Metals What are some properties of metals Good













- Slides: 16

Metals

What are some properties of metals? • • • Good conductors of heat and electricity Luster Malleable Ductile High Density

Good Conductors • How is this property used in everyday life? ?

Luster • Luster is the ability to reflect light well. • This property is used in jewelry and to make mirrors

Malleability and Ductility • Malleability is the ability to be compressed. It is often characterized as the materials ability to form a then sheet when rolled • Ductility is the ability to be stretched. It is often characterized as the materials ability to be pulled into a wire.

Density • Metals are usually very dense compared to non-metals. • This density is due to the tightly packed crystalline structure of their atoms • This property makes them useful in manufacturing and construction

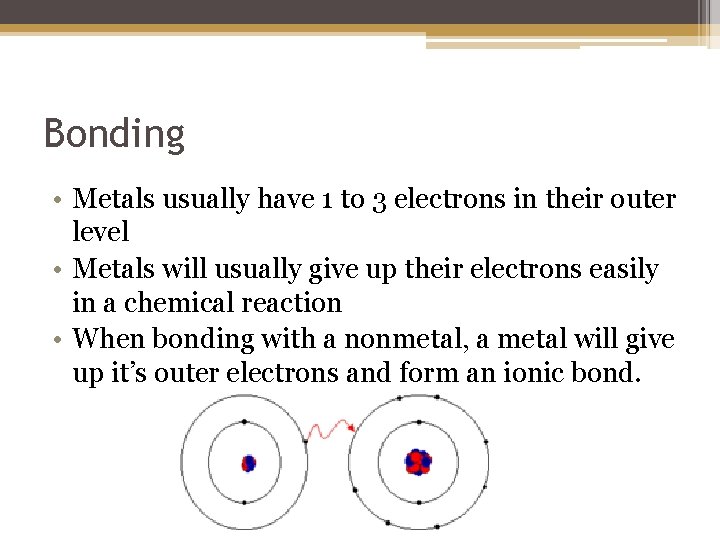
Bonding • Metals usually have 1 to 3 electrons in their outer level • Metals will usually give up their electrons easily in a chemical reaction • When bonding with a nonmetal, a metal will give up it’s outer electrons and form an ionic bond.

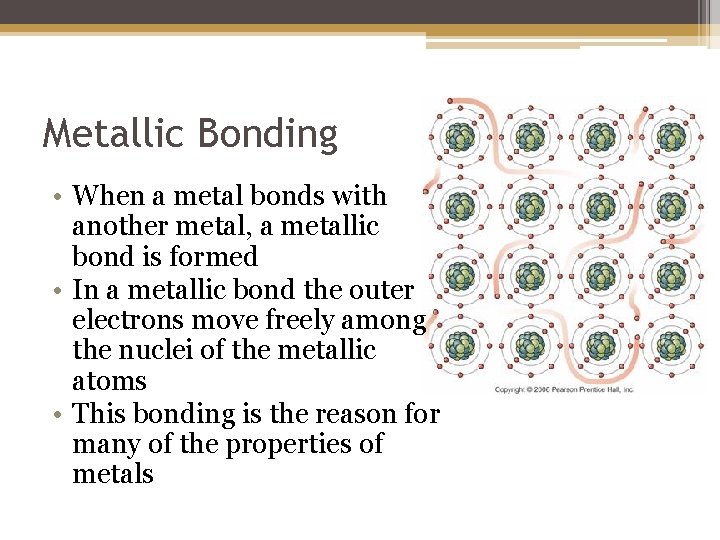
Metallic Bonding • When a metal bonds with another metal, a metallic bond is formed • In a metallic bond the outer electrons move freely among the nuclei of the metallic atoms • This bonding is the reason for many of the properties of metals

The Alkali Metals • The alkali metals are shiny, malleable, ductile, and good conductors • Alkali metals are softer than other metals • They are the most reactive of all metals, and easily react with oxygen and water • Francium is one of the rarest elements having only 25 -30 grams present in the Earth’s crust at any one time

The Alkaline Earth Metals • The alkaline earth metals are also shiny, malleable, ductile, and good conductors • They are also very active metals

Transition Metals • Transition metals are the most common metals found in their uncombined states because they are less reactive. • They transition between the groups 1 and 2 and 13 through 18 on the periodic table

Iron, Cobalt, & Nickel • These elements are used in the creation of steel and other metal mixtures. • Iron is the main component of steel and the most used of all metals. • It is the second most common metal in the Earth’s crust after aluminum • Nickel is added to other metals to give them strength or a shiny protective coating • Cobalt is used to make temperature resistant alloys

Copper, Silver, & Gold • These elements are very stable and malleable, and are easily found in nature • They also have a high luster which makes them useful in jewelry • Copper is very common and a good conductor of electricity

Zinc, Cadmium, & Mercury • Zinc and Cadmium are often used to coat other metals • This is because the react with oxygen and form a protective coating on the surface • Mercury is the only metal which is liquid at room temperature, and is used in thermometers, thermostats, and batteries

Lanthanides and Actinides • These elements are separated from the periodic table in order to make it easier to read • The lanthanides are mostly used in electronics and other high tech devices such as lasers and batteries • All of the actinides are radioactive and unstable. Their unstable nature makes them hard to study. • Uranium is one of the most commonly used actinides, used in weapons and nuclear reactors

Alloy • An alloy is a mixture of a metal and another element. • The second element makes the mixture stronger than it’s main element. • The second element enhances the properties of the main element • Two of the most well known alloys are steel and brass
 Insidan region jh
Insidan region jh Non metals periodic table
Non metals periodic table Ferrous metals
Ferrous metals Characteristics of metals
Characteristics of metals Matter and materials grade 7
Matter and materials grade 7 Grade 9 natural science term 2 notes
Grade 9 natural science term 2 notes Reactivity periodic trend
Reactivity periodic trend Google
Google C. good evening.
C. good evening. Good evening good morning good afternoon
Good evening good morning good afternoon If you are
If you are Good morning good afternoon
Good morning good afternoon Metals vs nonmetals properties
Metals vs nonmetals properties Alkaline metals reactivity
Alkaline metals reactivity Periodic table element families
Periodic table element families The physical properties of metals include luster and
The physical properties of metals include luster and Melting point of diamond
Melting point of diamond






























