Intra Inter Molecular Forces Intramolecular Forces are the











- Slides: 27

Intra & Inter Molecular Forces

Intramolecular Forces - are the forces that hold the atoms that make up a compound or molecule together (chemical bond) 3 Types of Intramolecular Forces (Within a molecule) 1. Ionic Bonding 2. Metallic Bonding 3. Covalent Bonding

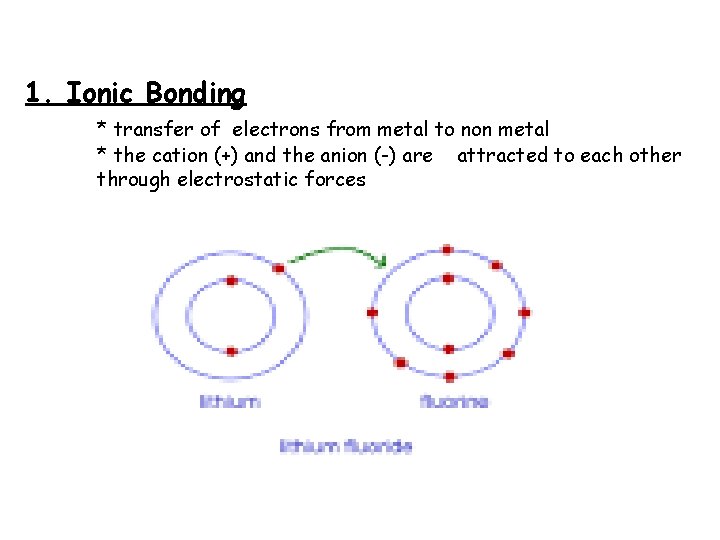
1. Ionic Bonding * transfer of electrons from metal to non metal * the cation (+) and the anion (-) are attracted to each other through electrostatic forces

• • Properties of Ionic Compounds High melting points and boiling points (Due to strong bonds holding the ions together) Good Conductors of electricity (when in solution) Hard, Rigid, Brittle solids Tend to be soluble in water

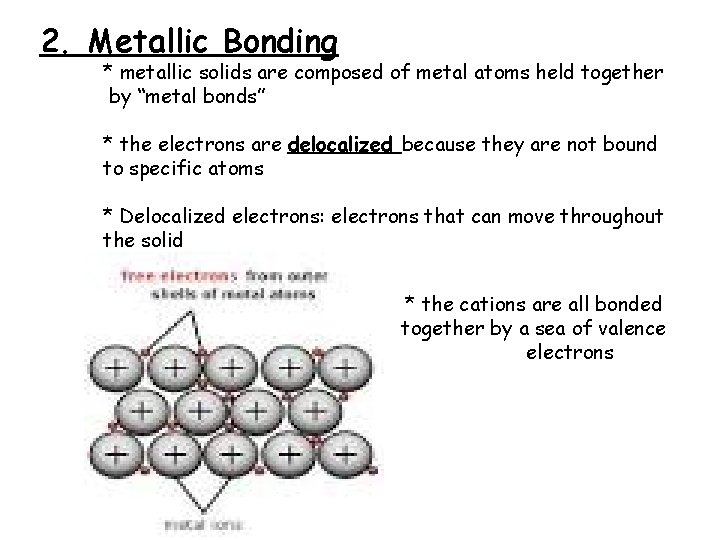
2. Metallic Bonding * metallic solids are composed of metal atoms held together by “metal bonds” * the electrons are delocalized because they are not bound to specific atoms * Delocalized electrons: electrons that can move throughout the solid * the cations are all bonded together by a sea of valence electrons

Properties of Metallic Bonds • moderately high melting Example: Metal Alloy points • high boiling points Alloy: mixture of a pure metal with • malleable into sheets) another (hammered metal • ductile (Able to be deformed without Brass ( Cu & Zn or Cu & Sn) losing toughness) • durable • hard & strong • good thermal & electrical conductivity

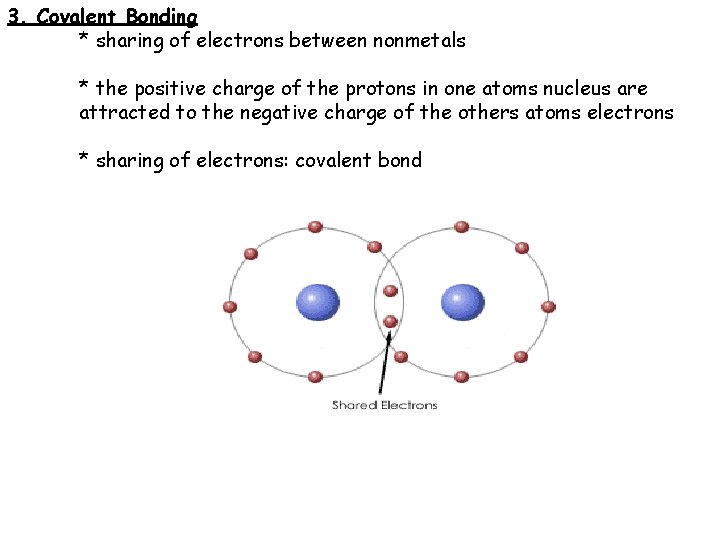
3. Covalent Bonding * sharing of electrons between nonmetals * the positive charge of the protons in one atoms nucleus are attracted to the negative charge of the others atoms electrons * sharing of electrons: covalent bond


Bond Polarity

Bond Polarity used to help describe the sharing of electrons between atoms. Not all covalent bonds are the same • The electrons are not always equally shared

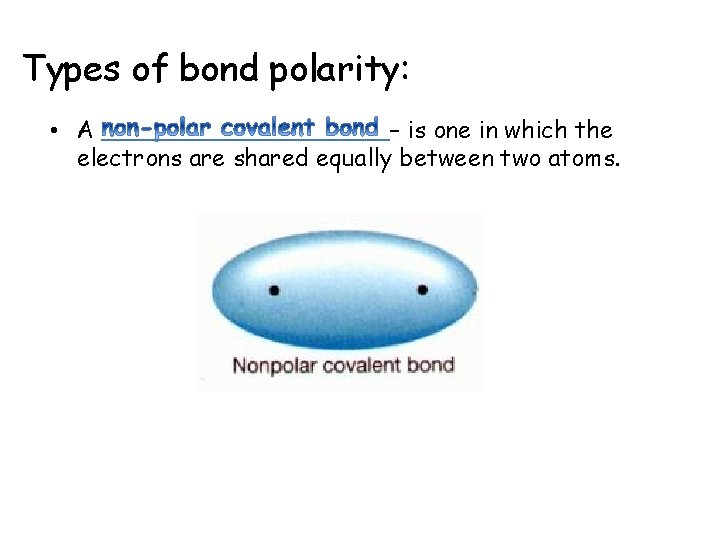
Types of bond polarity: • A – is one in which the electrons are shared equally between two atoms.

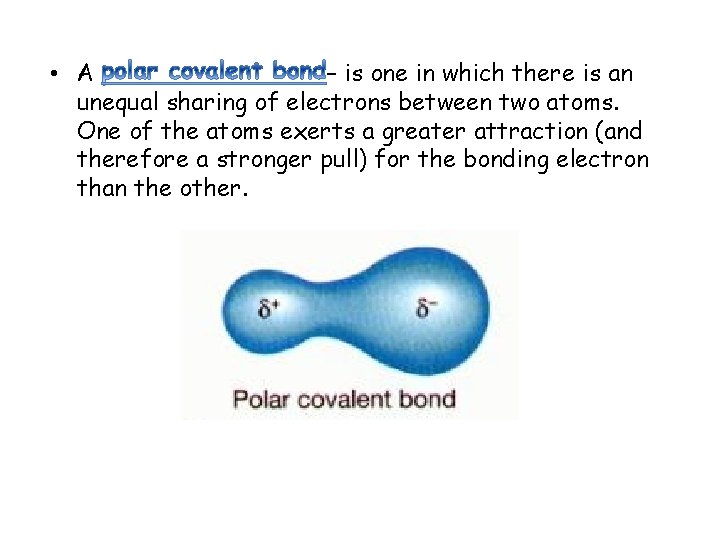
• A – is one in which there is an unequal sharing of electrons between two atoms. One of the atoms exerts a greater attraction (and therefore a stronger pull) for the bonding electron than the other.

Properties of Covalent Compounds • relatively low melting and boiling points • poor conductors of heat • do not conduct electricity in water • soft compared to ionic compounds • tend to be more flammable than ionic compounds

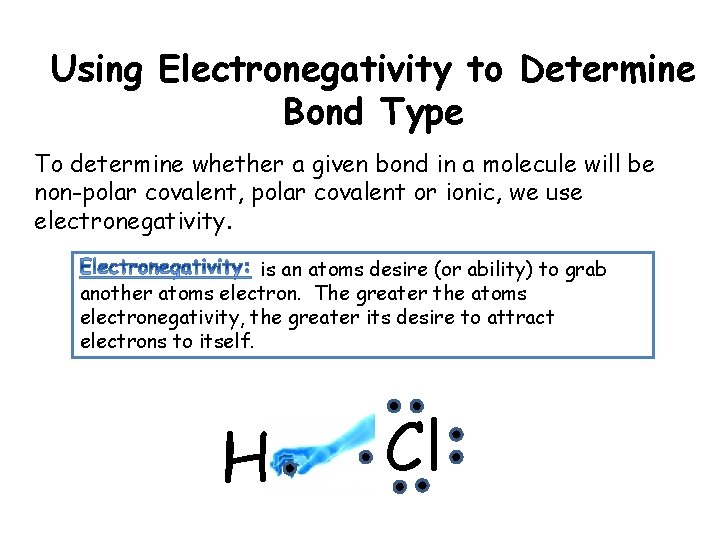
Using Electronegativity to Determine Bond Type To determine whether a given bond in a molecule will be non-polar covalent, polar covalent or ionic, we use electronegativity. is an atoms desire (or ability) to grab another atoms electron. The greater the atoms electronegativity, the greater its desire to attract electrons to itself. H Cl

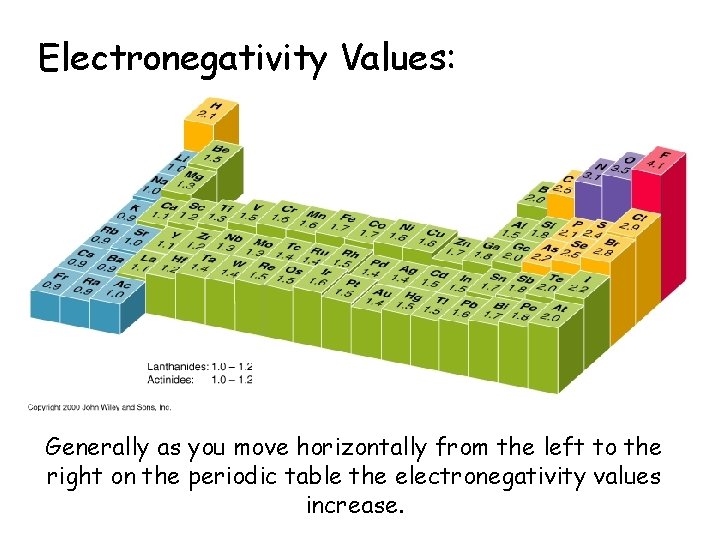
Electronegativity Values: Generally as you move horizontally from the left to the right on the periodic table the electronegativity values increase.

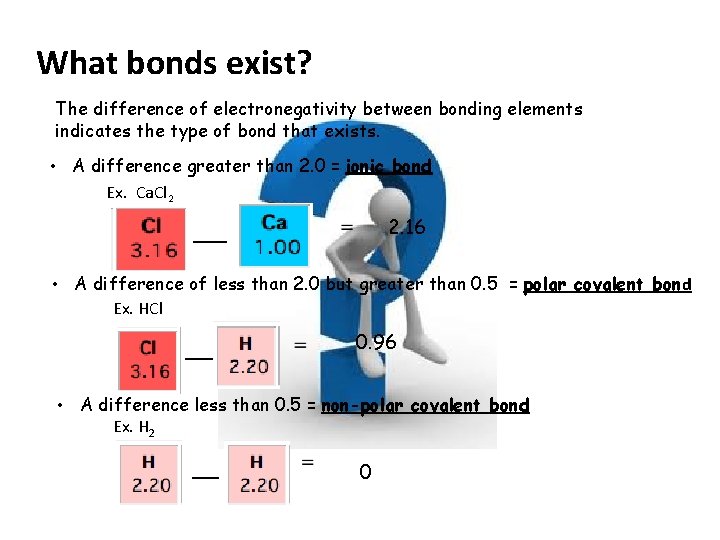
What bonds exist? The difference of electronegativity between bonding elements indicates the type of bond that exists. • A difference greater than 2. 0 = ionic bond Ex. Ca. Cl 2 2. 16 • A difference of less than 2. 0 but greater than 0. 5 = polar covalent bond Ex. HCl 0. 96 • A difference less than 0. 5 = non-polar covalent bond Ex. H 2 0


Intermolecular Forces * the forces of attraction or repulsion between neighbouring particles that aren’t actually bonded 3 Types of Intermolecular forces (between molecules) 1. Van Der Waals Forces a) Dispersion Forces (London Forces) b) Dipole Forces c) Hydrogen Bonding 2. Ionic Crystal 3. Network Covalent Crystals

1. Van der Waals Forces * They are attractive forces between covalent molecules. * The type of intermolecular forces depends on whether the molecule is polar or non-polar

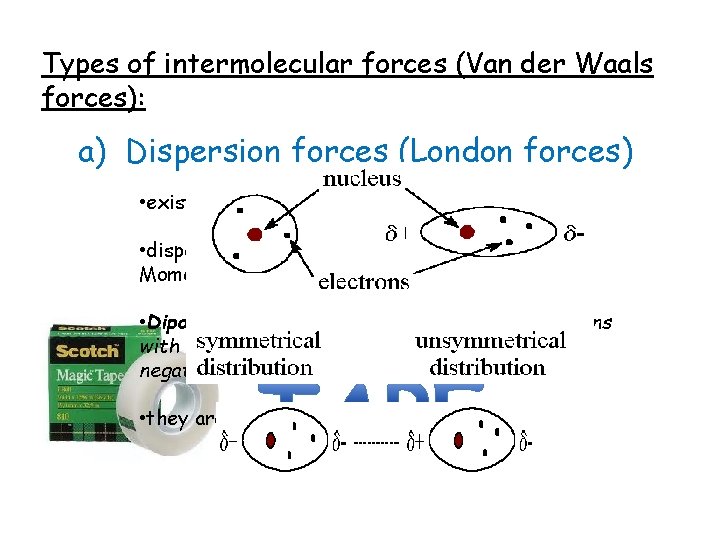
Types of intermolecular forces (Van der Waals forces): a) Dispersion forces (London forces) • exist between all covalent molecules • dispersion of the electrons causes a Dipole Moment • Dipole: refers to the unequal sharing of electrons with in a covalent molecule (causing a slight negative and slight positive) • they are very weak forces

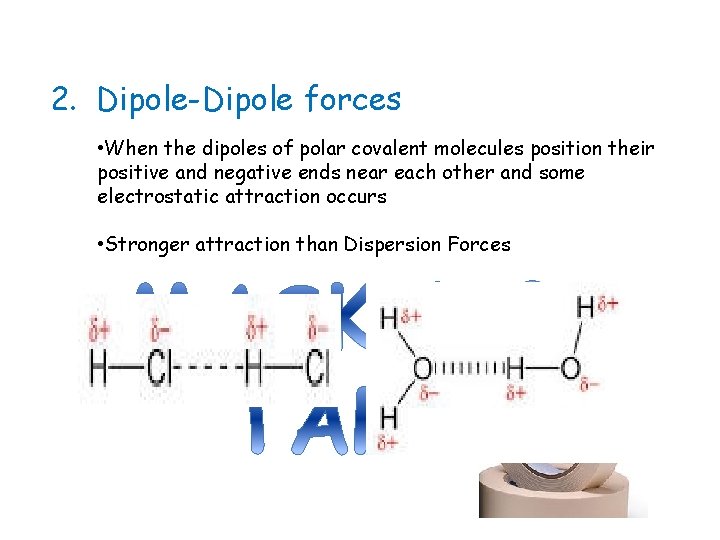
2. Dipole-Dipole forces • When the dipoles of polar covalent molecules position their positive and negative ends near each other and some electrostatic attraction occurs • Stronger attraction than Dispersion Forces

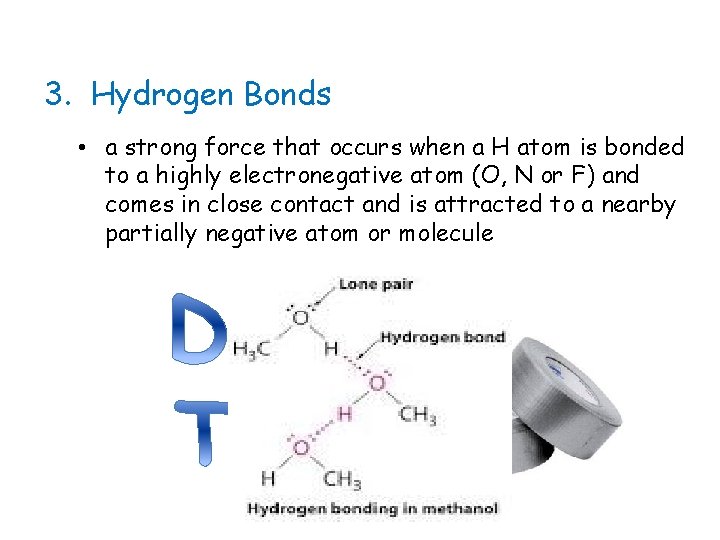
3. Hydrogen Bonds • a strong force that occurs when a H atom is bonded to a highly electronegative atom (O, N or F) and comes in close contact and is attracted to a nearby partially negative atom or molecule

Properties of Vander Waals Forces • the stronger the bond the higher the melting point, boiling point and surface area • Bond strength increases from London Dispersion to Dipole to H Bonding

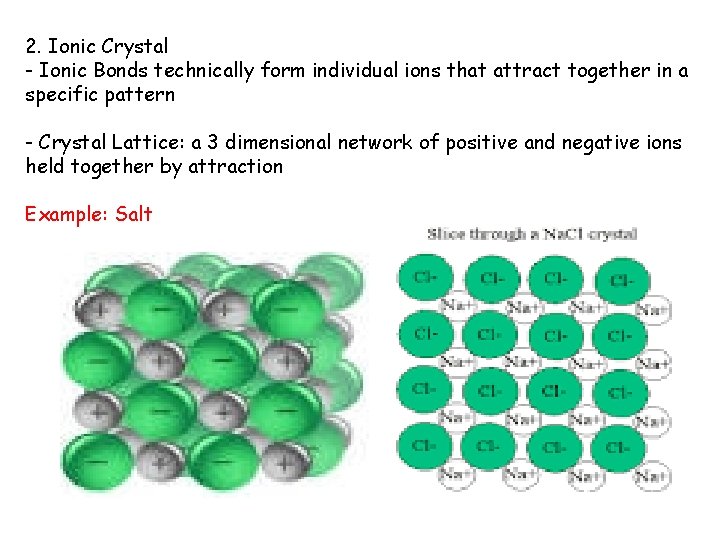
2. Ionic Crystal - Ionic Bonds technically form individual ions that attract together in a specific pattern - Crystal Lattice: a 3 dimensional network of positive and negative ions held together by attraction Example: Salt

Properties of Ionic Crystals • Soluble in water • the are good conductors of electricity when in solution • Hard & Brittle • High melting and boiling points

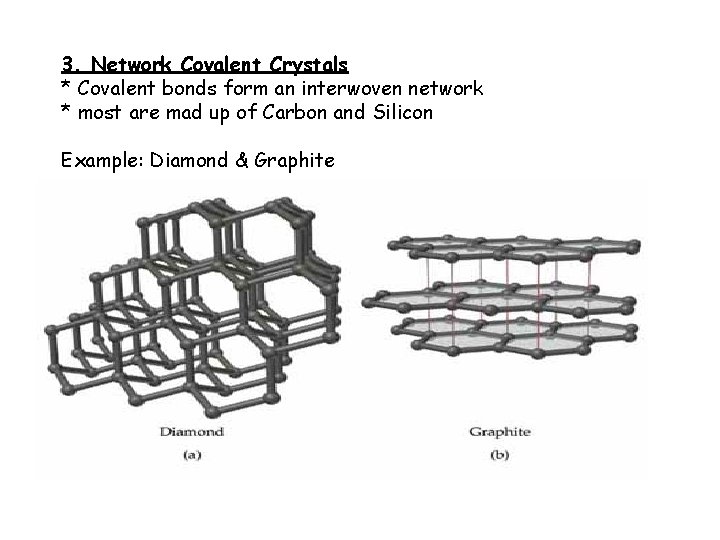
3. Network Covalent Crystals * Covalent bonds form an interwoven network * most are mad up of Carbon and Silicon Example: Diamond & Graphite

Properties of Network Covalent Crystals • Very high melting and boiling points • Extreme Hardness • Poor conductors • insoluble