EXTRACTION OF METALS PRINCIPLES OF METAL EXTRACTION Most





- Slides: 13

EXTRACTION OF METALS

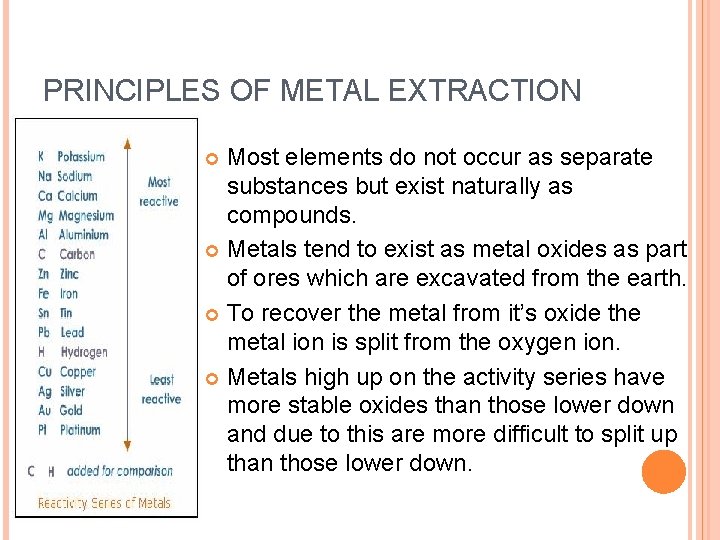
PRINCIPLES OF METAL EXTRACTION Most elements do not occur as separate substances but exist naturally as compounds. Metals tend to exist as metal oxides as part of ores which are excavated from the earth. To recover the metal from it’s oxide the metal ion is split from the oxygen ion. Metals high up on the activity series have more stable oxides than those lower down and due to this are more difficult to split up than those lower down.

METHODS OF METAL EXTRACTION: 1. Electrolyis 2. Reduction with carbon(carbon monoxide) 3. Heating of the ore.

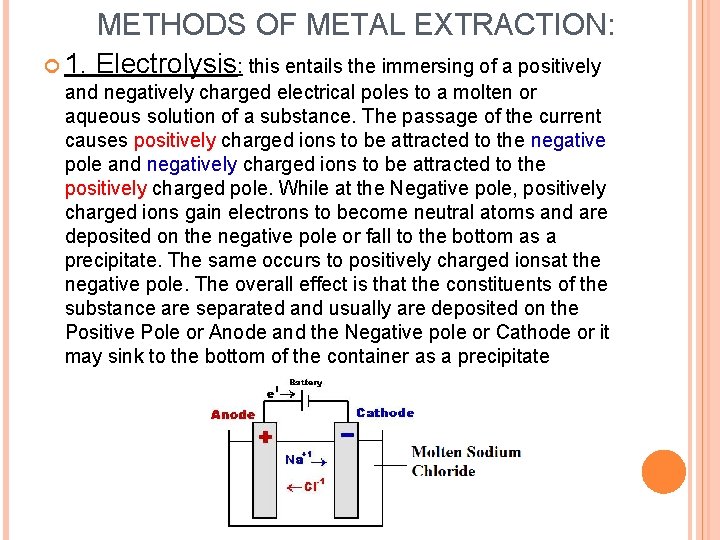
METHODS OF METAL EXTRACTION: 1. Electrolysis: this entails the immersing of a positively and negatively charged electrical poles to a molten or aqueous solution of a substance. The passage of the current causes positively charged ions to be attracted to the negative pole and negatively charged ions to be attracted to the positively charged pole. While at the Negative pole, positively charged ions gain electrons to become neutral atoms and are deposited on the negative pole or fall to the bottom as a precipitate. The same occurs to positively charged ionsat the negative pole. The overall effect is that the constituents of the substance are separated and usually are deposited on the Positive Pole or Anode and the Negative pole or Cathode or it may sink to the bottom of the container as a precipitate

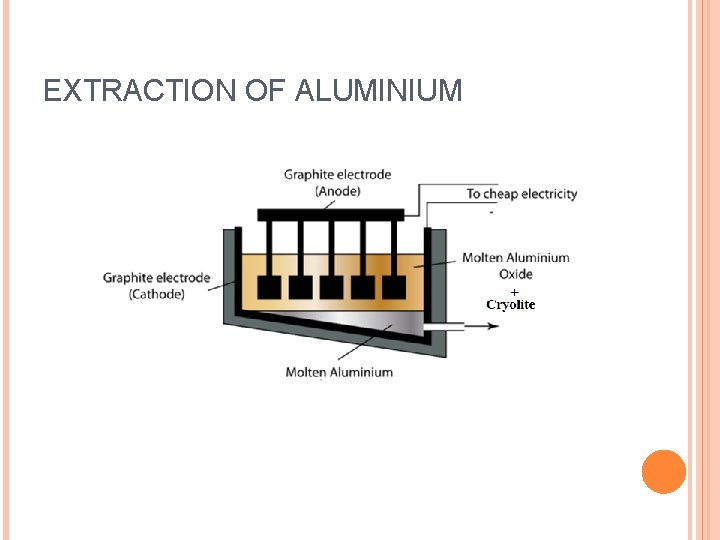
14 kilowat hours = 1 kg of Aluminium METHODS OF METAL EXTRACTION: 1. Electrolyis: Most powerful means of extraction. most expensive. Can only be used where electricity is abundant. 2. Reduction with carbon(carbon monoxide) Cheaper to operate than electrolysis. Labor intensive. Expensive to startup as large industrial equipment is used. 3. Heating of the ore. Cheap Can only be used on the most unreactive of metals(Mercury, gold, silver, etc. )

EXTRACTION OF ALUMINIUM

EXTRACTION OF IRON The extraction of Iron is a reductive process whereby oxygen is removed from the iron oxide by carbon monoxide. The process occurs within a Steel blast furnace lined with refractive(fire) bricks at temperatures from 800 C upto 1900 C. The Chamber is kept hot by jets of hot air at over 800 C, giving it the name “Blast” furnace.

Start materials: Iron Ore or Hematite Lime or Calcium carbonate Ca. CO 3 Coke a carbonaceous ashy substance Lime Iron Ore Coke

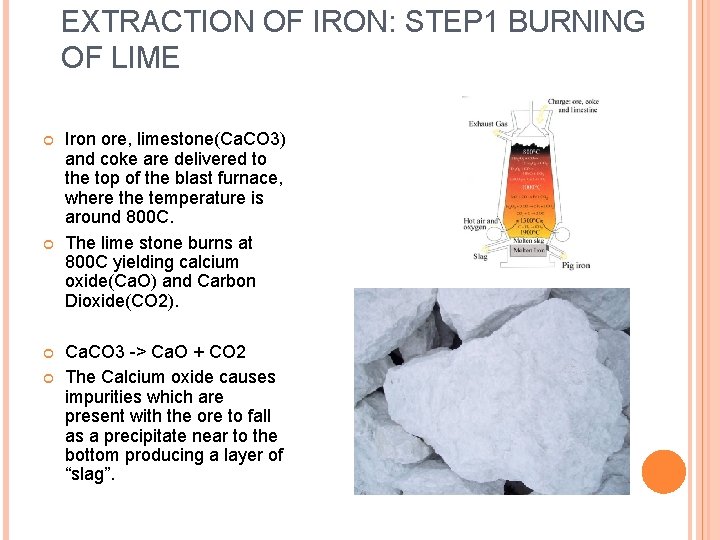
EXTRACTION OF IRON: STEP 1 BURNING OF LIME Iron ore, limestone(Ca. CO 3) and coke are delivered to the top of the blast furnace, where the temperature is around 800 C. The lime stone burns at 800 C yielding calcium oxide(Ca. O) and Carbon Dioxide(CO 2). Ca. CO 3 -> Ca. O + CO 2 The Calcium oxide causes impurities which are present with the ore to fall as a precipitate near to the bottom producing a layer of “slag”.

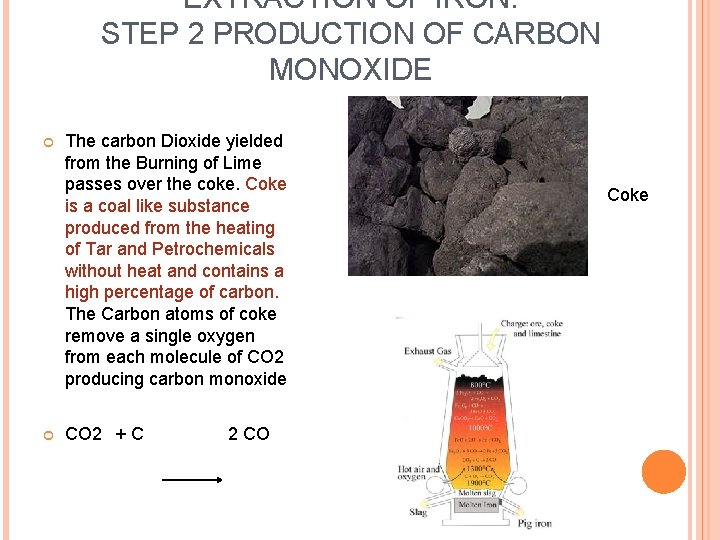
EXTRACTION OF IRON: STEP 2 PRODUCTION OF CARBON MONOXIDE The carbon Dioxide yielded from the Burning of Lime passes over the coke. Coke is a coal like substance produced from the heating of Tar and Petrochemicals without heat and contains a high percentage of carbon. The Carbon atoms of coke remove a single oxygen from each molecule of CO 2 producing carbon monoxide CO 2 + C 2 CO Coke

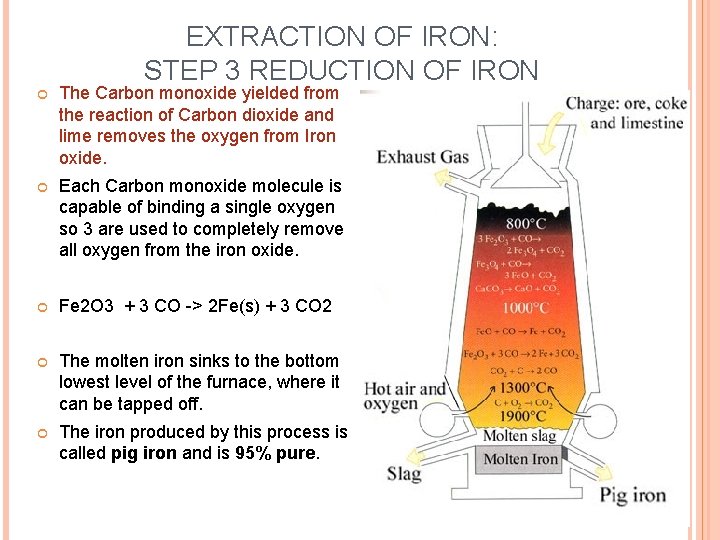
EXTRACTION OF IRON: STEP 3 REDUCTION OF IRON The Carbon monoxide yielded from the reaction of Carbon dioxide and lime removes the oxygen from Iron oxide. Each Carbon monoxide molecule is capable of binding a single oxygen so 3 are used to completely remove all oxygen from the iron oxide. Fe 2 O 3 + 3 CO -> 2 Fe(s) + 3 CO 2 The molten iron sinks to the bottom lowest level of the furnace, where it can be tapped off. The iron produced by this process is called pig iron and is 95% pure.

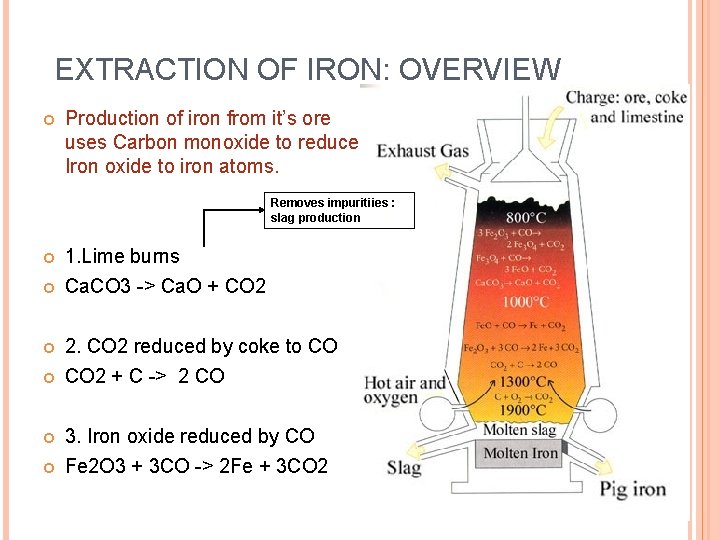
EXTRACTION OF IRON: OVERVIEW Production of iron from it’s ore uses Carbon monoxide to reduce Iron oxide to iron atoms. Removes impuritiies : slag production 1. Lime burns Ca. CO 3 -> Ca. O + CO 2 2. CO 2 reduced by coke to CO CO 2 + C -> 2 CO 3. Iron oxide reduced by CO Fe 2 O 3 + 3 CO -> 2 Fe + 3 CO 2

Q&A What is Limestone made of? How are impurities from the ore removed? What is the layer of precipitated impurities called? Where does the carbon dioxide produced during the process come from? What is the function of Coke during the process? What substance is responsible fo reducing the iron oxide to iron? What is the level of purity of the iron produced by this process?
 Non metals in the periodic table
Non metals in the periodic table Examples of alloy metals
Examples of alloy metals Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Natural science matter and materials
Natural science matter and materials Grade 7 term 2 natural science
Grade 7 term 2 natural science Examples of non metals
Examples of non metals Extraction of metals
Extraction of metals Extraction of metals class 10
Extraction of metals class 10 Method of metal extraction
Method of metal extraction Venn diagram of solid and liquid
Venn diagram of solid and liquid Difference between metal oxides and non metal oxides
Difference between metal oxides and non metal oxides Difference between metal oxides and non metal oxides
Difference between metal oxides and non metal oxides Deep n well
Deep n well Uses of nonmetals
Uses of nonmetals
























