IONS ISOTOPES Are atoms that differ in the






- Slides: 17

IONS

ISOTOPES • Are atoms that differ in the number of neutrons

• Atomic Number is to number of protons an atom has. It can also be the number of electrons an atom has in an atom if that atom is neutral • Mass Number is the sum of protons and neutrons. It is either given or it can be calculated. It is not on the periodic table • The atomic mass of an element listed in the periodic table is the weighted average of the atomic masses of all isotopes present in nature

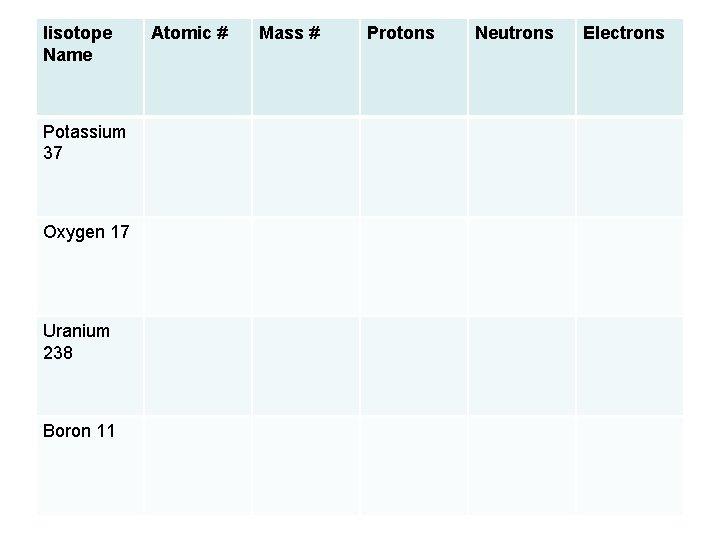
Iisotope Name Potassium 37 Oxygen 17 Uranium 238 Boron 11 Atomic # Mass # Protons Neutrons Electrons

Ions • Ions are charged atoms that are formed by either gaining or losing electrons • Cation is a positively charged atom formed by losing 1 or more electrons (metals) • Anion is a negatively charged atom formed by gaining 1 or more electrons (nonmetals) • Atoms gain or lose electrons so they can have the same number electrons as the closest noble gas

MONOATOMIC CATIONS • Positively charged atoms of just the 1 element • Monoatomic means just 1 atom • Are your metals • Lose electrons

NAMING MONOATOMIC CATIONS • To determine the charge you count “back” to the closest noble gas. That is the charge! • To Name – Name the element – Add ion!

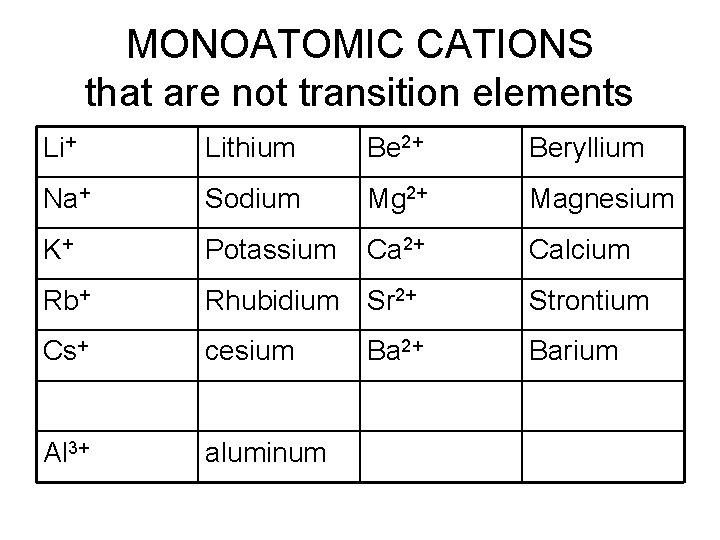
MONOATOMIC CATIONS that are not transition elements Li+ Lithium Be 2+ Beryllium Na+ Sodium Mg 2+ Magnesium K+ Potassium Ca 2+ Calcium Rb+ Rhubidium Sr 2+ Strontium Cs+ cesium Ba 2+ Barium Al 3+ aluminum

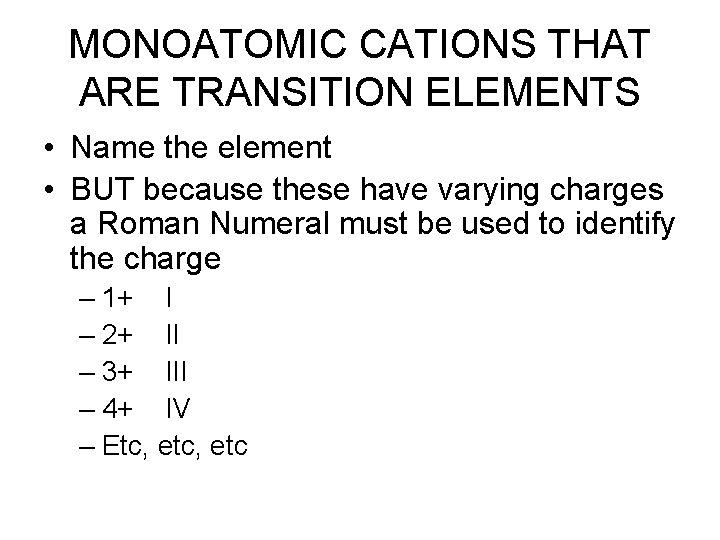
MONOATOMIC CATIONS THAT ARE TRANSITION ELEMENTS • Name the element • BUT because these have varying charges a Roman Numeral must be used to identify the charge – 1+ I – 2+ II – 3+ III – 4+ IV – Etc, etc

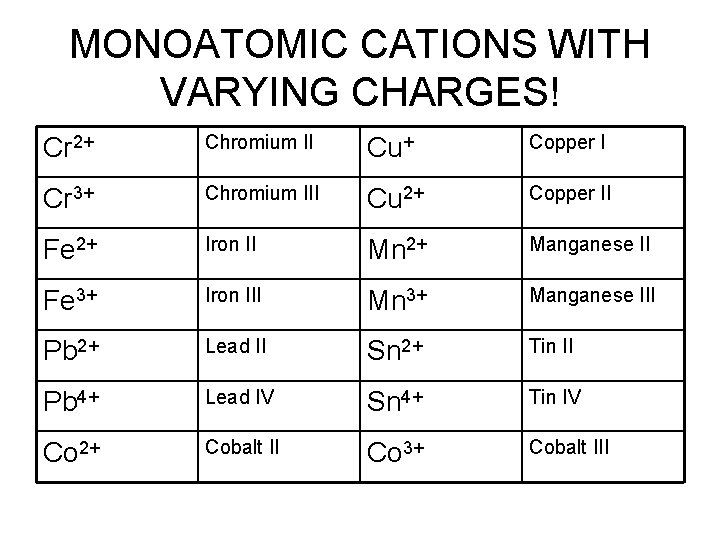
MONOATOMIC CATIONS WITH VARYING CHARGES! Cr 2+ Chromium II Cu+ Copper I Cr 3+ Chromium III Cu 2+ Copper II Fe 2+ Iron II Mn 2+ Manganese II Fe 3+ Iron III Mn 3+ Manganese III Pb 2+ Lead II Sn 2+ Tin II Pb 4+ Lead IV Sn 4+ Tin IV Co 2+ Cobalt II Co 3+ Cobalt III

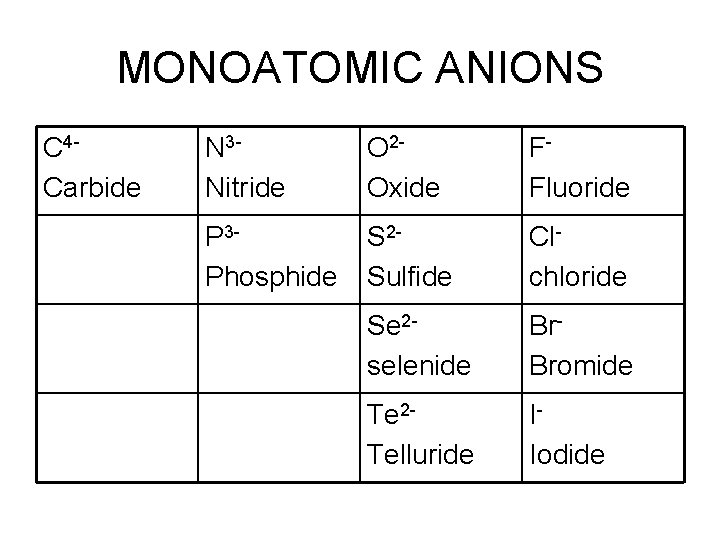
MONOATOMIC ANIONS • Anions have negative charges • Want to gain as many electrons as the closest noble gas • Names change here!!! – Name the element and get rid of its ending – Add –IDE – Add ion

MONOATOMIC ANIONS C 4 Carbide N 3 Nitride O 2 Oxide FFluoride P 3 Phosphide S 2 Sulfide Clchloride Se 2 selenide Br. Bromide Te 2 Telluride IIodide

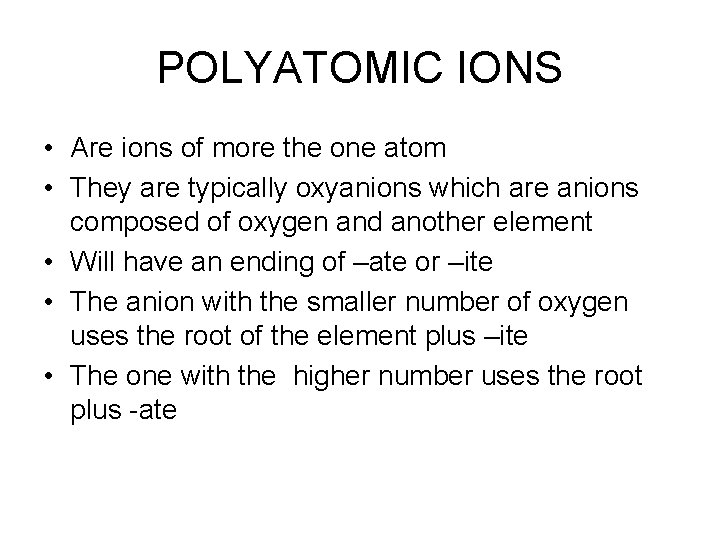
POLYATOMIC IONS • Are ions of more the one atom • They are typically oxyanions which are anions composed of oxygen and another element • Will have an ending of –ate or –ite • The anion with the smaller number of oxygen uses the root of the element plus –ite • The one with the higher number uses the root plus -ate

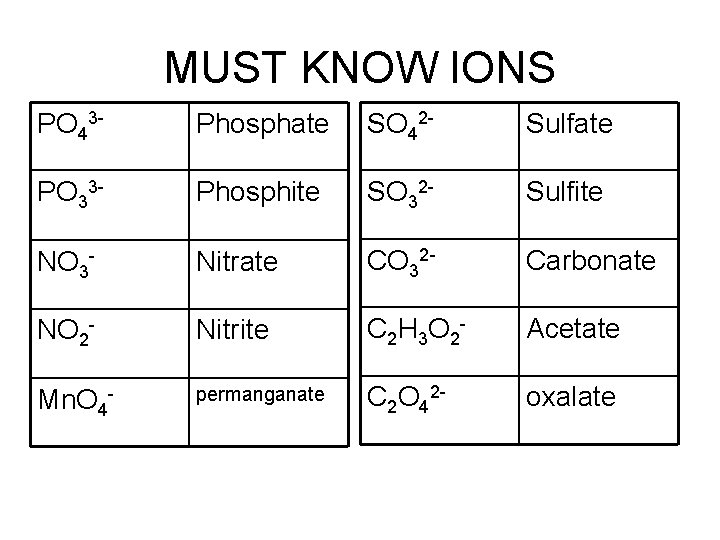
MUST KNOW IONS PO 43 - Phosphate SO 42 - Sulfate PO 33 - Phosphite SO 32 - Sulfite NO 3 - Nitrate CO 32 - Carbonate NO 2 - Nitrite C 2 H 3 O 2 - Acetate Mn. O 4 - permanganate C 2 O 42 - oxalate

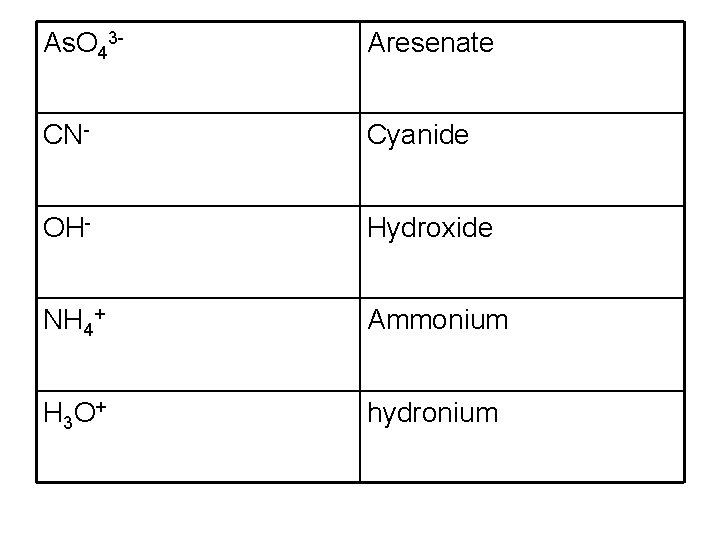
As. O 43 - Aresenate CN- Cyanide OH- Hydroxide NH 4+ Ammonium H 3 O + hydronium

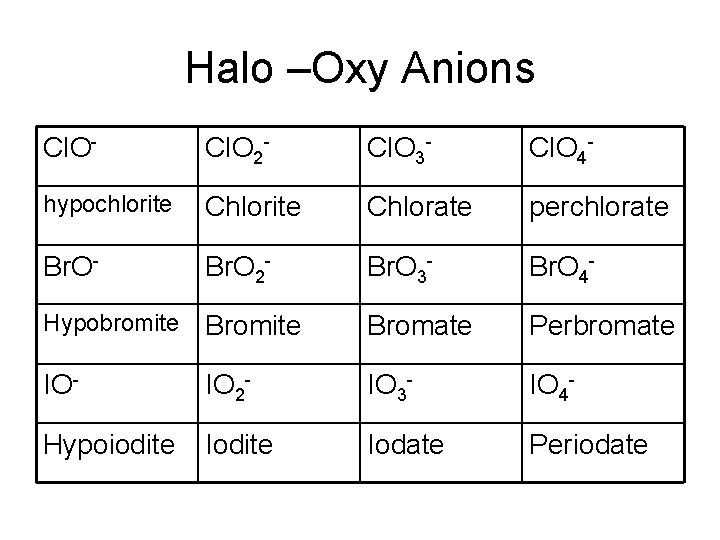
Halo –Oxy Anions Cl. O- Cl. O 2 - Cl. O 3 - Cl. O 4 - hypochlorite Chlorate perchlorate Br. O- Br. O 2 - Br. O 3 - Br. O 4 - Hypobromite Bromate Perbromate IO- IO 2 - IO 3 - IO 4 - Hypoiodite Iodate Periodate

ADDING HYDROGEN TO POLYATOMIC IONS 1. Put a hydrogen in front of the ion 2. Increase the charge by 1 each time 3. Name it - Hydrogen + ion for 1 hydrogen 2 hydrogens would be dihydrogen + ion