Temperature Conversions Temperature Definition Temperature is the average



- Slides: 12

Temperature Conversions

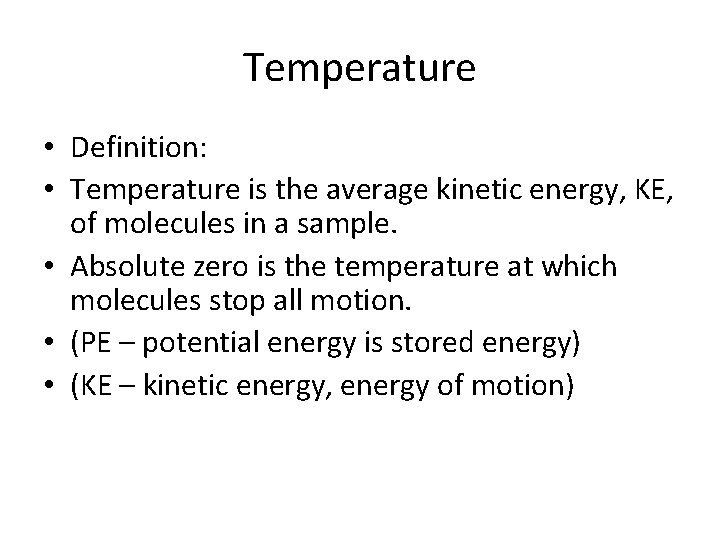
Temperature • Definition: • Temperature is the average kinetic energy, KE, of molecules in a sample. • Absolute zero is the temperature at which molecules stop all motion. • (PE – potential energy is stored energy) • (KE – kinetic energy, energy of motion)

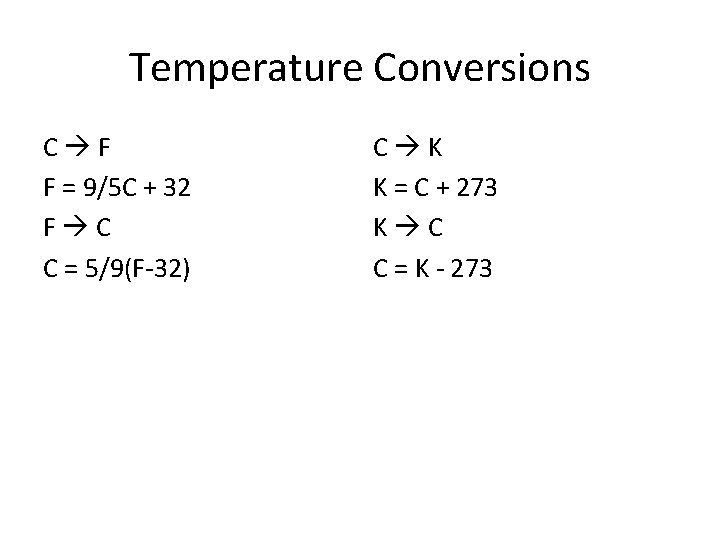
Temperature Conversions C F F = 9/5 C + 32 F C C = 5/9(F-32) C K K = C + 273 K C C = K - 273

Comparing Temperature Scales 212

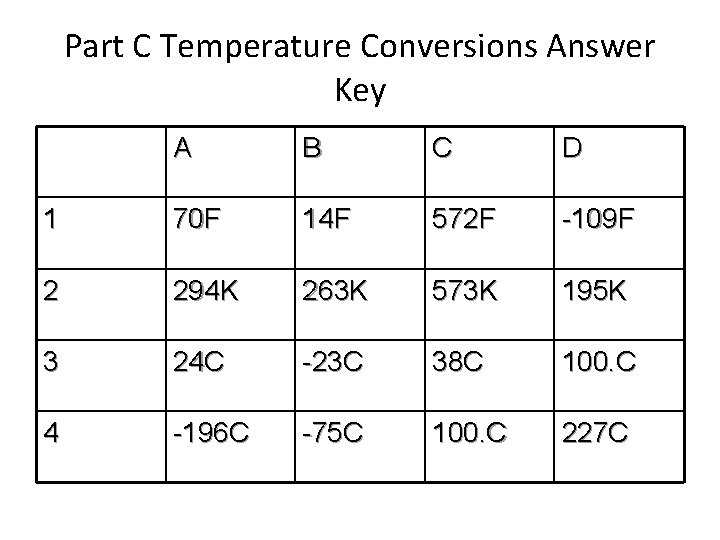
Temperature Conversions C. Do the following problems on another sheet of paper. Use the conversion equations from above. Show all your work: Knowns & Unknowns, Set-Up & Answer (K = C + 273, F = 9/5 C + 32) 1. Convert the following centigrade temperatures into fahrenheit. a. 21 'C (A: 70'F) b. -10 'C c. 300 'C d. -78. 5 'C 2. Convert the following centigrade temperatures into kelvin. a. 21 'C (A: 294 K) b. -10 'C c. 300 'C d. -78. 5 'C 3. Convert the following fahrenheit temperatures into celcius. a. 75 'F (A: 24 'C) b. -10 'F c. 100 'F d. 212 'F 4. Convert the following kelvin temperatures into celcius. a. 77 K (A: -196 'C) b. 198. 5 K c. 373 K d. 500 K

Part C Temperature Conversions Answer Key A B C D 1 70 F 14 F 572 F -109 F 2 294 K 263 K 573 K 195 K 3 24 C -23 C 38 C 100. C 4 -196 C -75 C 100. C 227 C

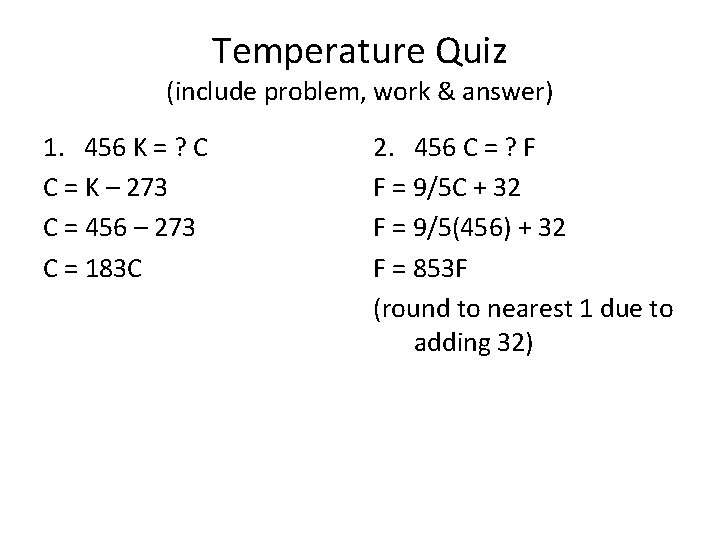
Temperature Quiz (include problem, work & answer) 1. 456 K = ? C C = K – 273 C = 456 – 273 C = 183 C 2. 456 C = ? F F = 9/5 C + 32 F = 9/5(456) + 32 F = 853 F (round to nearest 1 due to adding 32)

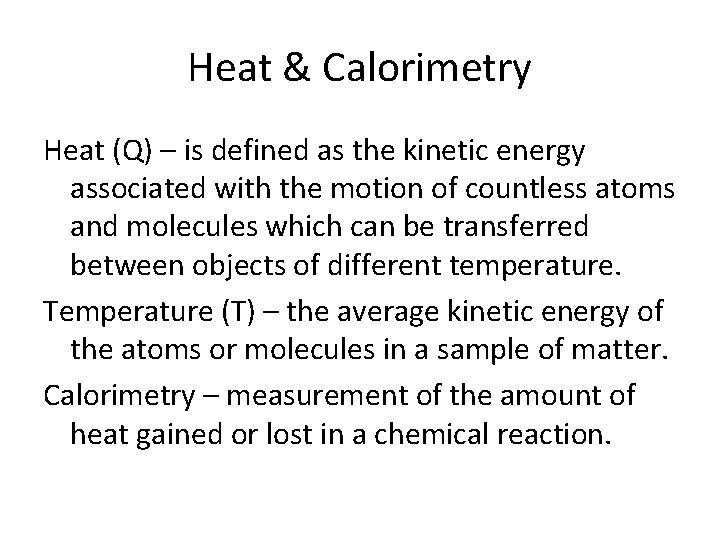
Heat & Calorimetry Heat (Q) – is defined as the kinetic energy associated with the motion of countless atoms and molecules which can be transferred between objects of different temperature. Temperature (T) – the average kinetic energy of the atoms or molecules in a sample of matter. Calorimetry – measurement of the amount of heat gained or lost in a chemical reaction.

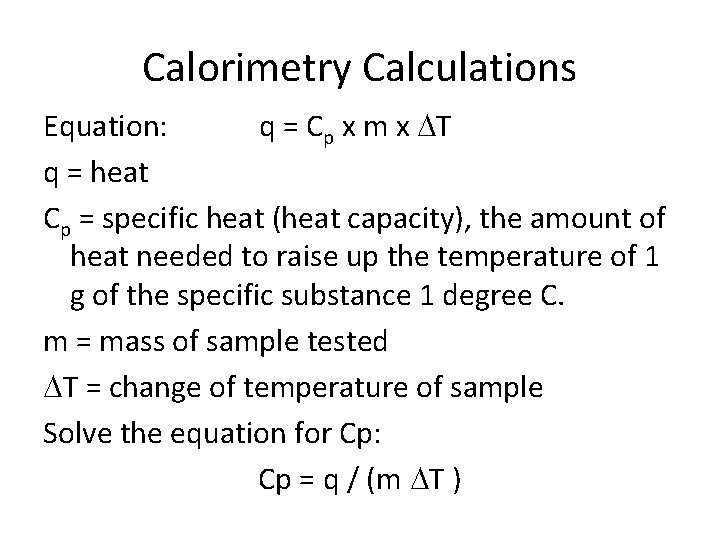
Calorimetry Calculations Equation: q = Cp x m x DT q = heat Cp = specific heat (heat capacity), the amount of heat needed to raise up the temperature of 1 g of the specific substance 1 degree C. m = mass of sample tested DT = change of temperature of sample Solve the equation for Cp: Cp = q / (m DT )

Specific Heat Lab • http: //sciencegeek. net/Virtual. Labs/Specific. He at. Lab. html Do 3 trials for each substance: wood, copper, glass, silver, sodium & water Make 2 tables for each substance: Observations & Data Table, Calculations Table Calculate Cp for all 6 substances with 3 trials each for a total of 18 calculations.

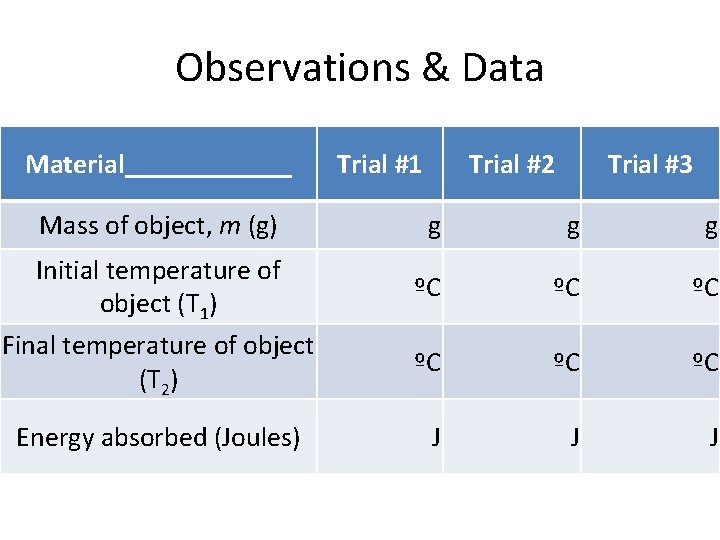
Observations & Data Material______ Mass of object, m (g) Initial temperature of object (T 1) Final temperature of object (T 2) Energy absorbed (Joules) Trial #1 Trial #2 Trial #3 g g g ºC ºC ºC J J J

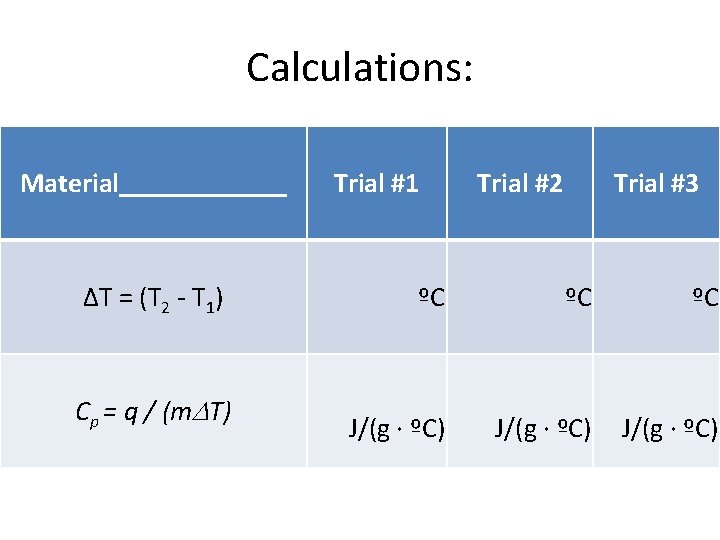
Calculations: Material______ Trial #1 Trial #2 Trial #3 ΔT = (T 2 - T 1) ºC ºC ºC Cp = q / (m. DT) J/(g · ºC)