Unit Conversions II 1 UNIT CONVERSIONS This is








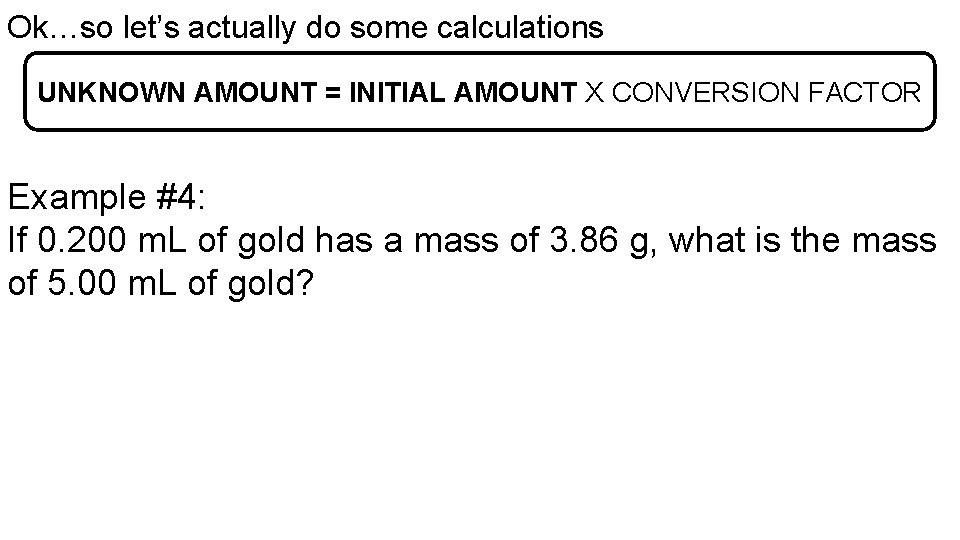
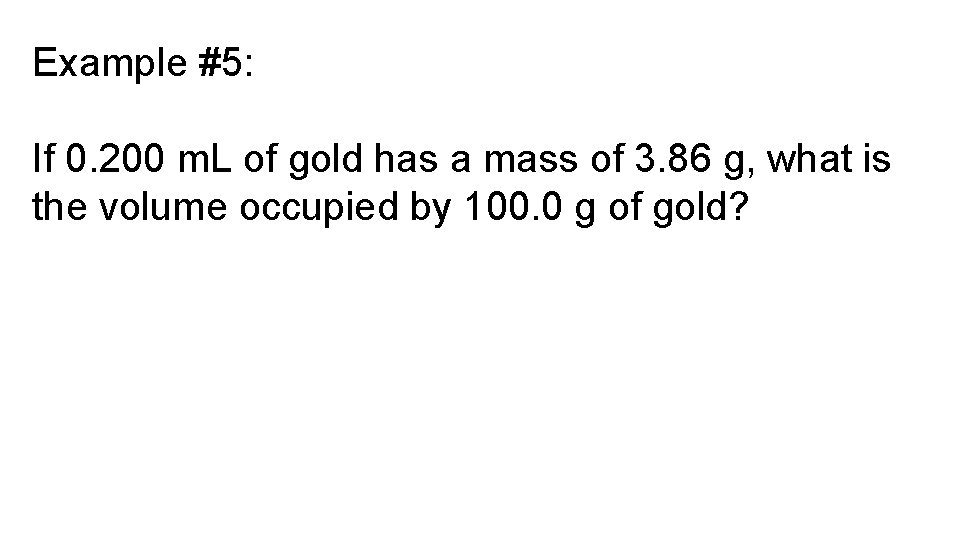













- Slides: 29

Unit Conversions

II. 1 UNIT CONVERSIONS • This is a mathematical process that can be used to solve questions in MANY other subjects besides chemistry • I DON’T CARE if you have a different method for solving these questions, YOU HAVE TO USE MINE!
The price of eggs is $1. 44 for 1 dozen eggs. You can show the relationship between the cost and the number of eggs in 2 different ways:

These are conversion factors “a fractional expression relating two different units” Using conversion factors makes changing between different units VERY EASY

Example #1: How many minutes are there in 3480 seconds?

Every unit conversion problem requires 3 main pieces of information: 1. the unknown amount (and its UNIT) 2. the initial amount (and its UNIT) 3. a conversion factor which relates #1 & 2

ALWAYS INCLUDE UNITS IN YOUR CALCULATIONS! IF YOU DON’T, YOU WON’T KNOW WHAT THE HECK YOU’RE CALCULATING!

Example #2: What is the cost of 2 dozen eggs if eggs are $1. 44/doz? Example #3: If a car can go 80 km in 1 h, how far can the car go in 8. 5 h?

Q. 1 pg. 11
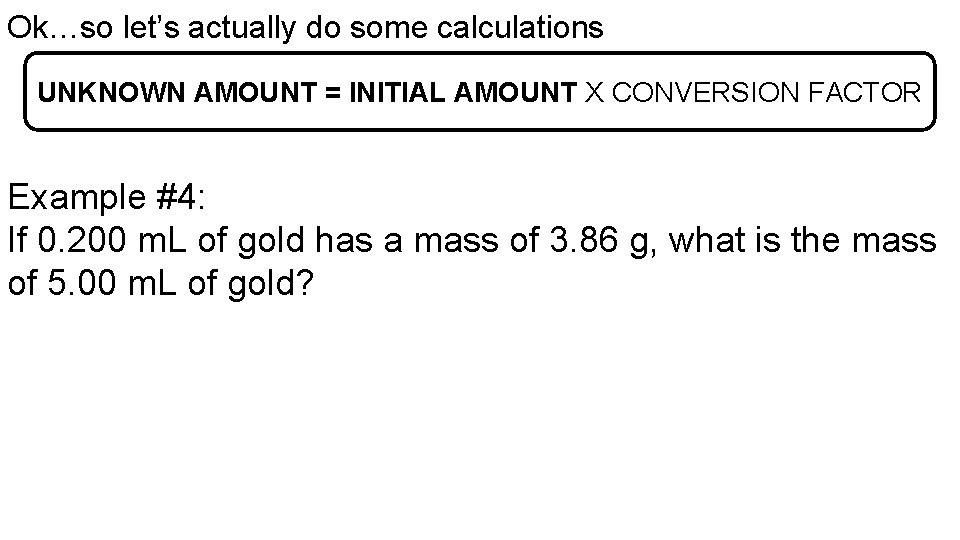
Ok…so let’s actually do some calculations UNKNOWN AMOUNT = INITIAL AMOUNT X CONVERSION FACTOR Example #4: If 0. 200 m. L of gold has a mass of 3. 86 g, what is the mass of 5. 00 m. L of gold?
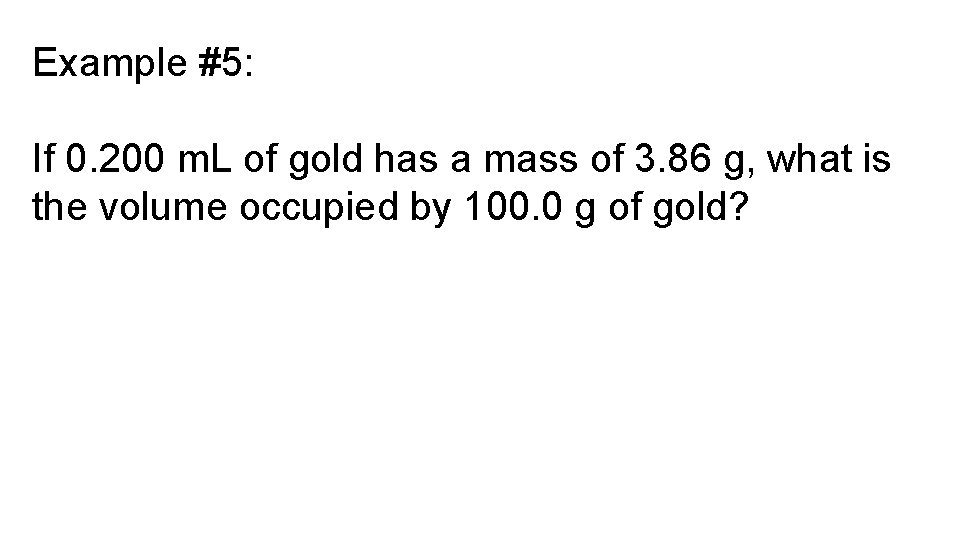
Example #5: If 0. 200 m. L of gold has a mass of 3. 86 g, what is the volume occupied by 100. 0 g of gold?

THE STEPS TO FOLLOW FOR UNIT CONVERSIONS 1. Identify the unknown amount (and its unit) 2. Identify the initial amount (and its unit) 3. Identify the conversion factor (and its unit) 4. Write down what you are trying to find 5. Write down an = sign 6. Write down your initial amount 7. Write down a X sign 8. Write down your conversion factor (in the correct form so that the units of your initial amount are cancelled) 9. Write down an = sign 10. Plug all the numbers into your calculator and then write out the answer.

Q. 2 pg. 14

MULTIPLE UNIT CONVERSIONS Don’t panic…these aren’t much worse than the questions you just finished… Example #6: If eggs are $1. 44/doz. , and if there are 12 eggs/doz. , how many individual eggs can be bought for $4. 32?

Example #7: The automobile gas tank of a Canadian tourist holds 39. 5 L of gas. If 1 L of gas is equal to 0. 264 gal in the United States, and gas is $1. 26/gal in Dallas, Texas, how much will it cost the tourist to fill his gas tank in Dallas?

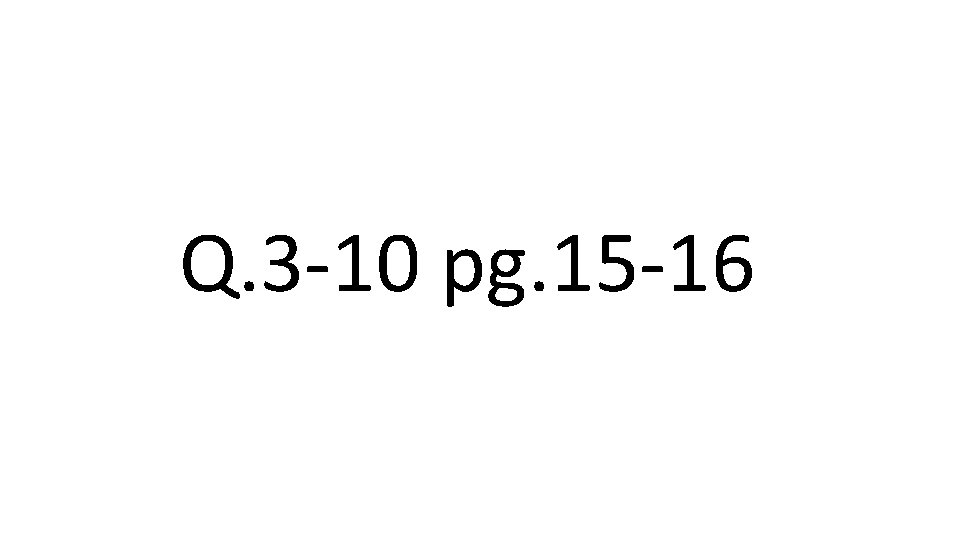
Q. 3 -10 pg. 15 -16

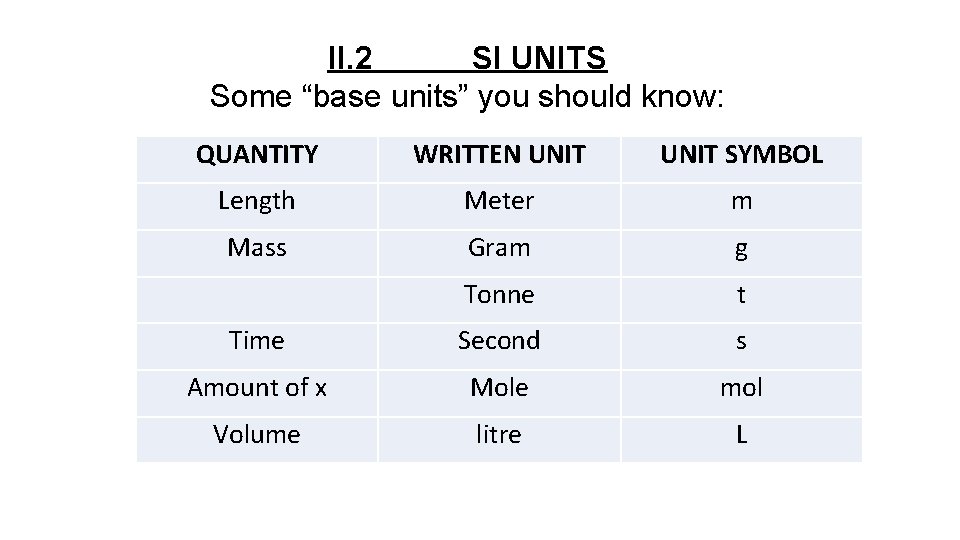
II. 2 SI UNITS Some “base units” you should know: QUANTITY WRITTEN UNIT SYMBOL Length Meter m Mass Gram g Tonne t Time Second s Amount of x Mole mol Volume litre L

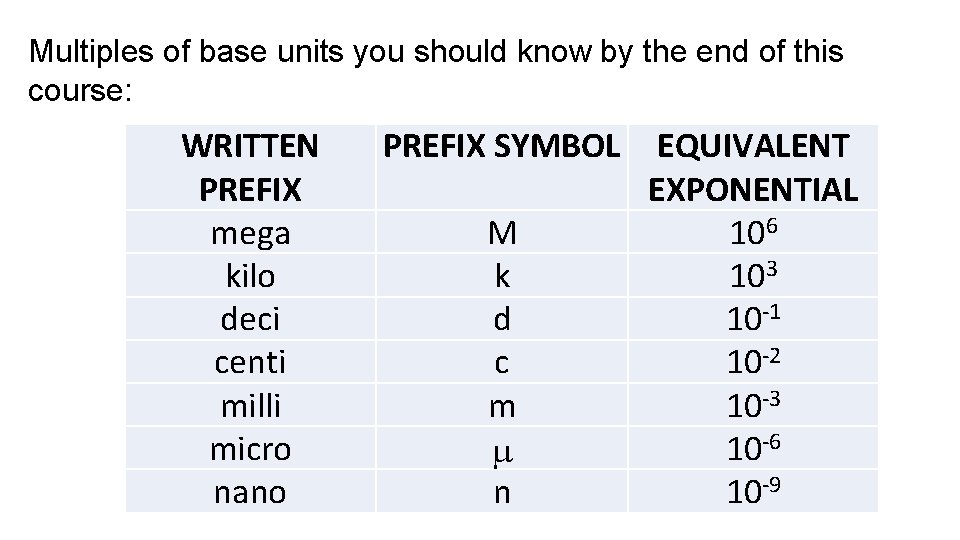
Multiples of base units you should know by the end of this course: WRITTEN PREFIX mega kilo deci centi milli micro nano PREFIX SYMBOL M k d c m n EQUIVALENT EXPONENTIAL 106 103 10 -1 10 -2 10 -3 10 -6 10 -9

3 Important facts to remember: 3 1 m. L = 1 cm 1 m 3 = 103 L 3 1 t = 10 kg

Metric conversions work the same as any other unit conversion. MOST IMPORTANT POINTS TO REMEMBER: 1. ALWAYS include units 2. ALWAYS cancel units 3. Prefixes NEVER go beside exponents

Example: 5 kg into g: 46. 0 L into n. L: 18. 2 mm into m:

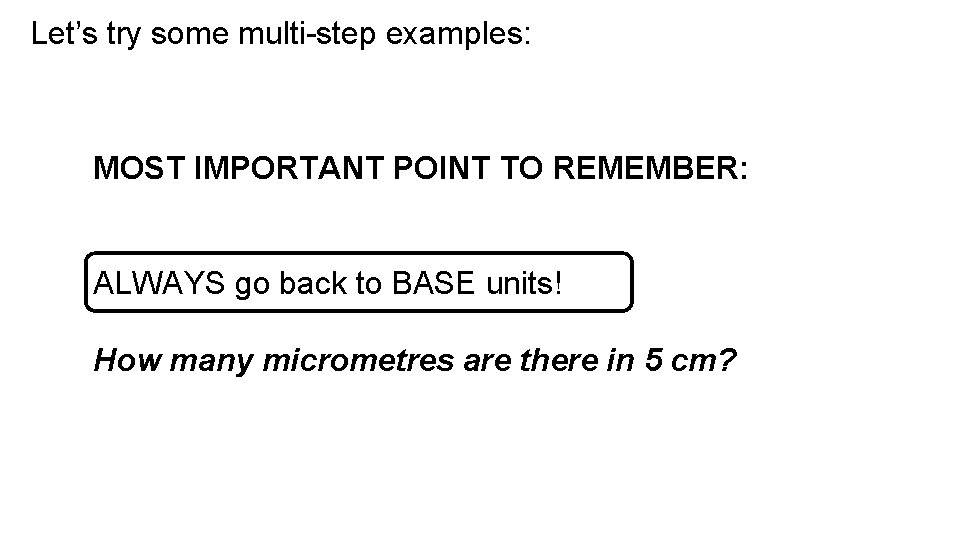
Let’s try some multi-step examples: MOST IMPORTANT POINT TO REMEMBER: ALWAYS go back to BASE units! How many micrometres are there in 5 cm?

Express 8 kg in milligrams Express 5 Mg/m. L in kilograms/litre

Shortcut! If aluminum is worth $0. 00116/g, what is the cost of 725 kg of aluminum? The “normal” way to solve the question…

The shortcut…

Q. 16 -19 pg. 21 -22 For extra practice, try Q: 20 - 28

Temperature Conversions This isn’t in your textbook so don’t search there for it! 1) Celcius to Fahrenheit °F = 9/5 (°C) + 32

2) Fahrenheit to Celsius °C = 5/9(°F – 32) 3) Celsius to Kelvin K = °C + 273. 15

Pressure Conversions This isn’t in your textbook either! 760 mm. Hg = 760 Torr = 1 Atm = 101325 Pa = 101. 325 k. Pa