Electrical conductivity in solids Solid metals such as

















- Slides: 22

Electrical conductivity in solids

Solid metals, such as aluminium, lead and sodium (right) are good conductors of electricity.

Metals continue to conduct electricity when melted. This is mercury, which is liquid at room temperature.

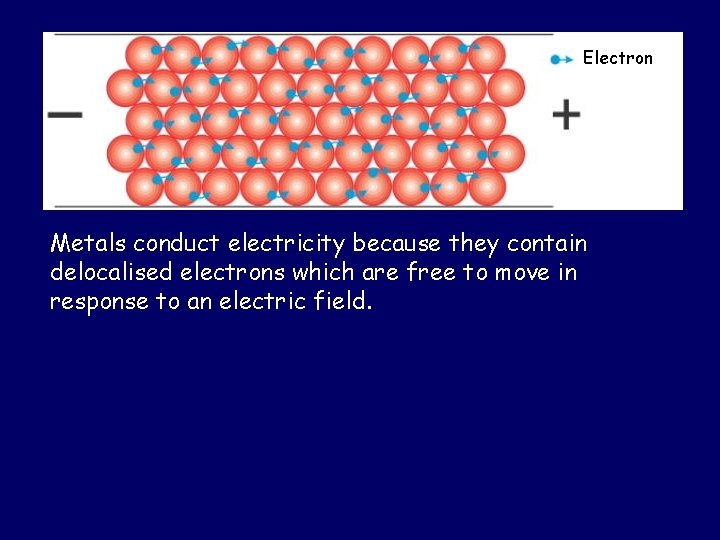
Electron Metals conduct electricity because they contain delocalised electrons which are free to move in response to an electric field.

Rock salt is solid sodium chloride. Solid sodium chloride does not conduct electricity.

We put a few drops of water onto the rock salt. As soon as some of the salt dissolves in the water, it begins to conduct electricity.

A teaspoon of salt crystals dissolved in 20 m. L of water in an evaporating dish is an excellent conductor of electricity.

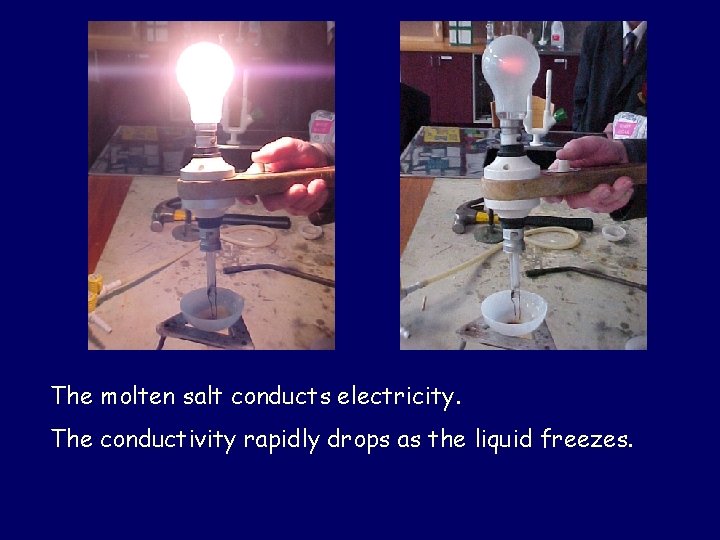
An oxyacetylene burner is needed to melt sodium chloride. As soon as the heat is removed, the liquid starts to set.

The molten salt conducts electricity. The conductivity rapidly drops as the liquid freezes.

Ionic crystals are composed of alternating positive and negative ions. In the solid state these ions are held in position by strong forces. Because they cannot move, the solid does not conduct electricity. When sufficient heat is supplied to overcome the forces holding the ions together, they are free to move around and so the liquid does conduct.

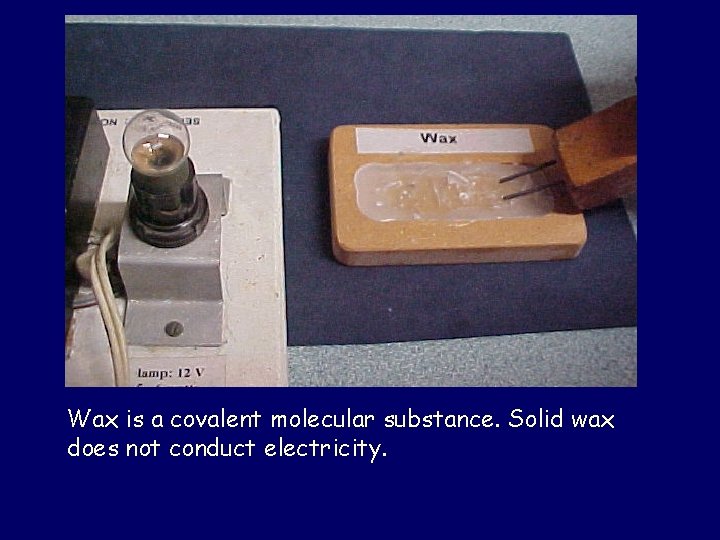
Wax is a covalent molecular substance. Solid wax does not conduct electricity.

Sugar is another molecular substance. Molten sugar does not conduct electricity.

When a teaspoon of sugar is dissolved in water the solution does not conduct electricity.

Iodine and carbon dioxide are also molecular substances. Molecular crystals are composed of a regular arrangement of neutral molecules. Whether solid, melted or in solution, these compounds cannot conduct electricity because the molecules are not charged.

Quartz, Si. O 2 (also known as silica), is a covalent network solid. It does not conduct electricity.

The element silicon is another network solid. It does not conduct electricity significantly. (Impure silicon does conduct a little, which is why it is sometimes called a semi-conductor. )

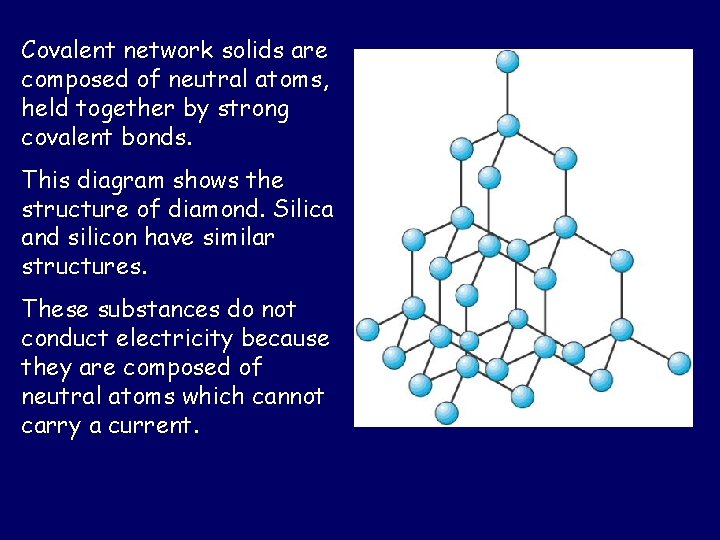
Covalent network solids are composed of neutral atoms, held together by strong covalent bonds. This diagram shows the structure of diamond. Silica and silicon have similar structures. These substances do not conduct electricity because they are composed of neutral atoms which cannot carry a current.

Diamond and graphite are two different allotropes of carbon. Their properties are different because their atoms are arranged in different ways. Graphite Diamond

Graphite is special because it is the only non-metal to conduct electricity well.

Notice the way the graphite crystals break (easily) into flat sheets.

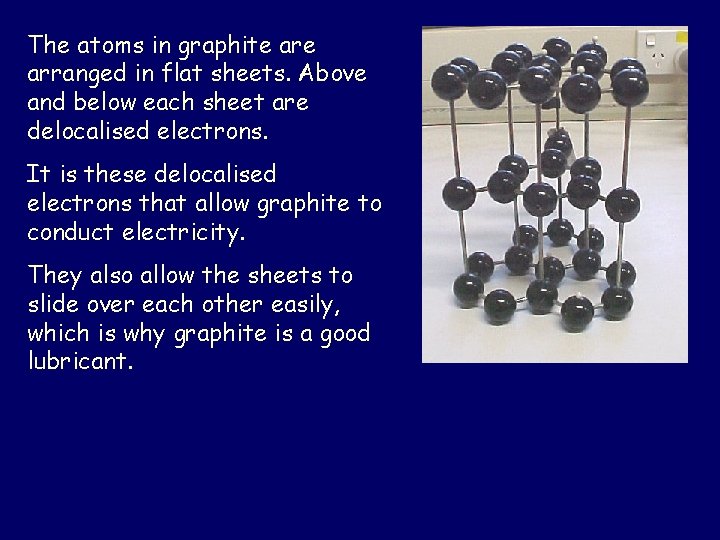
The atoms in graphite arranged in flat sheets. Above and below each sheet are delocalised electrons. It is these delocalised electrons that allow graphite to conduct electricity. They also allow the sheets to slide over each other easily, which is why graphite is a good lubricant.

Powdered graphite is very useful to unjam locks. The grey substance in ‘lead’ pencils is also graphite.