Extraction of metals Only some unreactive metals such






























- Slides: 36

Extraction of metals Only some unreactive metals such as silver, gold and platinum can occur freely in nature. Most metals react with other elements to form ores.

Major steps in extraction of metal u Ore concentration – Ore is purified and concentrated, unwanted rocks removed u Reduction to crude metal – Metal oxides to be reduced to metals, resulting in a mixture of metals collected u Refining to obtain pure metal – To obtain a specific metal, purify and remove unwanted metal impurities

the extraction of metals Method of extraction depends on the position of the metal in the reactivity series. § extraction of metal involves: o o getting rid of the unwanted rock to obtain concentrated form of the mineral obtaining pure metal from the mineral by chemical reactions

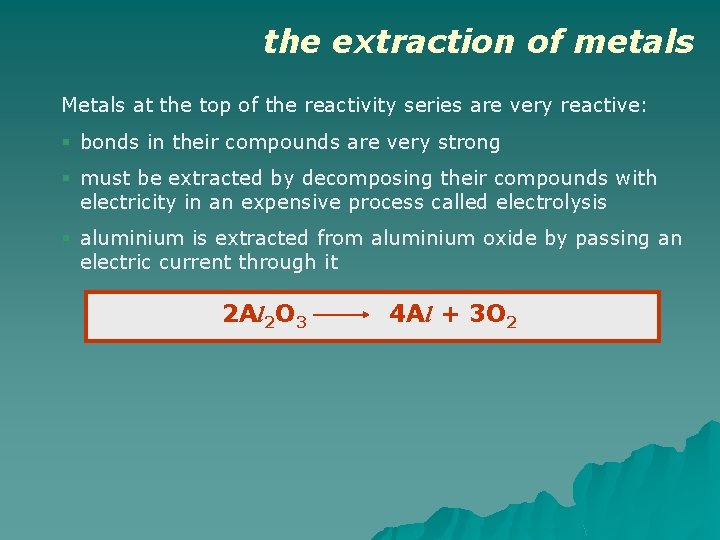
the extraction of metals Metals at the top of the reactivity series are very reactive: § bonds in their compounds are very strong § must be extracted by decomposing their compounds with electricity in an expensive process called electrolysis § aluminium is extracted from aluminium oxide by passing an electric current through it 2 Al 2 O 3 4 Al + 3 O 2

Ways of Extraction u u u Extraction by electrolysis of molten Al 2 O 3 dissolved in cryolite u u u Potassium Sodium Calcium Magnesium Aluminium Zinc Iron Tin Lead Copper Mercury Silver Gold Platinum K Na Ca Mg Al Zn Fe Sn Pb Cu Hg Ag Au Pt Extracted by electrolysis of molten chlorides Extraction by reduction of oxides using carbon Roasting ore by heating alone

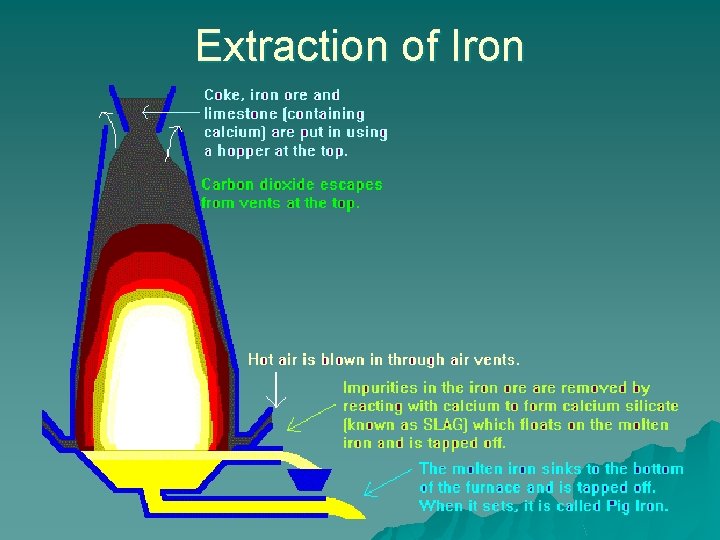
Extraction of Iron

Raw materials of extraction of Iron u Iron Ore – eg haematite ore [iron(III) oxide, Fe 2 O 3] u Coke – carbon, C u Hot air – for the O 2 in it u Limestone – calcium carbonate, Ca. CO 3

Stage 1 – Production of carbon dioxide u The coke is ignited at the base and hot air blown in to burn the coke (carbon) to form carbon dioxide – C(s) + O 2(g) CO 2(g) u The limestone is decomposed by heat to produce carbon dioxide & quicklime – Ca. CO 3(s) Ca. O(s) + CO 2(g)

Stage 2 – Production of carbon monoxide u At high temperature, the carbon dioxide formed reacts with more coke (carbon) to form carbon monoxide – CO 2(g) + C(s) 2 CO(g)

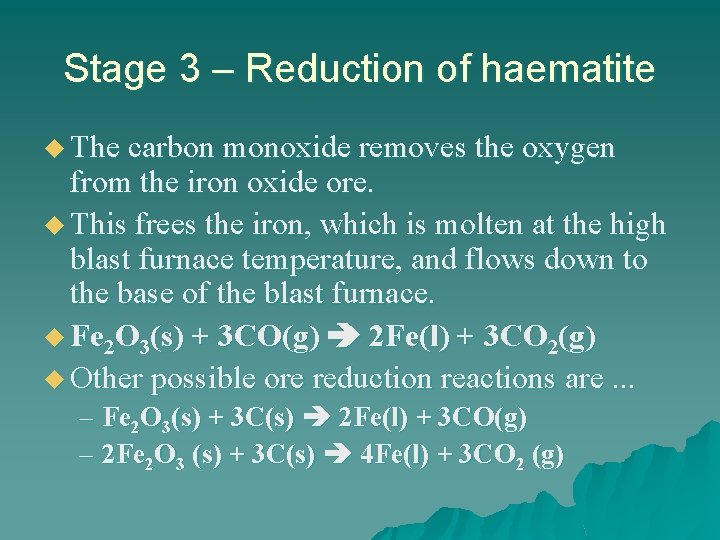
Stage 3 – Reduction of haematite u The carbon monoxide removes the oxygen from the iron oxide ore. u This frees the iron, which is molten at the high blast furnace temperature, and flows down to the base of the blast furnace. u Fe 2 O 3(s) + 3 CO(g) 2 Fe(l) + 3 CO 2(g) u Other possible ore reduction reactions are. . . – Fe 2 O 3(s) + 3 C(s) 2 Fe(l) + 3 CO(g) – 2 Fe 2 O 3 (s) + 3 C(s) 4 Fe(l) + 3 CO 2 (g)

Stage 3 – Reduction of haematite u Waste gases escape through the top of the furnace u Eg. Carbon monoxide, carbon dioxide, nitrogen…

Stage 4 – Removal of Impurities u The original ore contains silica (Si. O 2, silicon dioxide). These react with limestone to form a molten slag of e. g. calcium silicate in 2 stages – Ca. CO 3 Ca. O + CO 2 – Ca. O + Si. O 2 Ca. Si. O 3 The molten slag forms a layer above the more dense molten iron and can be separately, and regularly, drained away. The iron is cooled and cast into pig iron ingots / transferred directly to a steel producing furnace u Slag can be used for road surfacing u

http: //www. bbc. co. uk/history/games/blast. shtml

Why Steel? Steel is iron that has most of the impurities removed. Steel also has a consistent concentration of carbon throughout (0. 5 percent to 1. 5 percent) u Impurities like silica, phosphorous and sulphur weaken steel tremendously, so they must be eliminated u The advantage of steel over iron is greatly improved strength u

Pig Iron to Steel Using Basic Oxygen Furnace Pear-shaped furnace, lined with refractory bricks, that refines molten iron from the blast furnace and scrap into steel u Scrap is dumped into the furnace vessel u Followed by the hot metal from the blast furnace. u A high-pressure stream of oxygen is blown into it to cause chemical reactions that separate impurities as fumes or slag u Once refined, the liquid steel and slag are poured into separate containers u



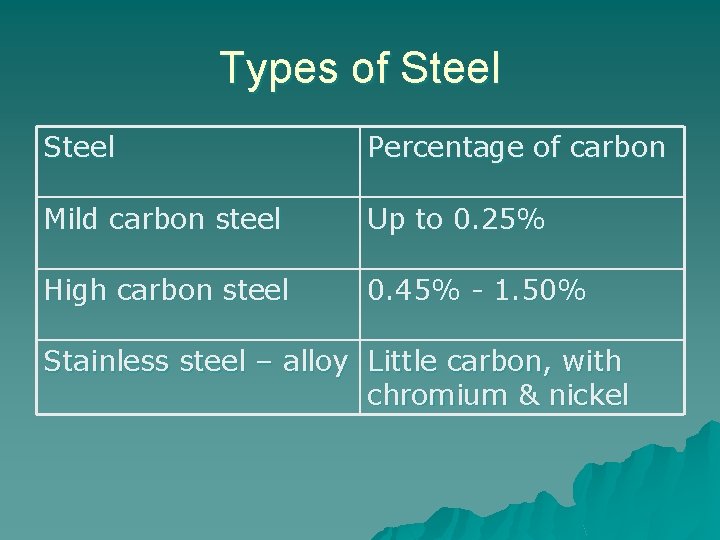
Types of Steel Percentage of carbon Mild carbon steel Up to 0. 25% High carbon steel 0. 45% - 1. 50% Stainless steel – alloy Little carbon, with chromium & nickel

Properties of Steel u Can be changed by the use of controlled additives u Eg. Carbon, chromium, nickel, manganese, silicon etc…

Uses of Steel Mild carbon steel – strong, hard & malleable High carbon steel – strong but brittle Stainless steel – does not rust Uses Make steel parts in car bodies , machineries Make knives, hammer, cutting tools Pipes & tanks in chemical plants, making cutlery, surgical instruments

Alloy u Mixture of a metal with other elements u Element in the largest proportion is the base metal u Elements in smaller proportions are the alloying elements


Metals u Soft u Low resistance to corrosion u High m. p u Easy to shape

Alloys u Have different physical properties compared to their constituent elements u Produce mainly for: – Improving strength and hardness – Improving resistance towards corrosion – Improving appearance of metal – Lower m. p of metal

Extraction of Aluminium from Bauxite u Raw materials – Bauxite: ore containing hydrated aluminium oxide Al 2 O 3. 2 H 2 O u M. p: ~2000 C – Molten Cryolite aka sodium aluminium fluoride Na 3 Al. F 6 used to lower m. p to ~900 C – Carbon electrodes u http: //www. patana. ac. th/parents/curriculu m/Chemistry/units/LR 803. html

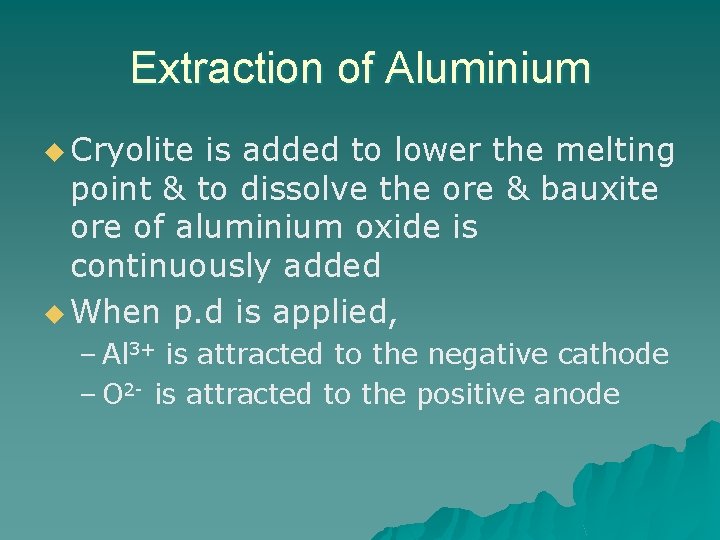
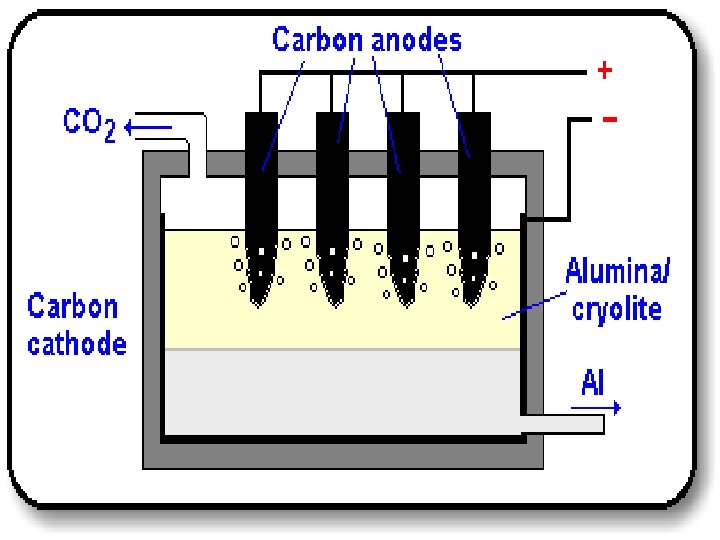
Extraction of Aluminium u Cryolite is added to lower the melting point & to dissolve the ore & bauxite ore of aluminium oxide is continuously added u When p. d is applied, – Al 3+ is attracted to the negative cathode – O 2 - is attracted to the positive anode

Extraction of Aluminium u At the cathode, – Al 3+ gains 3 electrons from the cathode to form molten aluminium, which is tapped off – Al 3+(l) + 3 e- Al (l) u At the anode, – O 2 - loses 2 electrons to the anode to form oxygen – 2 O 2 -(l) O 2(g) + 4 e– Oxygen released attacks carbon anode, to form Carbon monoxide/dioxide. Carbon anode dissolved. Needs to be replaced regularly


Anodising u Form of electroplating using oxygen, used commonly for aluminium u Aluminium when exposed in air forms a thin protective coat of aluminium oxide u For better protection, a thicker coat is made u Through the process: Anodising

Anodising Make aluminium the anode in sulphuric acid bath u Oxygen produced at the anode then combines with aluminium to form a protective porous layer aluminium oxide 1000 times thicker, compared when exposed to air u Pores can be sealed by dipping into hot water or coloured by using dyes which can be absorbed into it u

Uses of Aluminium Uses Properties Overhead electric cables Low density, light Resistant to corrosion (protected by aluminium oxide) Good electrical conductivity Food containers Non-toxic Resistant to corrosion Good conductor of heat Aircraft body Low density, light High tensile strength Resistant to corrosion

Conditions for Corrosion of Iron Presence of oxygen u Presence of water u Presence of sodium chloride/acidic pollutants speed up rusting u 4 Fe(s) + exothermic 3 O 2(g) + Rusting is an redox reaction where iron 2 x. H 2 to O(l) is oxidized form hydrated iron(III) oxide 2 Fe 2 O 3. x. H 2 O (s)

Prevention of rusting u Use of protective layer u Painting – Used in cars, ships, bridges u Greasing – Tools & machine parts u Zinc plating(Galvanising) – Zinc roofs u Tin plating – Food cans u Creates barrier around the metal preventing contact with oxygen and water

Sacrificial protection u More reactive metal, eg, Magnesium or zinc is attached to iron or steel u Protects by sacrificing itself, corrodes first since it is more reactive u Iron will not rust in the presence of a more reactive metal u Used in underground pipes, ships, steel piers

Alloying u Addition of nickel and chromium to iron u Chromium (III) oxide Cr 2 O 3 on the surface protects iron from corrosion u Used in cutlery, surgical instruments, pipes & tanks in chemical plants

Finite Resource u Metal ores – finite resource, will be used up u Need to recycle metals u Save resources and solves litter disposal u Saves energy u Saves costs
 Extraction of metals
Extraction of metals Extraction of metals class 10
Extraction of metals class 10 Properties of materials grade 7
Properties of materials grade 7 Periodic table with metals and nonmetals
Periodic table with metals and nonmetals Natural science grade 5 metals and non metals
Natural science grade 5 metals and non metals Examples of alloy metals
Examples of alloy metals Example of metal
Example of metal Density of metalloids
Density of metalloids Some natural resources such as wheat and cattle are
Some natural resources such as wheat and cattle are Contact vs noncontact forces
Contact vs noncontact forces They say sometimes you win some
They say sometimes you win some Fire and ice diamante poem
Fire and ice diamante poem They say it only takes a little faith to move a mountain
They say it only takes a little faith to move a mountain Some may trust in horses
Some may trust in horses Some say the world will end in fire some say in ice
Some say the world will end in fire some say in ice Cakecountable or uncountable
Cakecountable or uncountable Leave only footprints take only photos
Leave only footprints take only photos Cararella
Cararella Loveset pinard maneuver
Loveset pinard maneuver Extraction adn kiwi
Extraction adn kiwi O2 extraction ratio
O2 extraction ratio Temporal information extraction
Temporal information extraction Infusion in pharmaceutics
Infusion in pharmaceutics Caffeine partition coefficient
Caffeine partition coefficient Dexter data extraction
Dexter data extraction Chelex dna extraction advantages and disadvantages
Chelex dna extraction advantages and disadvantages Practical extraction and reporting language
Practical extraction and reporting language Dna extraction from wheat germ lab answers
Dna extraction from wheat germ lab answers Spooling dna definition
Spooling dna definition Ventouse extraction
Ventouse extraction Automatic wrappers for large scale web extraction
Automatic wrappers for large scale web extraction Compensatory extraction
Compensatory extraction Collapsible mandrel filament winding
Collapsible mandrel filament winding Operator position for extraction
Operator position for extraction Hepatic extraction ratio formula
Hepatic extraction ratio formula Accumulation index pharmacokinetics
Accumulation index pharmacokinetics Ecxessive
Ecxessive