Temperature Chapter 46 What is Temperature Temperature is




- Slides: 14

Temperature Chapter 46

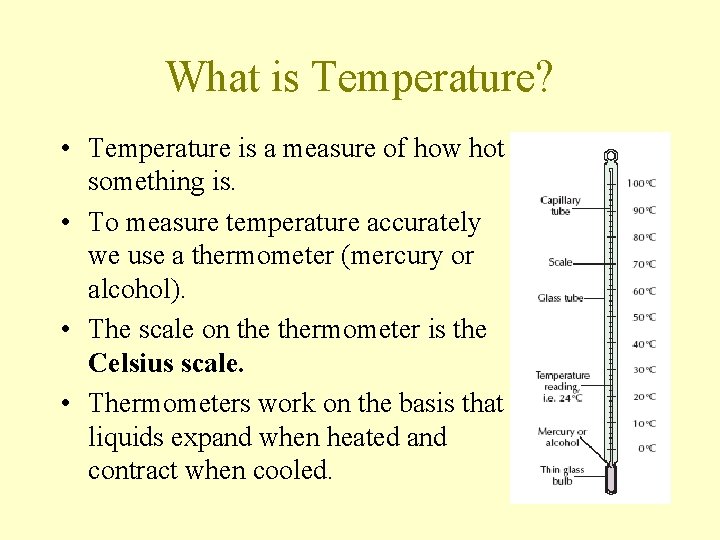
What is Temperature? • Temperature is a measure of how hot something is. • To measure temperature accurately we use a thermometer (mercury or alcohol). • The scale on thermometer is the Celsius scale. • Thermometers work on the basis that liquids expand when heated and contract when cooled.

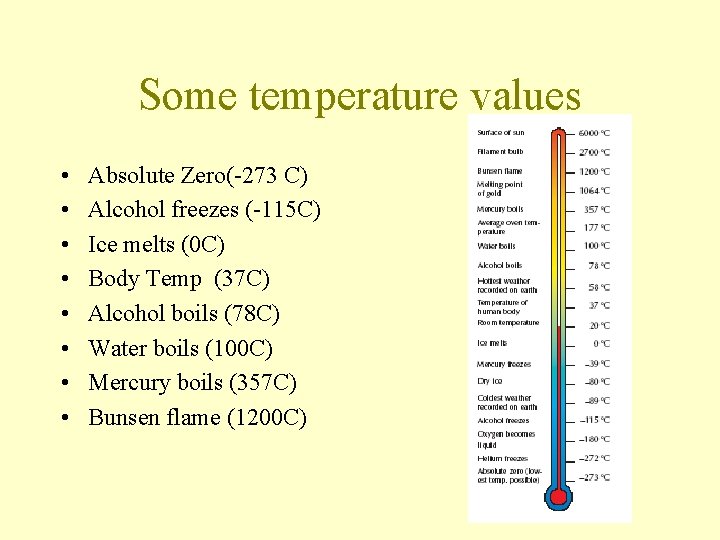
Some temperature values • • Absolute Zero(-273 C) Alcohol freezes (-115 C) Ice melts (0 C) Body Temp (37 C) Alcohol boils (78 C) Water boils (100 C) Mercury boils (357 C) Bunsen flame (1200 C)

Heat and Temperature • Heat and temperature are not the same thing. • The amount of heat in a substance depends on its temperature, its mass , and what the substance is. • A bath of water and a cup of water each have a temperature of 60 C. Which will cool down first? • A bath of water will contain more heat than a cup of water therefore the cup of heat will cool down first.

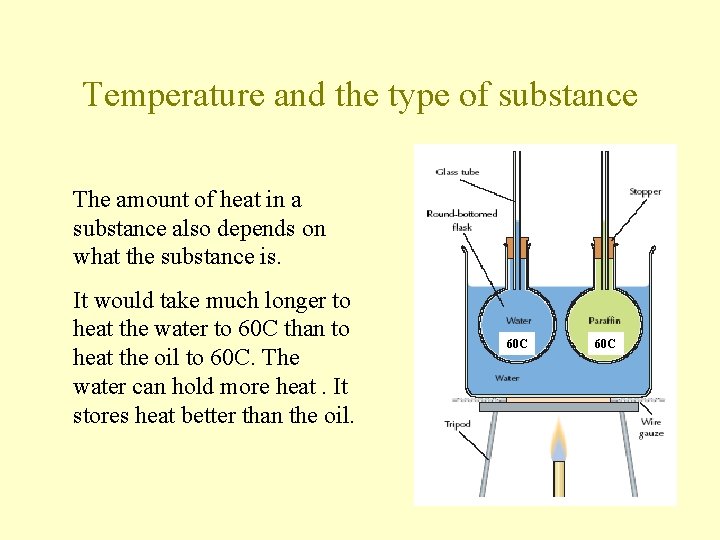
Temperature and the type of substance The amount of heat in a substance also depends on what the substance is. It would take much longer to heat the water to 60 C than to heat the oil to 60 C. The water can hold more heat. It stores heat better than the oil. 60 C

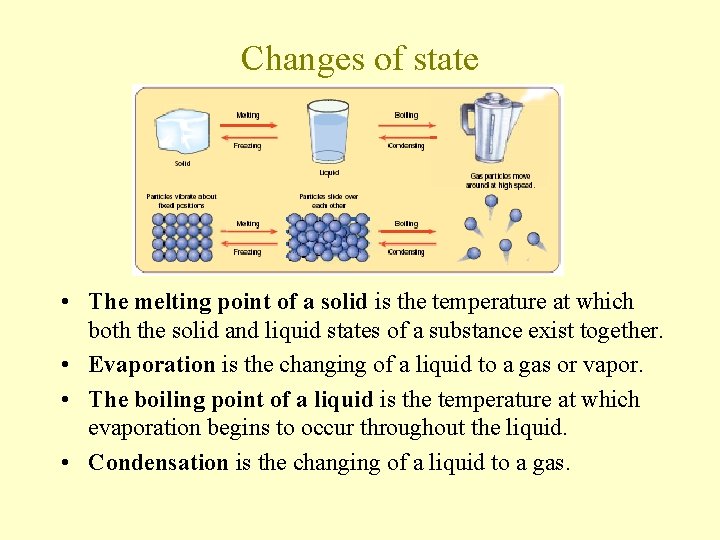
Changes of state • The melting point of a solid is the temperature at which both the solid and liquid states of a substance exist together. • Evaporation is the changing of a liquid to a gas or vapor. • The boiling point of a liquid is the temperature at which evaporation begins to occur throughout the liquid. • Condensation is the changing of a liquid to a gas.

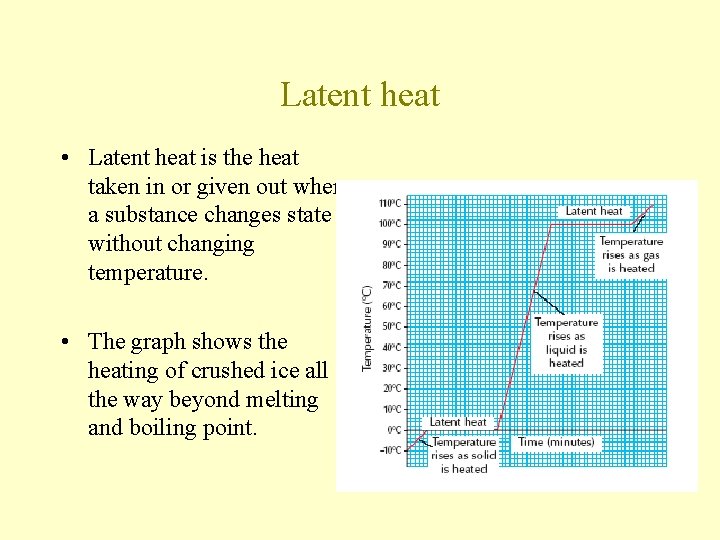
Latent heat • Latent heat is the heat taken in or given out when a substance changes state without changing temperature. • The graph shows the heating of crushed ice all the way beyond melting and boiling point.

Latent heat Placing large vats of water in greenhouses protects fruit from freezing; the heat liberated when the water freezes warms the air

Latent heat Panting wolf rids its body of 540 calories of heat energy with each gram of water vapor it exhales

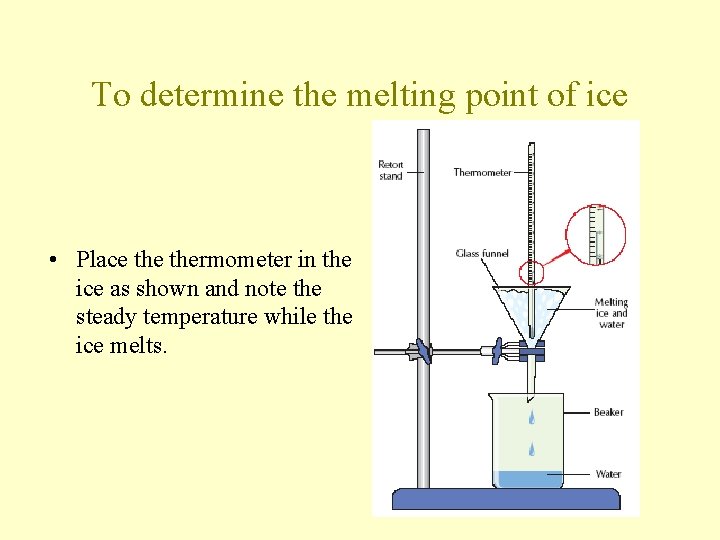
To determine the melting point of ice • Place thermometer in the ice as shown and note the steady temperature while the ice melts.

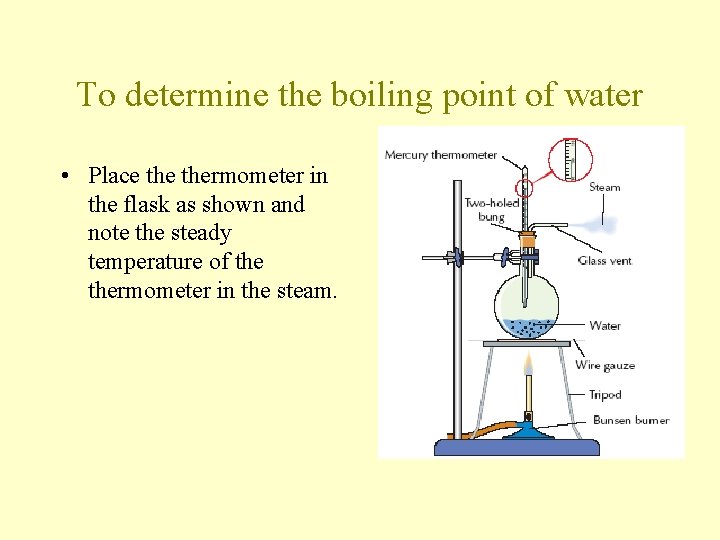
To determine the boiling point of water • Place thermometer in the flask as shown and note the steady temperature of thermometer in the steam.

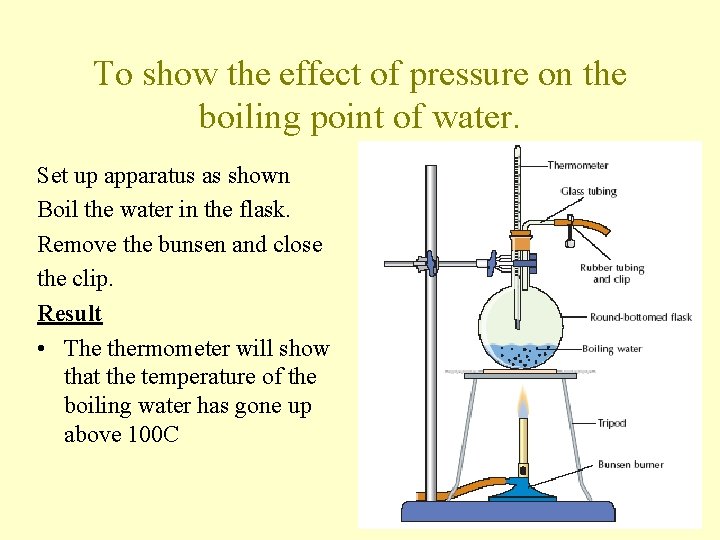
To show the effect of pressure on the boiling point of water. Set up apparatus as shown Boil the water in the flask. Remove the bunsen and close the clip. Result • The thermometer will show that the temperature of the boiling water has gone up above 100 C

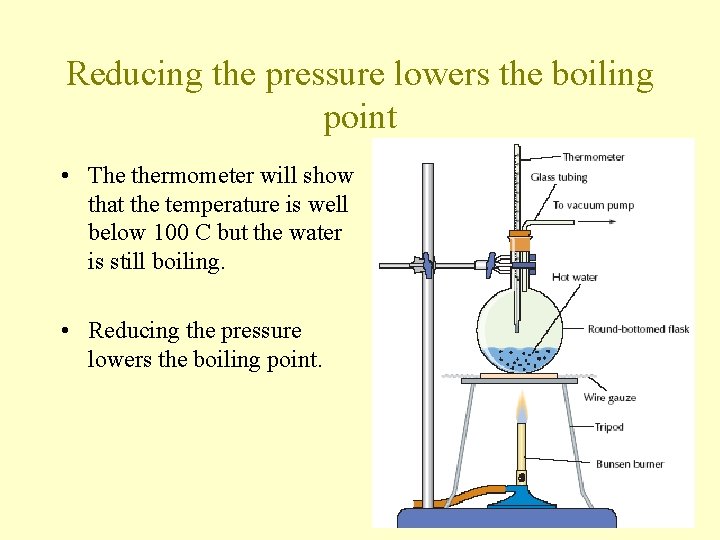
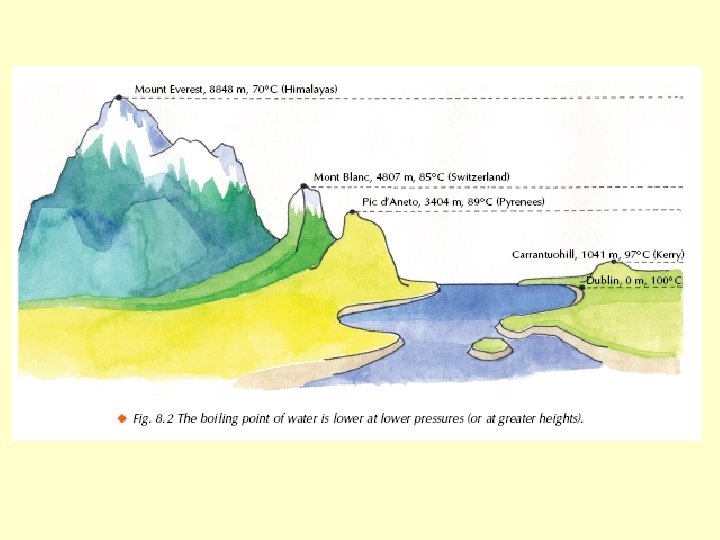
Reducing the pressure lowers the boiling point • The thermometer will show that the temperature is well below 100 C but the water is still boiling. • Reducing the pressure lowers the boiling point.
