Isotopes atoms of a given element that differ

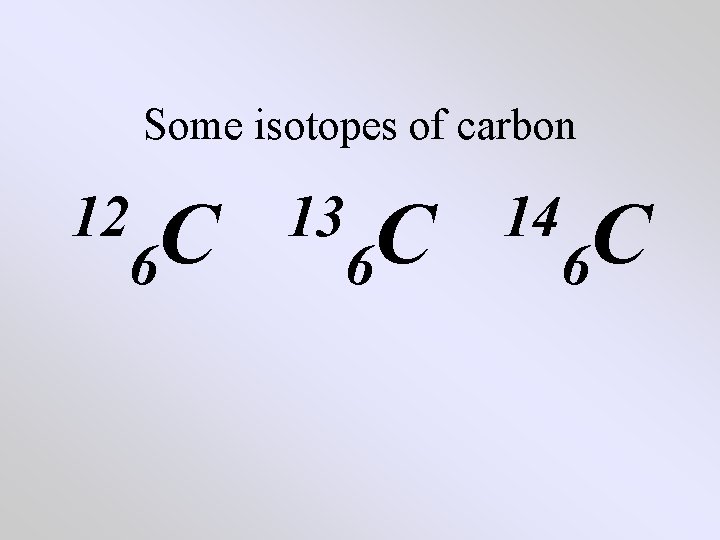
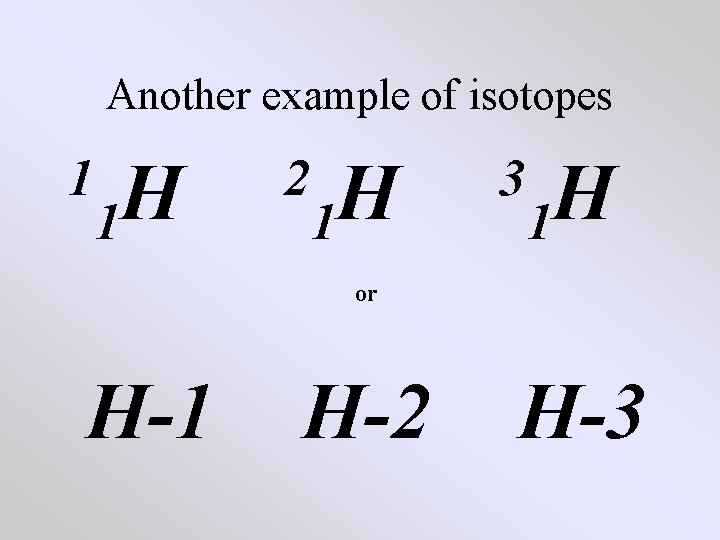










- Slides: 28

Isotopes atoms of a given element that differ in the number of neutrons …and consequently in mass.

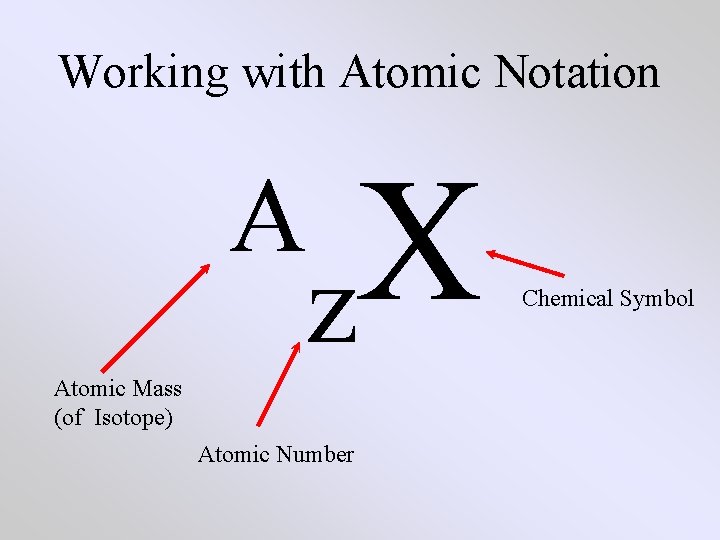
Working with Atomic Notation A X z Atomic Mass (of Isotope) Atomic Number Chemical Symbol

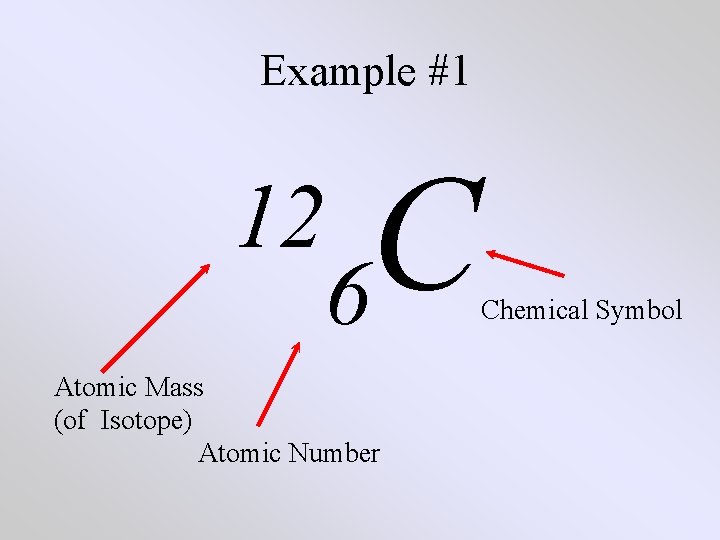
Example #1 C 12 6 Atomic Mass (of Isotope) Atomic Number Chemical Symbol

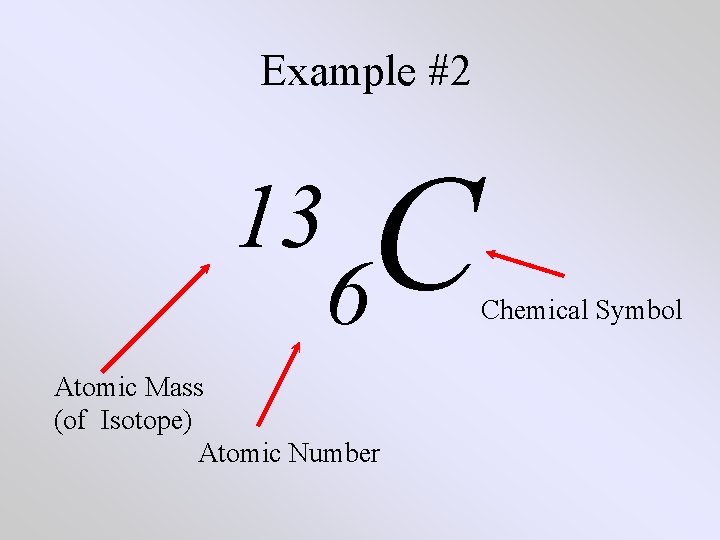
Example #2 C 13 6 Atomic Mass (of Isotope) Atomic Number Chemical Symbol

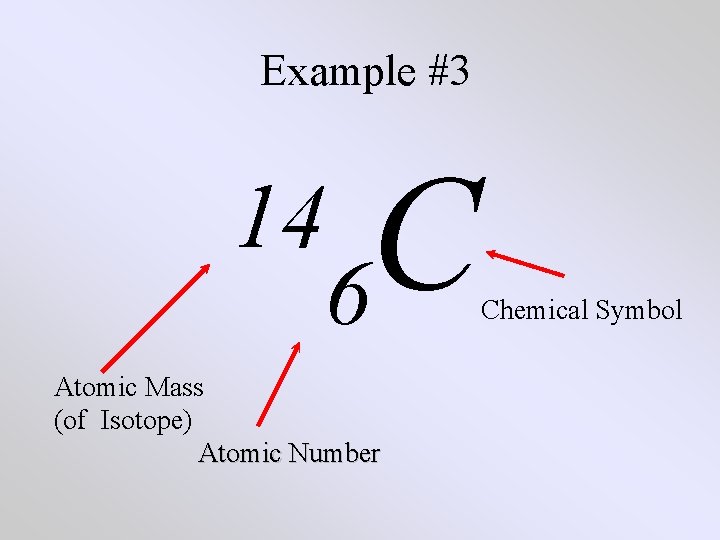
Example #3 C 14 6 Atomic Mass (of Isotope) Atomic Number Chemical Symbol
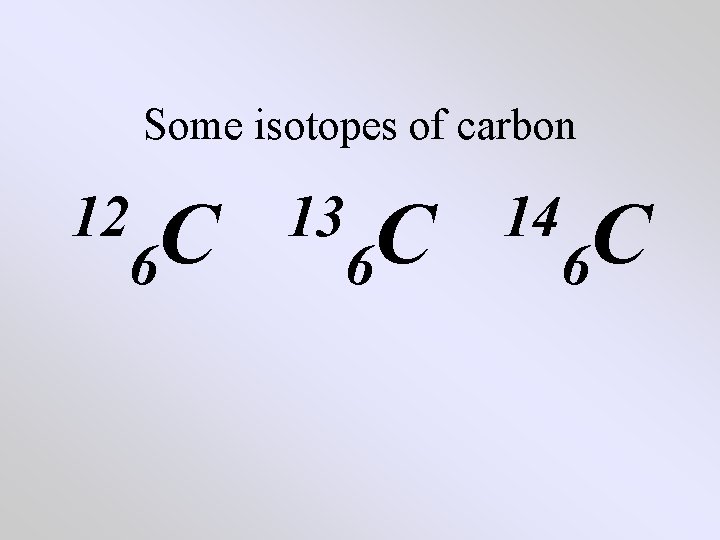
Some isotopes of carbon 12 C 6 13 C 6 14 C 6

10 47 Ne Ag 20. 1797 107. 8682 3 11 Li Na 6. 941 22. 98977 Neon Lithium Silver Sodium

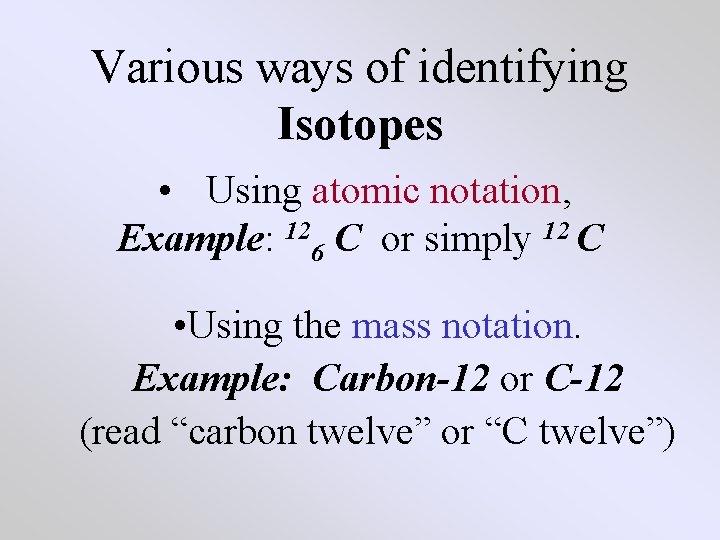
Various ways of identifying Isotopes • Using atomic notation, Example: 126 C or simply 12 C • Using the mass notation. Example: Carbon-12 or C-12 (read “carbon twelve” or “C twelve”)

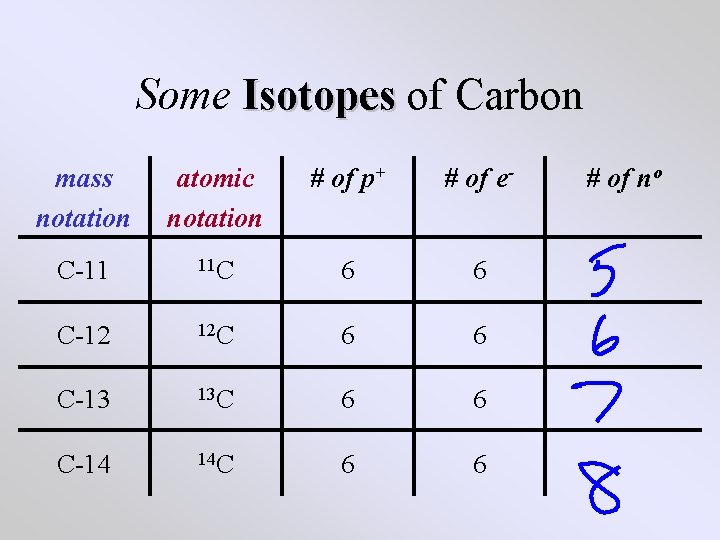
Some Isotopes of Carbon mass notation atomic notation # of p+ # of e- C-11 11 C 6 6 C-12 12 C 6 6 C-13 13 C 6 6 C-14 14 C 6 6 # of no
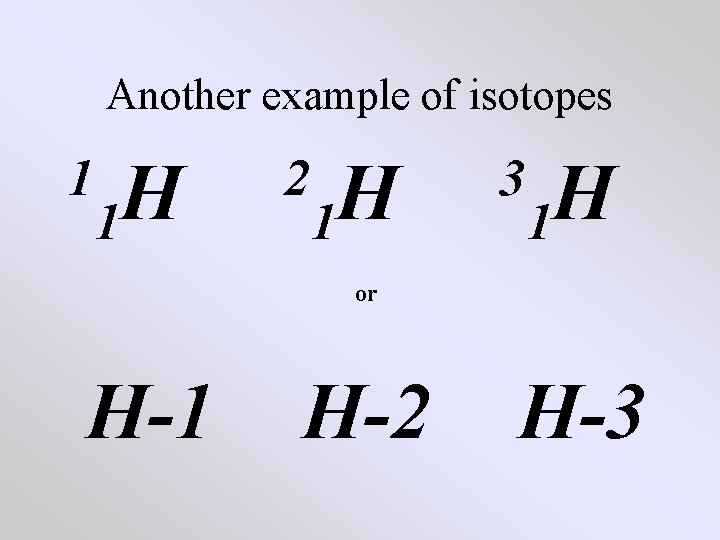
Another example of isotopes 1 H 1 2 H 1 3 H 1 or H-1 H-2 H-3

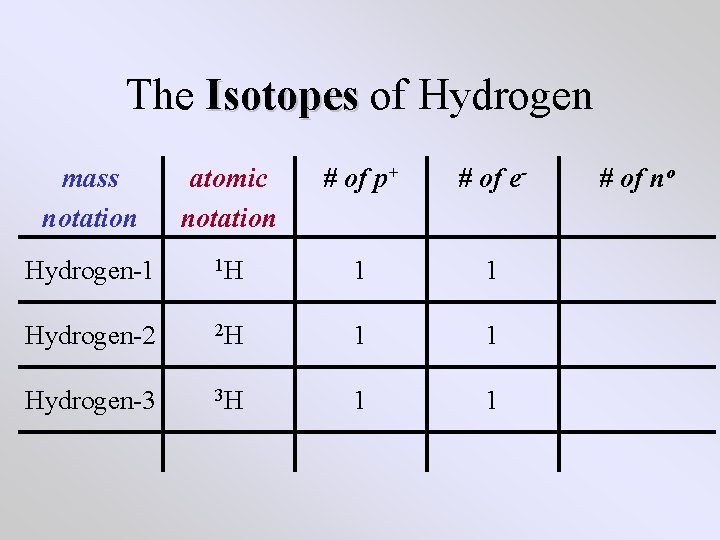
The Isotopes of Hydrogen mass notation atomic notation # of p+ # of e- Hydrogen-1 1 H 1 1 Hydrogen-2 2 H 1 1 Hydrogen-3 3 H 1 1 # of no

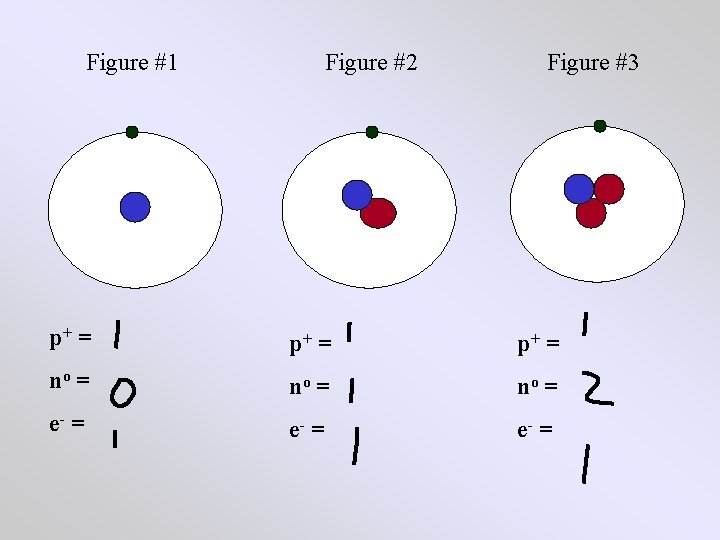
Figure #1 Figure #2 Figure #3 p+ = no = e- =

Isotopes atoms of a given element that differ in the number of neutrons …and consequently in mass.

Why are masses on the periodic table usually expressed as decimal numbers? • masses on the table are weighted averages of all known isotopes of the element of interest

Keep in mind: §It is not possible to determine how many different isotopes exist by looking at the periodic table. §It is not possible to determine the frequency of various nuclides by looking at the periodic table.

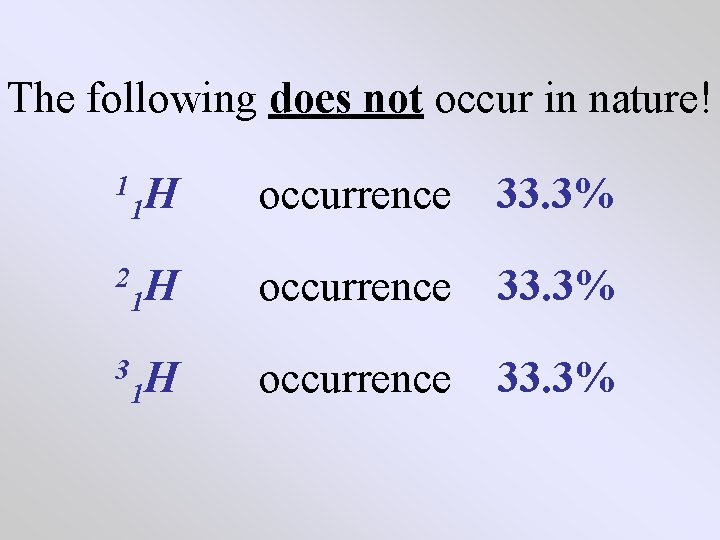
The following does not occur in nature! 1 H 1 occurrence 33. 3% 2 H 1 occurrence 33. 3% 3 H 1 occurrence 33. 3%

The following does occur in nature! 1 H 1 occurrence 99. 98% 2 H 1 occurrence 0. 0156% 3 H 1 occurrence 0. 0044%

another way of looking at it: Imagine having 10, 000 H atoms 1 H 1 occurrence 9, 998 2 H 1 occurrence 1. 56 3 H 1 occurrence 0. 44

That means the weighted average is: 1 H 1 1 x 0. 9998 = 0. 9998 2 H 1 2 x 0. 00156 = 0. 00312 3 H 1 3 x 0. 00004 = 0. 00012 Weighted Average (0. 9998 + 0. 00312 + 0. 00012) 1. 01

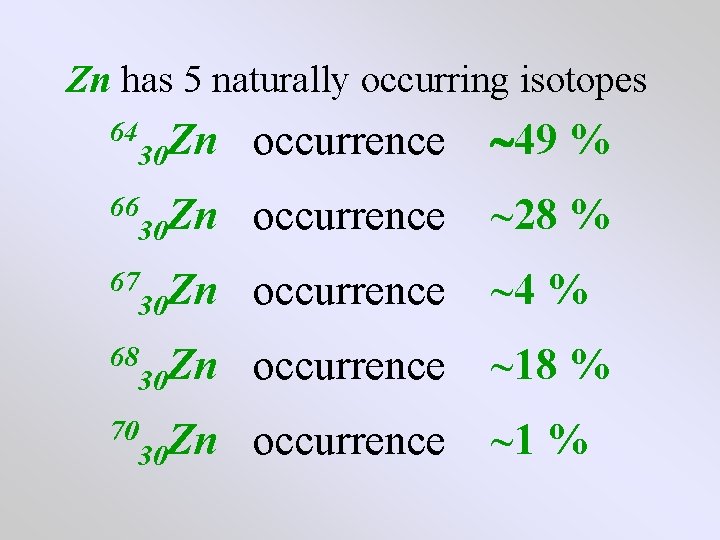
Zn has 5 naturally occurring isotopes 30 Zn occurrence 30 Zn occurrence 64 66 67 68 70

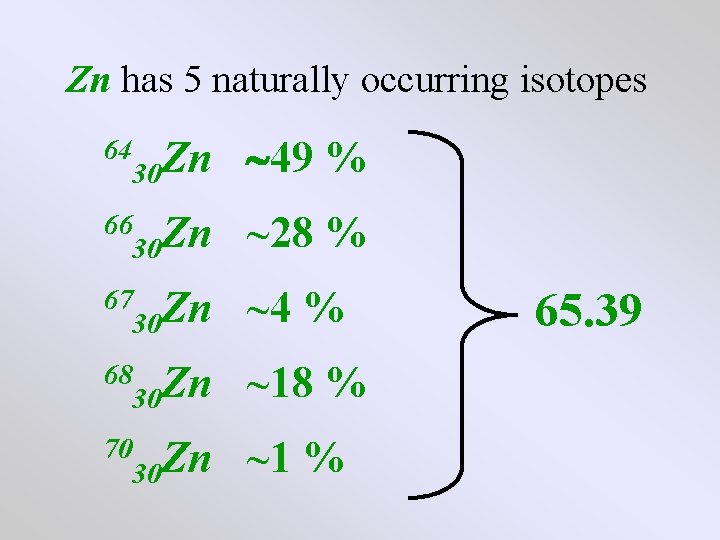
Zn has 5 naturally occurring isotopes 30 Zn occurrence 49 % 30 Zn occurrence ~28 % 30 Zn occurrence ~4 % 30 Zn occurrence ~18 % 30 Zn occurrence ~1 % 64 66 67 68 70

Zn has 5 naturally occurring isotopes 64 30 Zn 49 % 66 30 Zn ~28 % 67 30 Zn ~4 % 68 30 Zn ~18 % 70 30 Zn ~1 % 65. 39

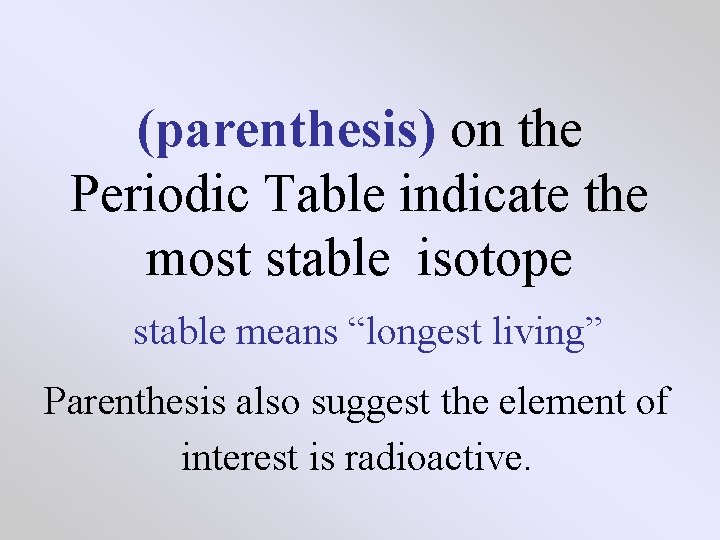
(parenthesis) on the Periodic Table indicate the most stable isotope stable means “longest living” Parenthesis also suggest the element of interest is radioactive.

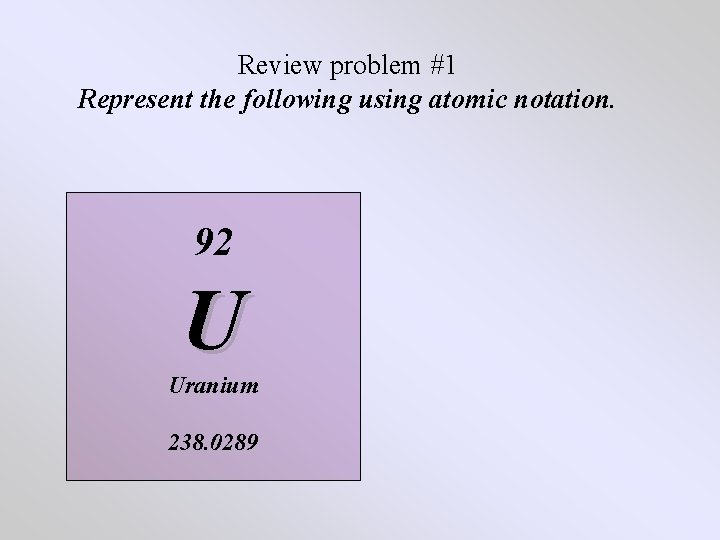
Review problem #1 Represent the following using atomic notation. 92 U Uranium 238. 0289

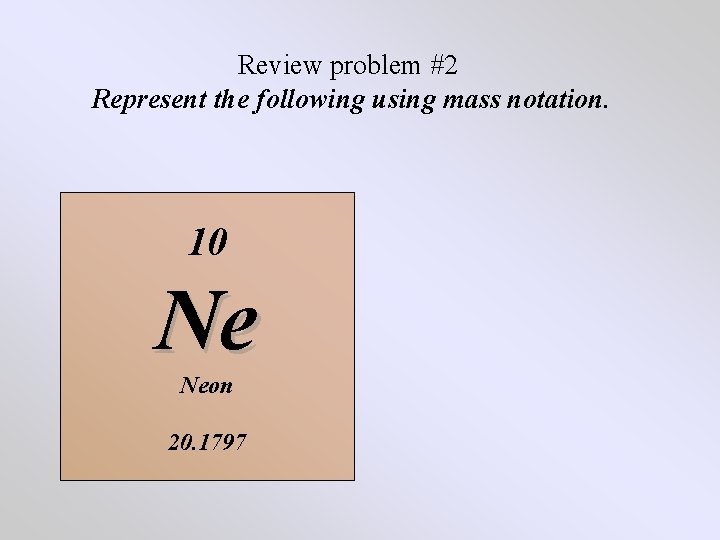
Review problem #2 Represent the following using mass notation. 10 Ne Neon 20. 1797

Review problem #3 If the atom described below had 2 naturally occurring isotopes, which of the 2 would have a greater frequency of occurrence? Express your answer in atomic and mass notation. 3 Li Lithium 6. 941

Review problem #4 How many total subatomic particles are in the following “neutral” atoms of Fe-55 and Fe-57?
