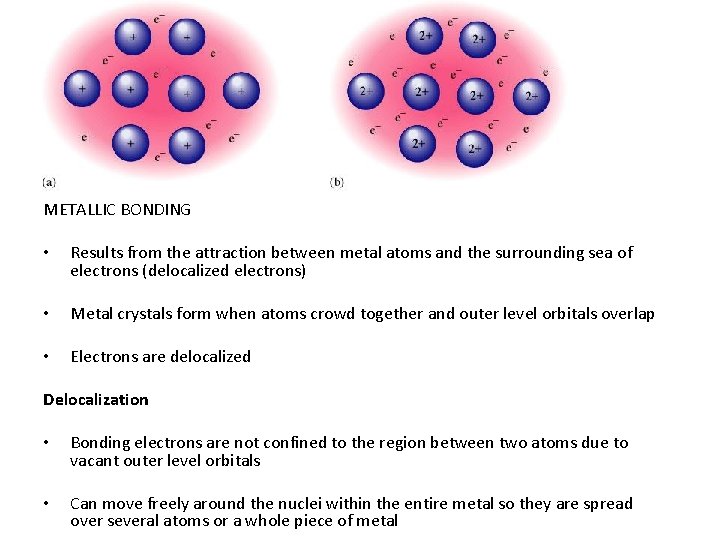
METALLIC BONDING • Results from the attraction between metal atoms and the surrounding sea of electrons (delocalized electrons) • Metal crystals form when atoms crowd together and outer level orbitals overlap • Electrons are delocalized Delocalization • Bonding electrons are not confined to the region between two atoms due to vacant outer level orbitals • Can move freely around the nuclei within the entire metal so they are spread over several atoms or a whole piece of metal

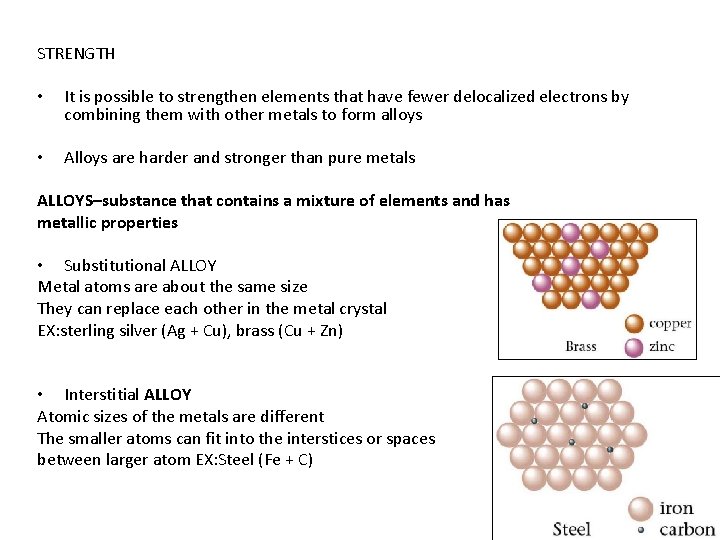
STRENGTH • It is possible to strengthen elements that have fewer delocalized electrons by combining them with other metals to form alloys • Alloys are harder and stronger than pure metals ALLOYS–substance that contains a mixture of elements and has metallic properties • Substitutional ALLOY Metal atoms are about the same size They can replace each other in the metal crystal EX: sterling silver (Ag + Cu), brass (Cu + Zn) • Interstitial ALLOY Atomic sizes of the metals are different The smaller atoms can fit into the interstices or spaces between larger atom EX: Steel (Fe + C)

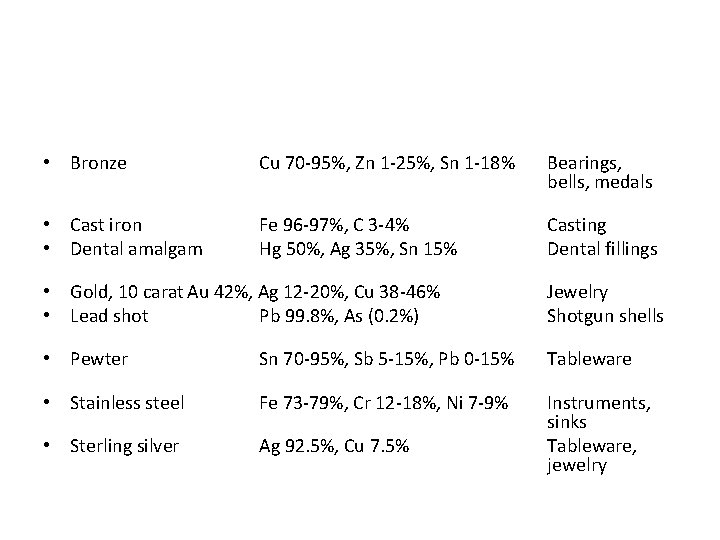
• Bronze Cu 70 -95%, Zn 1 -25%, Sn 1 -18% Bearings, bells, medals • Cast iron • Dental amalgam Fe 96 -97%, C 3 -4% Hg 50%, Ag 35%, Sn 15% Casting Dental fillings • Gold, 10 carat Au 42%, Ag 12 -20%, Cu 38 -46% • Lead shot Pb 99. 8%, As (0. 2%) Jewelry Shotgun shells • Pewter Sn 70 -95%, Sb 5 -15%, Pb 0 -15% Tableware • Stainless steel Fe 73 -79%, Cr 12 -18%, Ni 7 -9% • Sterling silver Ag 92. 5%, Cu 7. 5% Instruments, sinks Tableware, jewelry

• Electrical conductivity is a measure of the material’s ability to conduct current…metals have a larger electrical conductivity than non metals/ insulators • Thermal conductivity, k is a property of the material’s ability to conduct heat. • Melting point is the temperature at which a material changes state from solid to liquid. • Density is the mass per unit volume of a material. It is a measure of how heavy or light a material is and thus if it will sink or float in another substance. • Tensile strength is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract…break

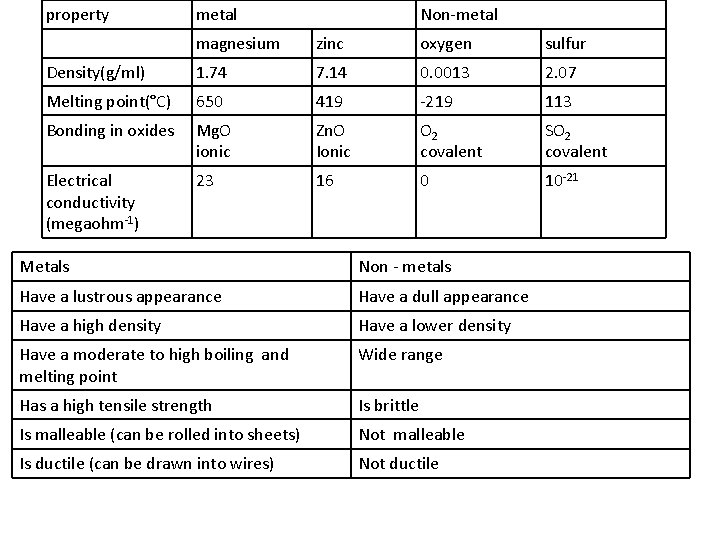
property metal Non-metal magnesium zinc oxygen sulfur Density(g/ml) 1. 74 7. 14 0. 0013 2. 07 Melting point(°C) 650 419 -219 113 Bonding in oxides Mg. O ionic Zn. O Ionic O 2 covalent SO 2 covalent Electrical conductivity (megaohm-1) 23 16 0 10 -21 Metals Non - metals Have a lustrous appearance Have a dull appearance Have a high density Have a lower density Have a moderate to high boiling and melting point Wide range Has a high tensile strength Is brittle Is malleable (can be rolled into sheets) Not malleable Is ductile (can be drawn into wires) Not ductile

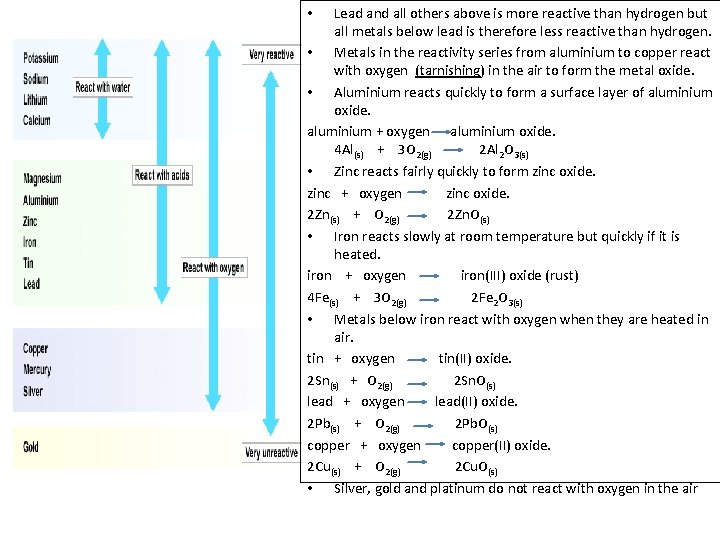
Lead and all others above is more reactive than hydrogen but all metals below lead is therefore less reactive than hydrogen. • Metals in the reactivity series from aluminium to copper react with oxygen (tarnishing) in the air to form the metal oxide. • Aluminium reacts quickly to form a surface layer of aluminium oxide. aluminium + oxygen aluminium oxide. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) • Zinc reacts fairly quickly to form zinc oxide. zinc + oxygen zinc oxide. 2 Zn(s) + O 2(g) 2 Zn. O(s) • Iron reacts slowly at room temperature but quickly if it is heated. iron + oxygen iron(III) oxide (rust) 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) • Metals below iron react with oxygen when they are heated in air. tin + oxygen tin(II) oxide. 2 Sn(s) + O 2(g) 2 Sn. O(s) lead + oxygen lead(II) oxide. 2 Pb(s) + O 2(g) 2 Pb. O(s) copper + oxygen copper(II) oxide. 2 Cu(s) + O 2(g) 2 Cu. O(s) • Silver, gold and platinum do not react with oxygen in the air •

• Copper cookware is still the preferred choice by many of the world's professional chefs because copper is the best conductor of heat, especially for top-of-range cooking where the food must be cooked at precisely controlled temperatures. To prevent copper from reacting with foods, the pans should be lined with tin or stainless steel. Tin lined pans will wear over time and require retinning. Stainless steel lined copper pans are generally more expensive, but never need retinning. The major disadvantage of copper cookware is maintenance. They should not be placed in the dishwasher nor left to air dry as this causes spotting and they will have to be polished more frequently. Copper should have the lacquer finish removed before it's first use. One of the best products to use is called Klean Strip, Premium Stripper. This can be found at most hardware stores. However, most Lacquer strippers will work just as well. Cookware made of copper is expensive. Aluminum cookware is second only to copper in it's ability to conduct heat. Also, like copper it reacts with food and being a soft metal, it easily scratches. For these reasons it should be lined and have a harder exterior finish. Today's quality aluminum cookware will have an aluminum core sandwiched between layers of other metals, like stainless steel, or treated in a process called hard anodization. Over 50% of the cookware sold today contains aluminum. Stainless Steel cookware is easy to care for, exceptionally durable and light, won't corrode or tarnish and it's nonporous surface is resistant to wear. But it is a poor conductor of heat and does not distribute heat evenly. Quality stainless steel cookware will usually have heavy bottoms with a core of copper or aluminum sandwiched between layers of stainless steel.

Cast Iron The major advantage of cast iron is its ability to retain and evenly disburse heat. It is excellent for browning, frying and baking various foods. The major disadvantage is it's weight. Cast Iron also reacts with food and can absorb the flavors of what was cooked in it unless it is "seasoned". Before a raw cast iron pan is used it should be "seasoned". To season it, wash and dry the pan thoroughly, lightly rub shortening into the pans surface, and bake the pan in a moderate oven, 300 degrees, for 60 to 75 minutes. This seasoning process gives the pan a non-stick surface that can last indefinitely. (I still use my grandmother's cast iron skillets and muffin tins on a regular basis and don't believe there is another skillet made that can brown and fry a trout like cast iron. Also, if you like crusty corn muffins and corn sticks as we do, heat the cast iron muffin tin in the oven for 20 min, then pour the batter in and bake as usual. )Enameled cast iron cookware has a hard porcelain enamel coating that provides a permanent finish to prevent the cast iron from reacting with food. It also comes in many colors and doesn't require seasoning. • Non-Stick Non-stick can refer to any cookware which has a non-stick surface applied to it. The advantages of nonstick cookware include ease of cleaning and food requires less fat when cooking. The disadvantage is the non-stick surface will eventually wear off and the pan will have to be replaced. There are many grades of non-stick cookware and some of the high quality lines produce a coating that will last a very long time.

Alloys are • Stronger than the pure metal • Less reactive than the pure metal. Such as oxidation of iron increased in moist conditions. (to reduce chances of rusting, painting, galvanizing, covering with oil reduces the risk of the iron rusting. • Cheaper to produce and • Lighter (cheaper to ship) So for use in kitchen utensils and cookware it would be more efficient to use aluminum

• Factors affecting rusting Exposure to air Exposure to water Increased exposure to salt content in air/water • To reduce or prevent rusting - Painting - Covering with oil - Electroplating - Galvanizing – zinc plating - Tin plating (tin is lower in the reactivity series, so less reactive if the iron is scratched) - Using alloys of iron eg. Stainless steel -Using a sacrificial protection – placing a more reactive metal near the iron