Temperature Control Devices Preheaters convert sample into its










- Slides: 22

Temperature Control Devices Preheaters: convert sample into its vapour form, present along with injecting devices Thermostatically controlled oven: temperature maintenance in a column is highly essential for efficient separation. Two types of operations Isothermal programming: Linear programming: this method is efficient for separation of complex mixtures

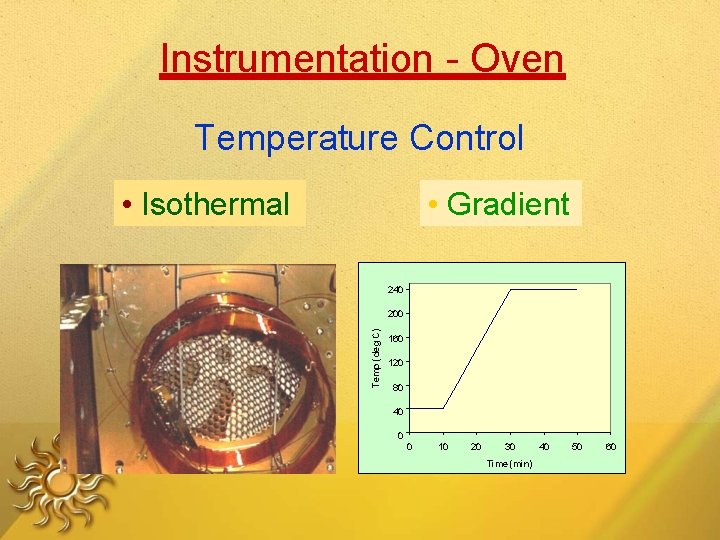
Instrumentation - Oven Temperature Control • Isothermal • Gradient 240 Temp (deg C) 200 160 120 80 40 0 0 10 20 30 Time (min) 40 50 60

DETECTORS Heart of the apparatus The requirements of an ideal detector are Applicability to wide range of samples Rapidity High sensitivity Linearity Response should be unaffected by temperature, flow rate… Non destructive Simple & inexpensive

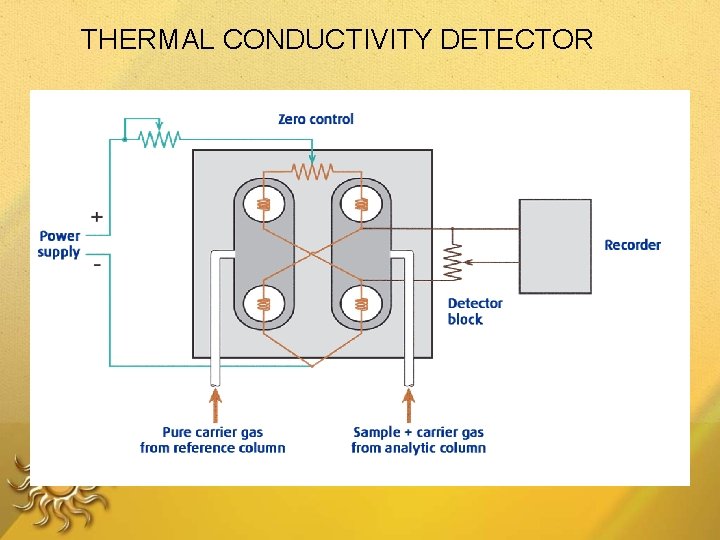
1. Thermal Conductivity Detector (Katharometer, Hot Wire Detector) Measures the changes of thermal conductivity due to the sample ( g). Sample can be recovered.

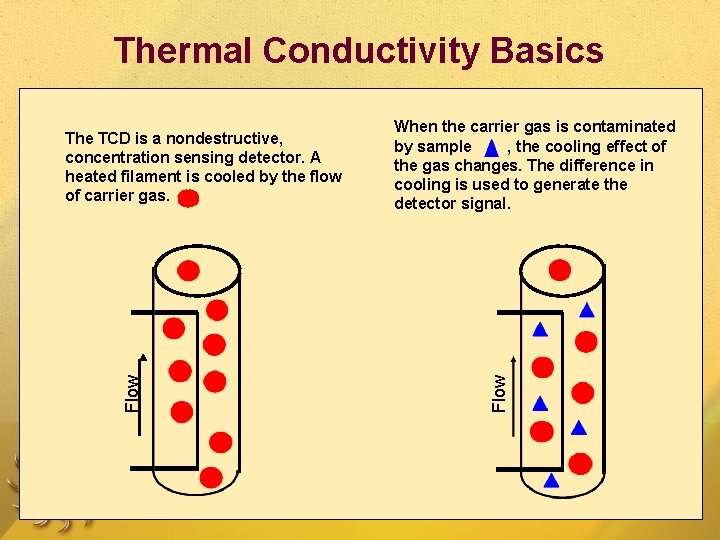
Thermal Conductivity Basics When the carrier gas is contaminated by sample , the cooling effect of the gas changes. The difference in cooling is used to generate the detector signal. Flow The TCD is a nondestructive, concentration sensing detector. A heated filament is cooled by the flow of carrier gas.

Thermal Conductivity Detector When a separated compound elutes from the column , thermal conductivity of the mixture of carrier gas and compound gas is lowered. The filament in the sample column becomes hotter than the control column. The imbalance between control and sample filament temperature is measured by a simple gadget and a signal is recorded

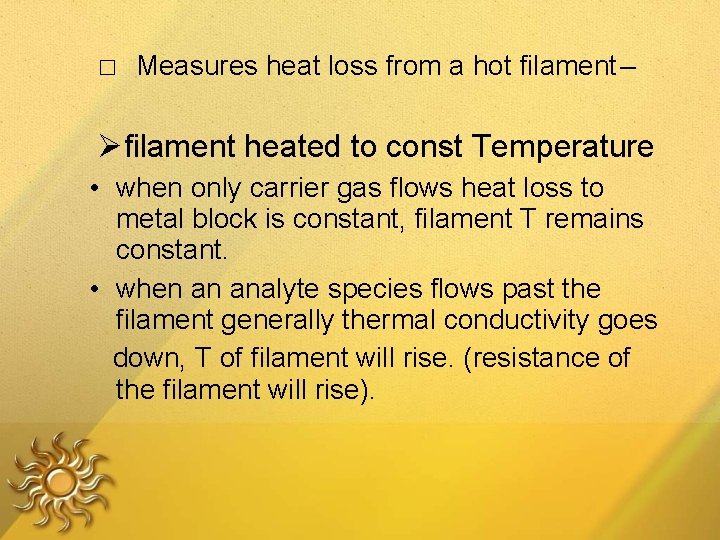
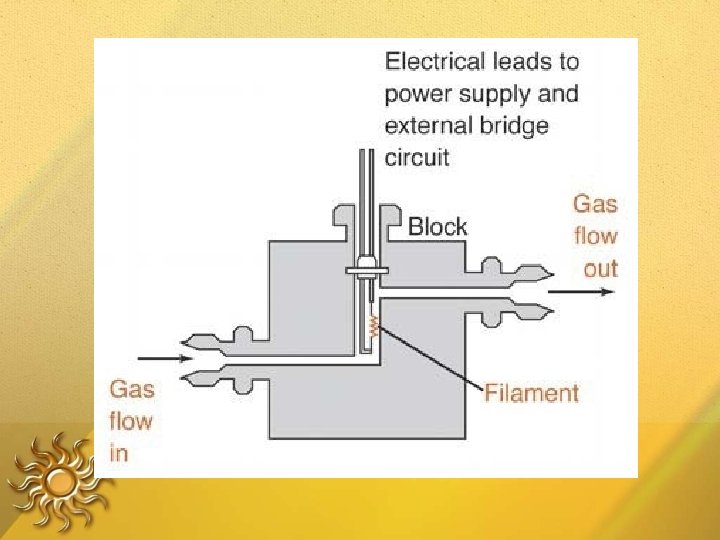
□ Measures heat loss from a hot filament – filament heated to const Temperature • when only carrier gas flows heat loss to metal block is constant, filament T remains constant. • when an analyte species flows past the filament generally thermal conductivity goes down, T of filament will rise. (resistance of the filament will rise).

THERMAL CONDUCTIVITY DETECTOR


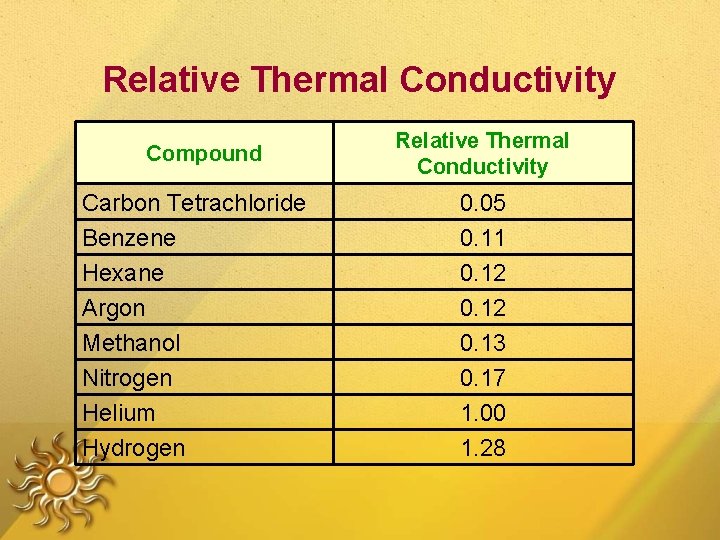
Relative Thermal Conductivity Compound Carbon Tetrachloride Benzene Hexane Argon Methanol Nitrogen Helium Hydrogen Relative Thermal Conductivity 0. 05 0. 11 0. 12 0. 13 0. 17 1. 00 1. 28

Advantages of Katharometer (TCD) Linearity is good Applicable to most compounds Non destructive Simple & inexpensive Disadvantages Low sensitivity Affected by fluctuations in temperature and flow rate Biological samples cannot be analyzed

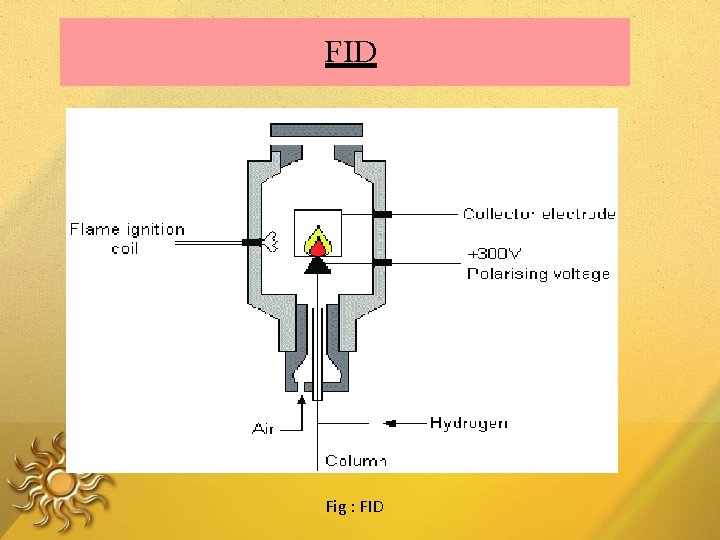
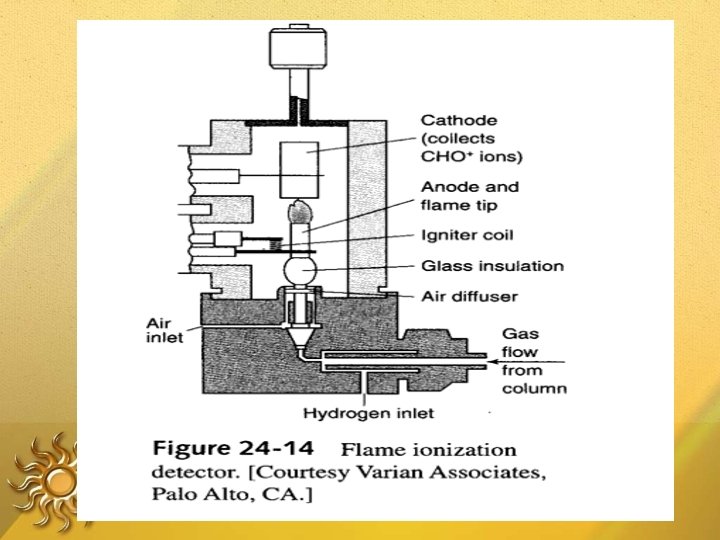
Flame Ionization Detector Destructive detector The effluent from the column is mixed with H & air, and ignited. Organic compounds burning in the flame produce ions and electrons, which can conduct electricity through the flame. A large electrical potential is applied at the burner tip The ions collected on collector or electrode and were recorded on recorder due to electric current.

FIDs are mass sensitive rather than conc. sensitive ADVANTAGES: • µg quantities of the solute can be detected • Stable • Responds to most of the organic compounds • Linearity is excellent • DA: Destroy the sample

FID Fig : FID


Argon ionization detector Depends on the excitation of argon atoms to a metastable state, by using radioactive energy. Argon→ irradiation Argon + e- →collision Metastable Argon→ collision of sub. → Ionization →↑Current ADVANTAGES 1. Responds to organic compounds 2. High sensitivity DISADVANTAGES 1. Response is not absolute 2. Linearity is poor 3. Sensitivity is affected by water

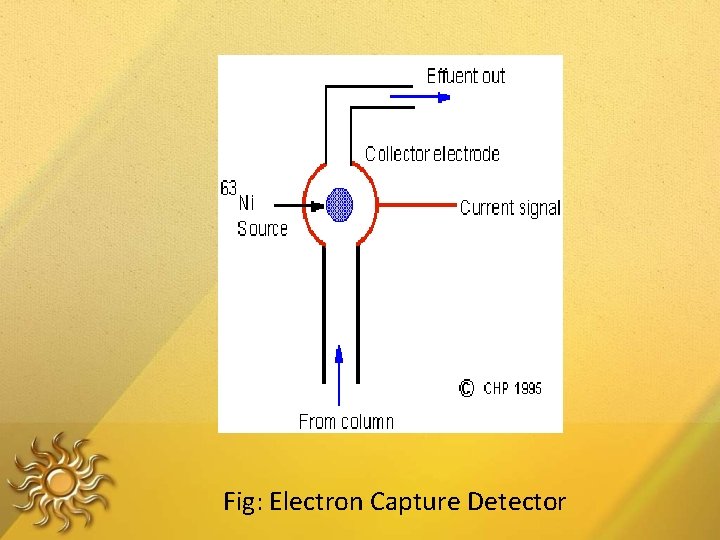
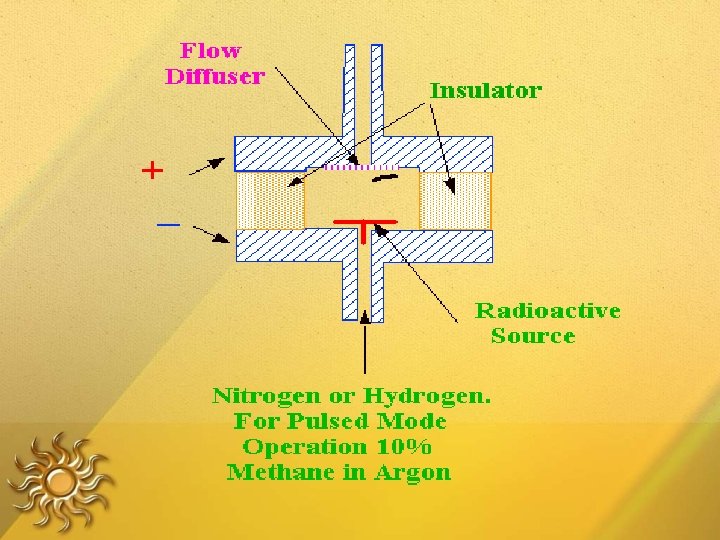
ELECTRON CAPTURE DETECTOR The detector consists of a cavity that contains two electrodes and a radiation source that emits - radiation (e. g. 63 Ni, 3 H) The collision between electrons and the carrier gas (methane plus an inert gas) produces a plasma containing electrons and positive ions.

• If a compound is present that contains electronegative atoms, those electrons are captured and negative ions are formed, and rate of electron collection decreases • The detector selective for compounds with atoms of high electron affinity. • This detector is frequently used in the analysis of chlorinated compounds • e. g. – pesticides, polychlorinated biphenyls

Fig: Electron Capture Detector



ADVANTAGE Highly sensitive DISADVANTAGE Used only for compounds with electron affinity