Reading Materials Chapter 4 LECTURES 4 5 Chapter




















![Illustration 10 Ideal Gas Equation Parameters Pressure Symbols P Dimensions [M] [L]-1 [T] -2 Illustration 10 Ideal Gas Equation Parameters Pressure Symbols P Dimensions [M] [L]-1 [T] -2](https://slidetodoc.com/presentation_image_h/a0c6e35707dde90a4f8d2b69dcc49e77/image-39.jpg)




- Slides: 49

Reading Materials: Chapter 4 LECTURES 4 -5 Chapter 4 CHEM ENG 1007 1

Class Activity 1. How tall are you? 2. How long does it take you to get to Adelaide Uni every morning? 3. How many days are there in one year? 4. How heavy are you? 5. What is the distance from the earth to the moon? 6. What is the average weight of the new-born baby? Chapter 4 CHEM ENG 1007 2

Objective At the end of these lectures you should be able to: q convert between sets of units by using dimensional equation q add, subtract, multiply and divide units q use SI, American Engineering and cgs units q understand dimensional homogeneity Chapter 4 CHEM ENG 1007 3

§ 4. 1 Units & Dimensions Quick Quiz 1: True or False A quantity is meaningless without units! "the distance from my house to school is six" unless we follow that statement with "miles" or "kilometres", or whichever unit, the above statement is meaningless. Chapter 4 CHEM ENG 1007 4

§ 4. 1 Units & Dimensions Quick Quiz 2: True or False All additive/subtractive terms must have the same dimension! Chapter 4 CHEM ENG 1007 5

Illustration 1 Every Monday you buy three litres of milk and five packages of Tim Tam. Since you have to carry your purchases home, you’d like to know how many kilograms of food you’ve bought. Can we just add together litres of milk plus packages of tim tam to get kilograms of food? (3 L milk) + (5 packages of tim tam) = ? kg of food Chapter 4 CHEM ENG 1007 6

Illustration 2 Assignment 1, Question 7 a) b) c) d) e) f) g) h) i) 6. 201 cm + 7. 4 cm + 0. 68 cm + 12. 0 cm = ? 1. 6 km + 1. 62 m + 1200 cm = ? 8. 264 g - 7. 8 g = ? 10. 4168 m - 6. 0 m = ? 12. 00 m + 15. 001 kg = ? 131 cm x 2. 3 cm = ? 5. 7621 m x 6. 201 m = ? 20. 2 cm divided by 7. 41 s = ? 40. 002 g divided by 13. 000005 ml = ? Chapter 4 CHEM ENG 1007 7

Case Study 1: Feet or Meters? SEOUL, South Korea – A mix up in the cockpit over whether altitude guidance was measured in feet or meters led to the crash of a Korean Air Lines Mc. Donnell Douglas MD-11 freighter soon after takeoff in Shanghai in April 1999. The crash killed all 3 crew-members. Five people on the ground were killed and 40 more were injured when the plane went down in light rain onto a construction site near Shanghai’s Hongqiao Airport. Chapter 4 CHEM ENG 1007 8

Case Study 1: Feet or Meters? A Chinese air-traffic controller directed the pilots to an altitude of 1, 500 meters (4, 950 feet). The plane was climbing rapidly to that level when the co-pilot told the pilot he thought the instructed height was 1, 500 feet (455 meters). The international aviation industry commonly measures altitude in feet, and the confusion led the pilot to conclude the jet was almost 1, 000 meters too high, so he quickly moved the controls to lower the plane. As the plane descended, the pilot realized the error but couldn’t correct the mistake in time. South Korea’s Ministry of Construction and Transportation said Korean Air Lines would lose the right to serve the Seoul. Shanghai cargo route for at least two years because of errors by the pilots. Korean Air Lines said it would appeal the decision…. . Wall Street Journal, June 6, 2001 Chapter 4 CHEM ENG 1007 9

Case Study 2 Mars Probe Lost Due to Simple Math Error NASA lost its $125 -million Mars Climate Orbiter because spacecraft engineers failed to convert from English to metric measurements when exchanging vital data before the craft was launched. pound-seconds versus newton-seconds Want to know more…. . http: //articles. latimes. com/1999/oct/01/news/mn-17288 Chapter 4 CHEM ENG 1007 10

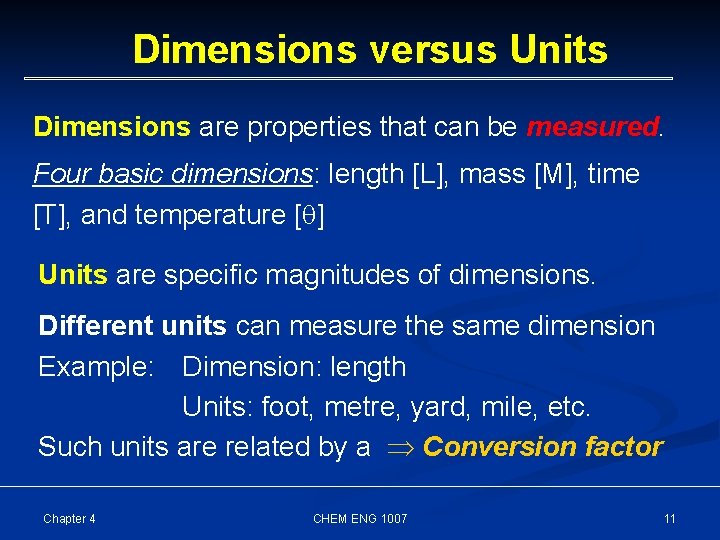
Dimensions versus Units Dimensions are properties that can be measured. Four basic dimensions: length [L], mass [M], time [T], and temperature [ ] Units are specific magnitudes of dimensions. Different units can measure the same dimension Example: Dimension: length Units: foot, metre, yard, mile, etc. Such units are related by a Conversion factor Chapter 4 CHEM ENG 1007 11

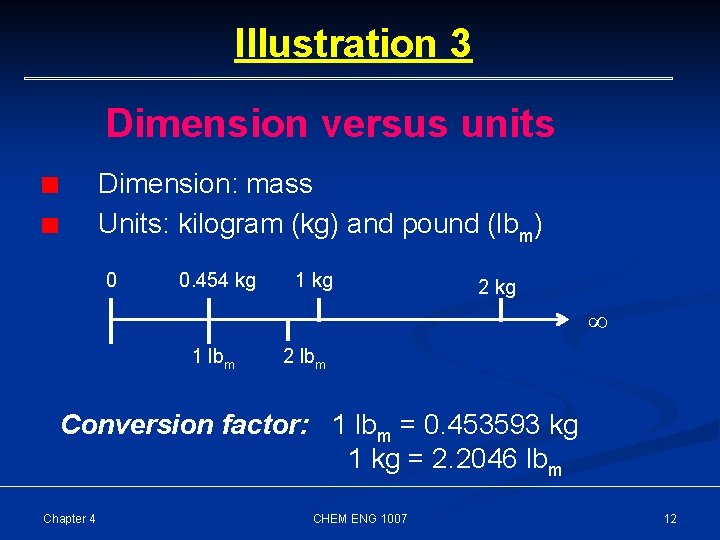
Illustration 3 Dimension versus units Dimension: mass Units: kilogram (kg) and pound (lbm) 0 0. 454 kg 1 kg 2 kg 1 lbm 2 lbm Conversion factor: 1 lbm = 0. 453593 kg 1 kg = 2. 2046 lbm Chapter 4 CHEM ENG 1007 12

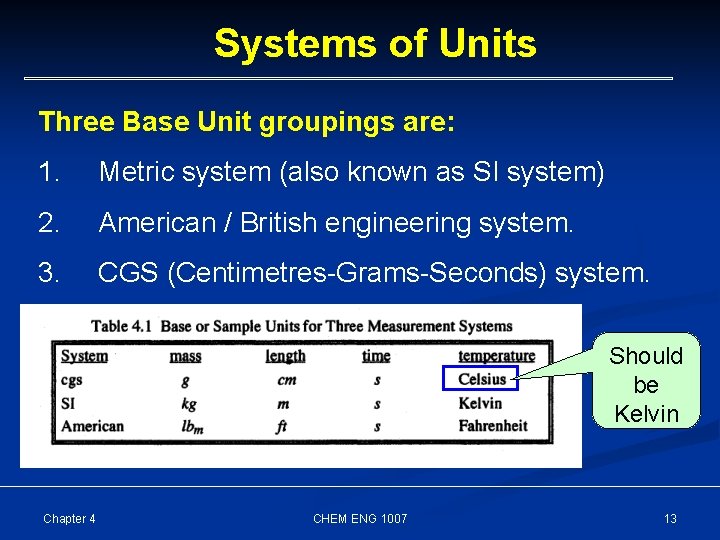
Systems of Units Three Base Unit groupings are: 1. Metric system (also known as SI system) 2. American / British engineering system. 3. CGS (Centimetres-Grams-Seconds) system. Should be Kelvin Chapter 4 CHEM ENG 1007 13

Concept Inventory 1. Which of the following set of units is in CGS system a) kg, m, s, N b) lbm, ft, s, lbf c) g, m, s, N d) g, cm, s, dyne e) kg, cm, s, dyne Chapter 4 CHEM ENG 1007 14

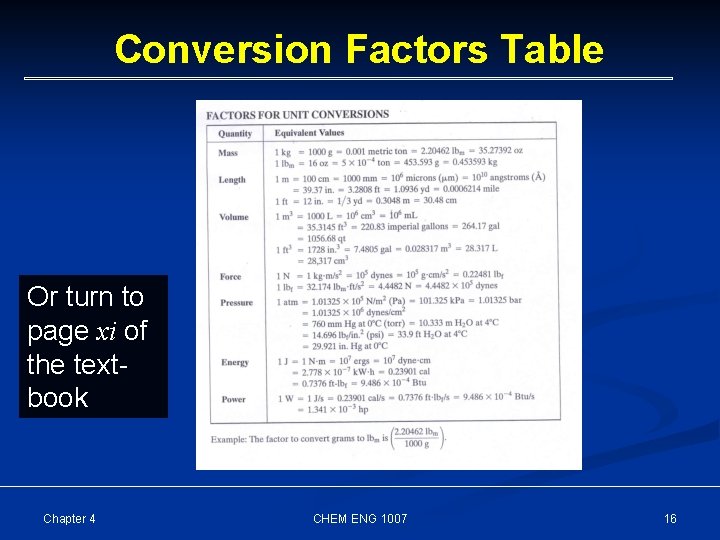
§ 4. 1. 1 Conversion Factors A conversion factor is an equation that equates two quantities expressed in different units where, in fact, the two quantities are exactly equivalent. Examples: 12 in = 1 ft Chapter 4 1000 g = 1 kg CHEM ENG 1007 60 s = 1 min 15

Conversion Factors Table Or turn to page xi of the textbook Chapter 4 CHEM ENG 1007 16

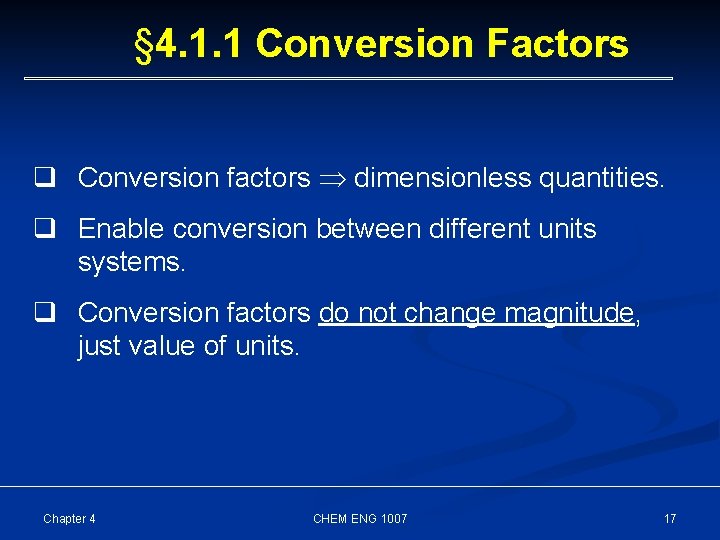
§ 4. 1. 1 Conversion Factors q Conversion factors dimensionless quantities. q Enable conversion between different units systems. q Conversion factors do not change magnitude, just value of units. Chapter 4 CHEM ENG 1007 17

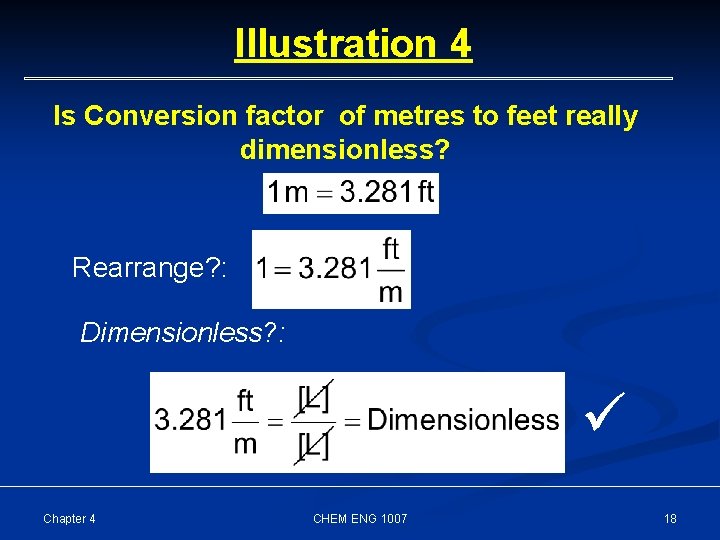
Illustration 4 Is Conversion factor of metres to feet really dimensionless? Rearrange? : Dimensionless? : Chapter 4 CHEM ENG 1007 18

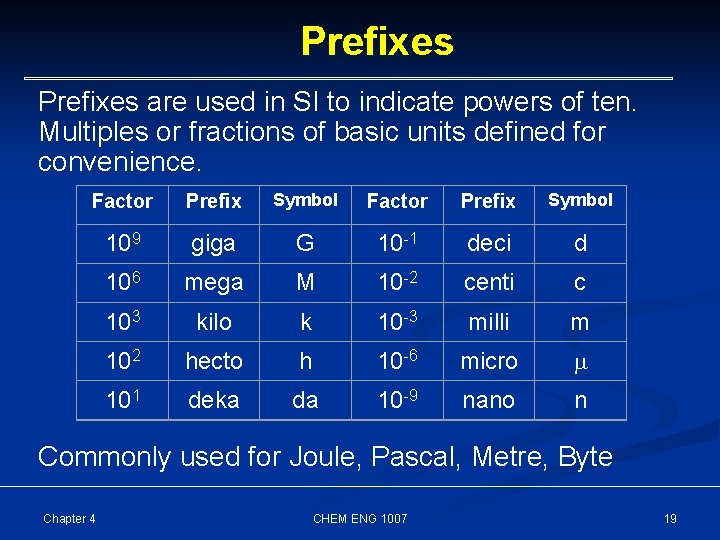
Prefixes are used in SI to indicate powers of ten. Multiples or fractions of basic units defined for convenience. Factor Prefix Symbol 109 giga G 10 -1 deci d 106 mega M 10 -2 centi c 103 kilo k 10 -3 milli m 102 hecto h 10 -6 micro 101 deka da 10 -9 nano n Commonly used for Joule, Pascal, Metre, Byte Chapter 4 CHEM ENG 1007 19

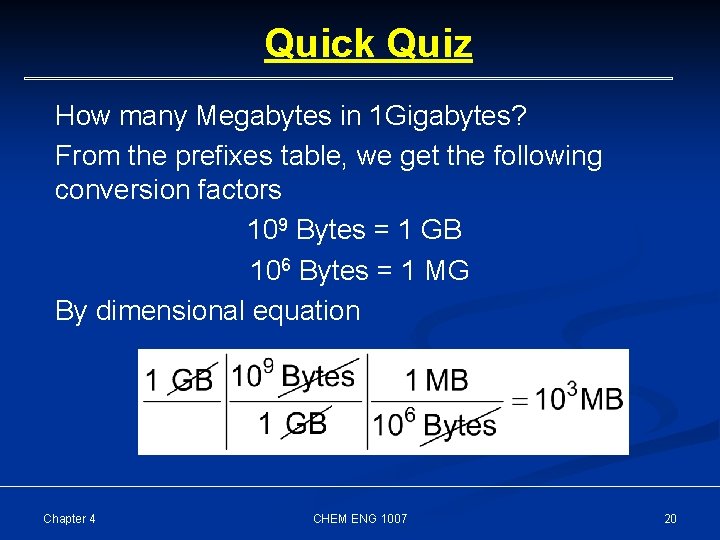
Quick Quiz How many Megabytes in 1 Gigabytes? From the prefixes table, we get the following conversion factors 109 Bytes = 1 GB 106 Bytes = 1 MG By dimensional equation Chapter 4 CHEM ENG 1007 20

Quick Quiz How many micrograms in 5 kilograms? Again from the prefixes table, we get the following conversion factors 103 g = 1 kg 10 -6 g = 1 g Chapter 4 CHEM ENG 1007 21

Quick Quiz How many millimetres in 1 nanometres? Again from the prefixes table, we get the following conversion factors 10 -3 m = 1 mm 10 -9 m = 1 nm Chapter 4 CHEM ENG 1007 22

Quick Quiz Write conversion factors that relate to each of the following pairs of units: 1. Litres and m. L 1 L = 1000 m. L 2. Hours and seconds 1 h = 3600 s 3. mg and kg 106 mg = 1 kg 4. Nanometers and kilometers 1012 nm = 1 km Chapter 4 CHEM ENG 1007 23

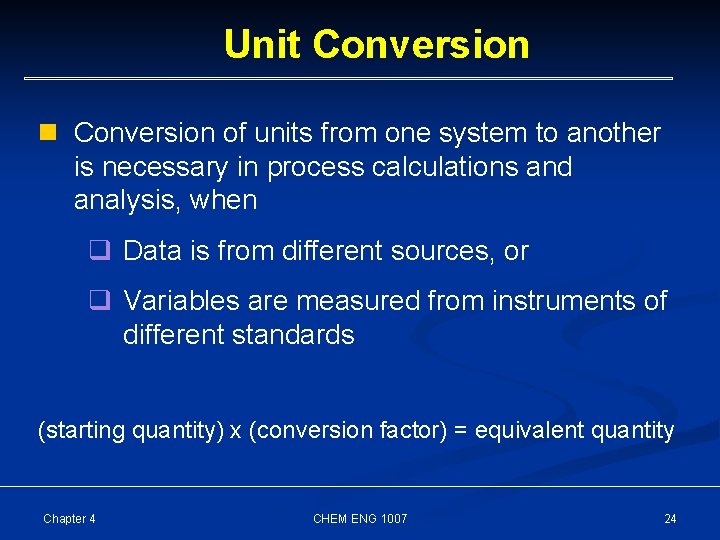
Unit Conversion n Conversion of units from one system to another is necessary in process calculations and analysis, when q Data is from different sources, or q Variables are measured from instruments of different standards (starting quantity) x (conversion factor) = equivalent quantity Chapter 4 CHEM ENG 1007 24

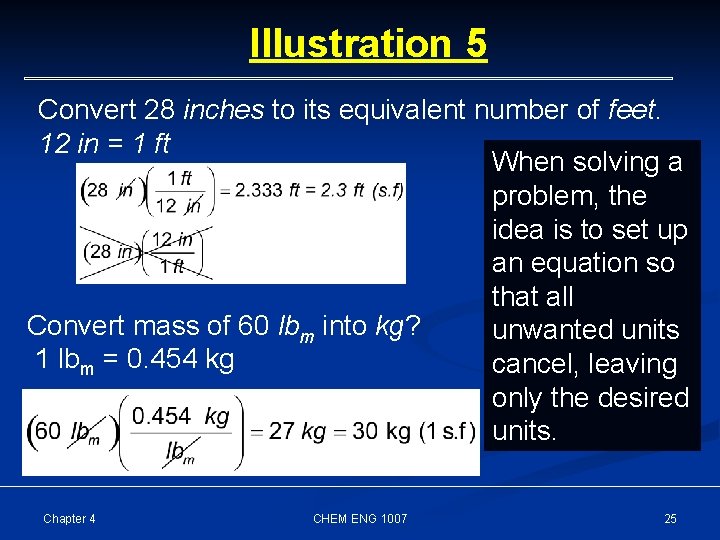
Illustration 5 Convert 28 inches to its equivalent number of feet. 12 in = 1 ft When solving a problem, the idea is to set up an equation so that all Convert mass of 60 lbm into kg? unwanted units 1 lbm = 0. 454 kg cancel, leaving only the desired units. Chapter 4 CHEM ENG 1007 25

Dimensional Equation A convenient method for unit conversion. How to set up a dimensional equation? Write the given quantity and its units on the left, write the units of conversion factors that cancel the old units and replace them with the described ones, fill in the values of the conversion factors, and carry out the indicated arithmetic to find the desired value. Chapter 4 CHEM ENG 1007 26

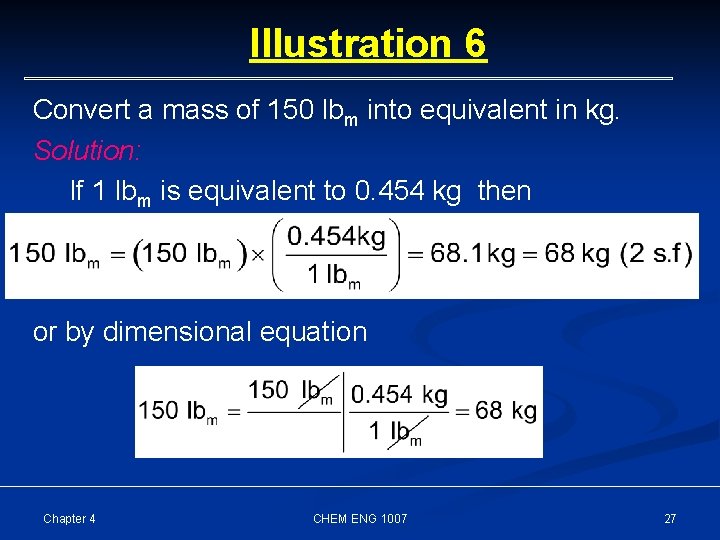
Illustration 6 Convert a mass of 150 lbm into equivalent in kg. Solution: If 1 lbm is equivalent to 0. 454 kg then or by dimensional equation Chapter 4 CHEM ENG 1007 27

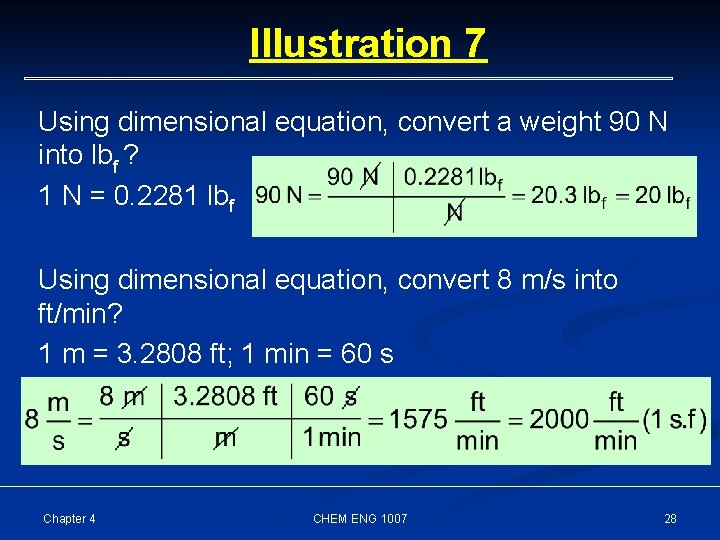
Illustration 7 Using dimensional equation, convert a weight 90 N into lbf ? 1 N = 0. 2281 lbf Using dimensional equation, convert 8 m/s into ft/min? 1 m = 3. 2808 ft; 1 min = 60 s Chapter 4 CHEM ENG 1007 28

Quick Quiz Convert a 40 mile/gal into km/L Convert a force of 40 lbf into equivalent in N. Chapter 4 CHEM ENG 1007 29

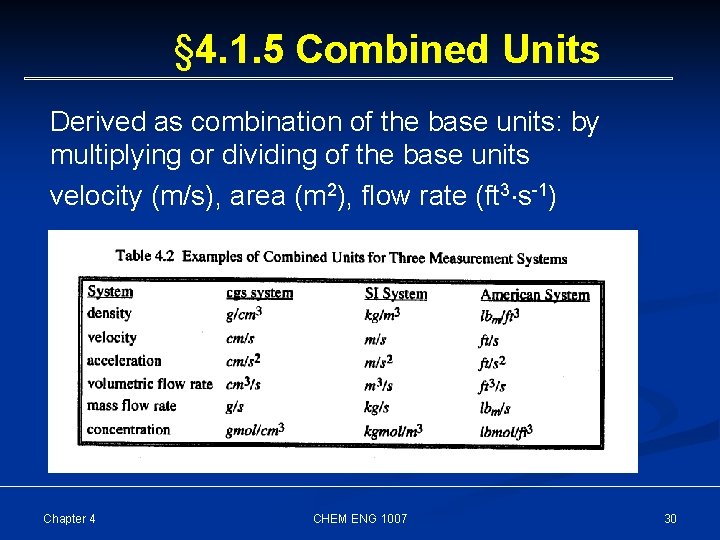
§ 4. 1. 5 Combined Units Derived as combination of the base units: by multiplying or dividing of the base units velocity (m/s), area (m 2), flow rate (ft 3 s-1) Chapter 4 CHEM ENG 1007 30

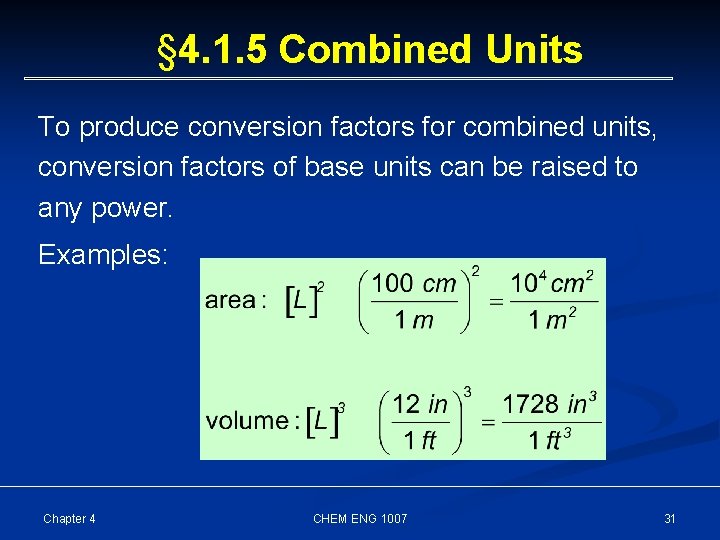
§ 4. 1. 5 Combined Units To produce conversion factors for combined units, conversion factors of base units can be raised to any power. Examples: Chapter 4 CHEM ENG 1007 31

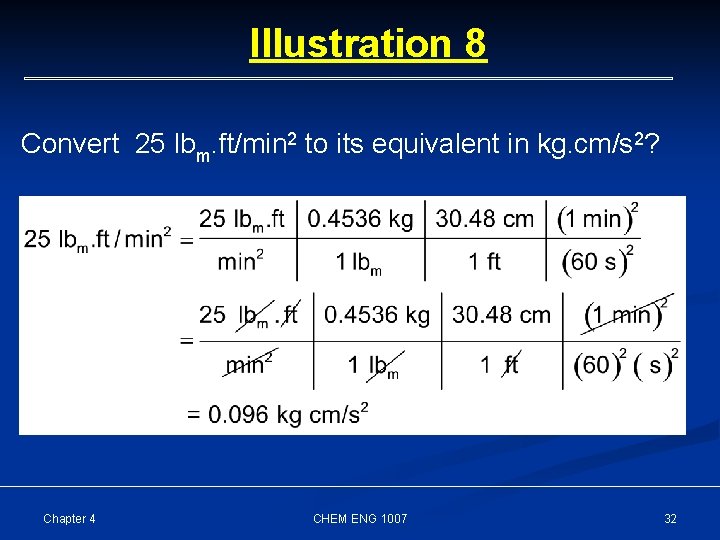
Illustration 8 Convert 25 lbm. ft/min 2 to its equivalent in kg. cm/s 2? Chapter 4 CHEM ENG 1007 32

Quick Quiz Convert 45 m 3/d 2 to its equivalent in mm 3/s 2? Chapter 4 CHEM ENG 1007 33

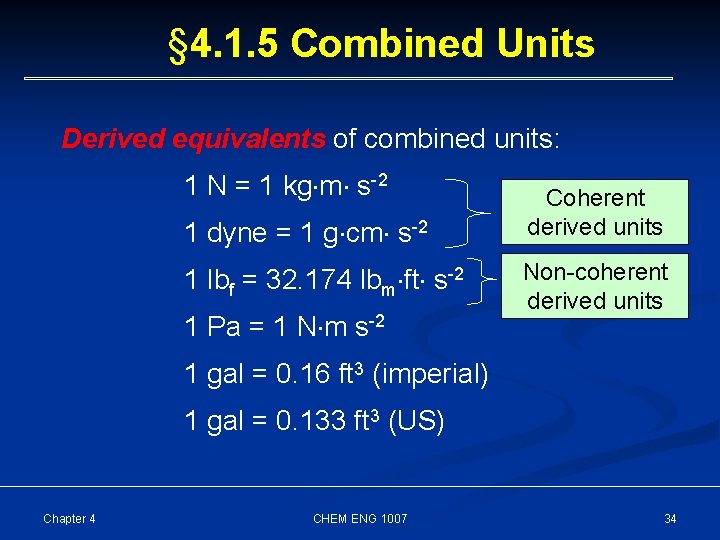
§ 4. 1. 5 Combined Units Derived equivalents of combined units: 1 N = 1 kg m s-2 1 dyne = 1 g cm s-2 1 lbf = 32. 174 lbm ft s-2 1 Pa = 1 N m s-2 Coherent derived units Non-coherent derived units 1 gal = 0. 16 ft 3 (imperial) 1 gal = 0. 133 ft 3 (US) Chapter 4 CHEM ENG 1007 34

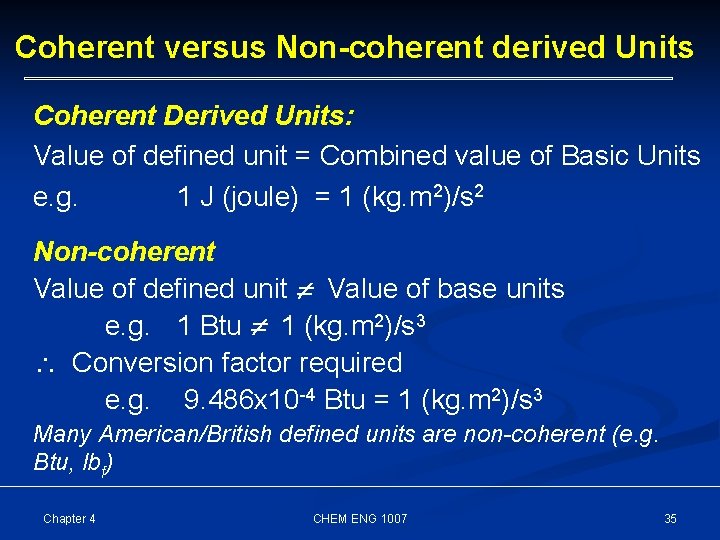
Coherent versus Non-coherent derived Units Coherent Derived Units: Value of defined unit = Combined value of Basic Units e. g. 1 J (joule) = 1 (kg. m 2)/s 2 Non-coherent Value of defined unit Value of base units e. g. 1 Btu 1 (kg. m 2)/s 3 Conversion factor required e. g. 9. 486 x 10 -4 Btu = 1 (kg. m 2)/s 3 Many American/British defined units are non-coherent (e. g. Btu, lbf) Chapter 4 CHEM ENG 1007 35

Summary for Units Conversion To convert a quantity expressed in terms of one unit to its equivalent in terms of another unit, multiply the given quantity by the conversion factor (new unit/old unit). Alternative way: Chapter 4 CHEM ENG 1007 36

§ 4. 2. 5 Dimensional Consistency Rules of dimensional consistency 1. Terms that are added together (or subtracted) must have the same units. For example, in the equation Q = ab + c 2, the units of ab must be the same as those of c 2. 2. Exponents must be unitless (dimensionless). Thus, if an exponent consists of several terms, the units of all those terms must cancel. For example, in the equation y = xab/c, the units in the term ab/c must all cancel out to leave no units. Chapter 4 CHEM ENG 1007 37

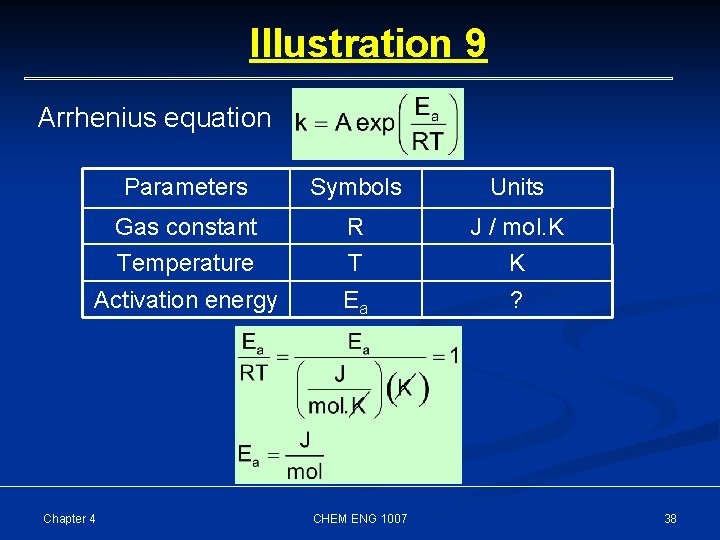
Illustration 9 Arrhenius equation Parameters Symbols Units Gas constant R J / mol. K Temperature T K Activation energy Ea ? Chapter 4 CHEM ENG 1007 38
![Illustration 10 Ideal Gas Equation Parameters Pressure Symbols P Dimensions M L1 T 2 Illustration 10 Ideal Gas Equation Parameters Pressure Symbols P Dimensions [M] [L]-1 [T] -2](https://slidetodoc.com/presentation_image_h/a0c6e35707dde90a4f8d2b69dcc49e77/image-39.jpg)
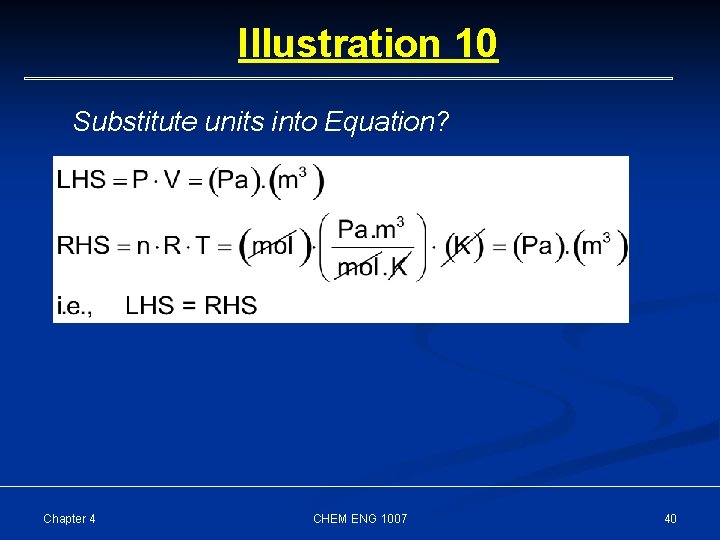
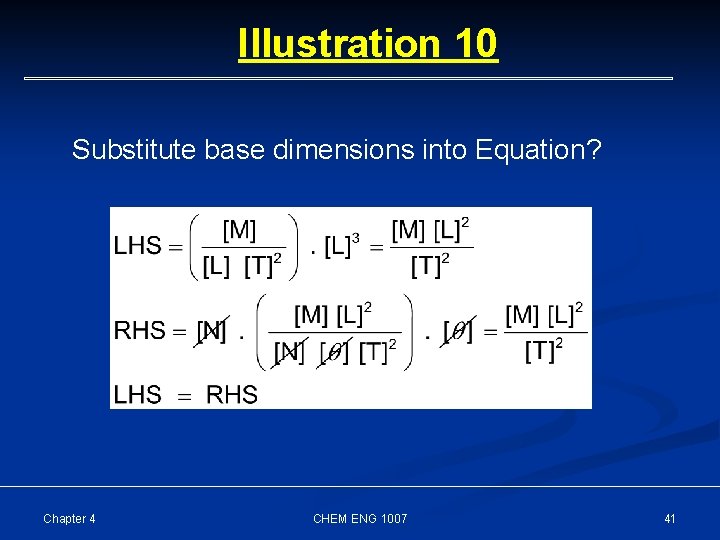
Illustration 10 Ideal Gas Equation Parameters Pressure Symbols P Dimensions [M] [L]-1 [T] -2 Units Pa Volume V [L]3 m 3 # of moles n [N] mol Gas constant R [M] [L] 2 [T] -2 [N]-1 [ ]-1 Pa. m 3/mol. K Temperature T [ ] K In terms of these parameters, show that ideal gas equation obeys the laws of dimensional consistency Chapter 4 CHEM ENG 1007 39

Illustration 10 Substitute units into Equation? Chapter 4 CHEM ENG 1007 40

Illustration 10 Substitute base dimensions into Equation? Chapter 4 CHEM ENG 1007 41

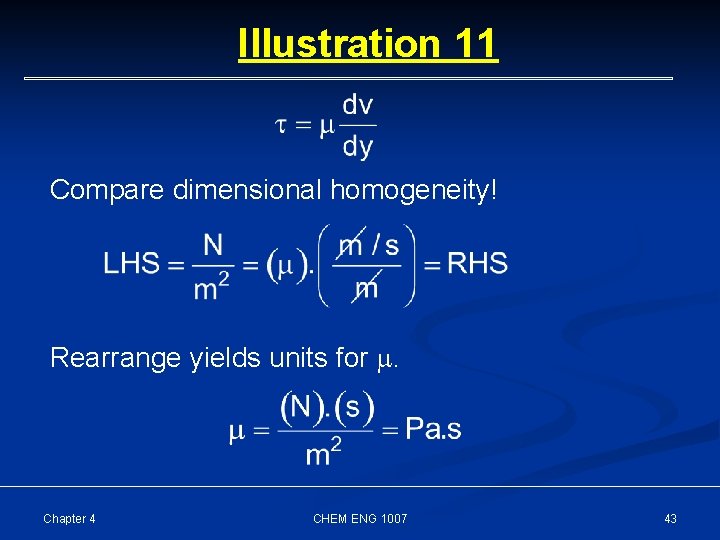
Illustration 11 Consider this equation What are SI units of ? Chapter 4 CHEM ENG 1007 42

Illustration 11 Compare dimensional homogeneity! Rearrange yields units for . Chapter 4 CHEM ENG 1007 43

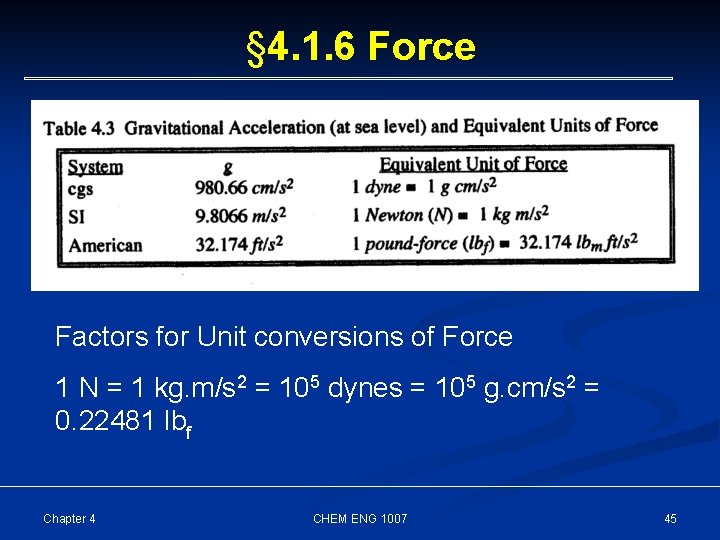
§ 4. 1. 6 Force The force “F” to accelerate a mass “m” at an acceleration rate “a” is defined by Newton’s second law as F = ma [M. L. T-2] One form of acceleration is that associated with earth’s gravity. The rate of acceleration is described by the gravitational acceleration, g, and the force that an object exerts on the earth’s surface (its “weight”), is W = mg Chapter 4 CHEM ENG 1007 44

§ 4. 1. 6 Force Factors for Unit conversions of Force 1 N = 1 kg. m/s 2 = 105 dynes = 105 g. cm/s 2 = 0. 22481 lbf Chapter 4 CHEM ENG 1007 45

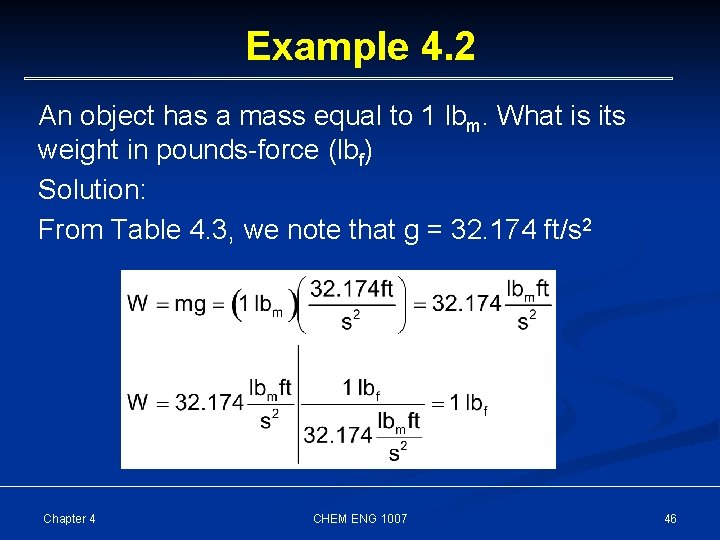
Example 4. 2 An object has a mass equal to 1 lbm. What is its weight in pounds-force (lbf) Solution: From Table 4. 3, we note that g = 32. 174 ft/s 2 Chapter 4 CHEM ENG 1007 46

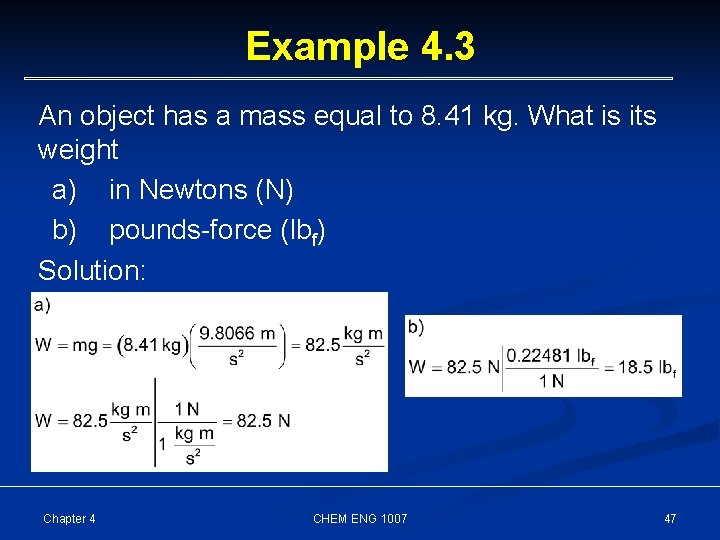
Example 4. 3 An object has a mass equal to 8. 41 kg. What is its weight a) in Newtons (N) b) pounds-force (lbf) Solution: Chapter 4 CHEM ENG 1007 47

Quick Quiz From Reading Question 4: The weight of an astronaut is measured on a distant planet and found to be one-fifth of his weight on the earth’s surface. Is his mass different on that distant planet than on earth? What does the weight difference imply about the acceleration of gravity on the distant planet? Chapter 4 CHEM ENG 1007 48

Summary P 2 case-studies PIdentified the difference in SI, American Engineering, and CGS units system PUnderstand Ø Ø Ø conversion factors dimensional equations dimensionless dimensional consistency coherent and non-coherent derived units Force/Weight PAble to convert between sets of units Chapter 4 CHEM ENG 1007 49