Identifying and Managing Nitrate in Drinking Water Workshop
































- Slides: 61

Identifying and Managing Nitrate in Drinking Water Workshop developed by RCAP/AWWA and funded by the USEPA

Purpose Addressing the growing challenge from elevated levels of nitrate in source water. Numerous water systems do not realize that finished water has elevated levels of nitrate until they face a notice of violation. Describe steps water systems can take to understand the challenge they face and begin to evaluate treatment alternatives appropriate to their local circumstances and budgets.

Learning Objectives 1. Understand differences between acute and chronic drinking water contaminants 2. 3. Identify the typical sources of elevated nitrate in source water Compare system’s distribution of nitrate occurrence with the regulatory limits Analyze treatment methods to mitigate elevated influent nitrate Understand factors to consider in selecting a nitrate mitigation or treatment strategy Recognize elements of an initial response plan and long-term compliance 4. 5. 6.

Agenda • Introduction to sources of elevated nitrate levels • Monitoring for nitrate and regulatory limits • Alternative water supply options • Centralized water treatment technologies • Exercise 1 • Recap • Exercise 2

The Contaminant • The federal MCL for nitrate is 10 mg/L as nitrogen (N) • Some states express their MCL as nitrate (e. g. , NO 3 -) 10 mg/L as nitrogen can be expressed as 45 mg/L as nitrate

Helpful Hint • The atomic weight of nitrogen is 14. 01 and the molar mass of nitrate anion (NO 3 -) is 62. 01 g/mole • Therefore, to convert Nitrate- NO 3 - (mg/L) to Nitrate. N (mg/L): – Nitrate-N (mg/L) = 0. 22 x Nitrate- NO 3 - (mg/L) • And to convert Nitrate-N (mg/L) to Nitrate- NO 3(mg/L): – Nitrate- NO 3 - (mg/L) = 4. 43 x Nitrate-N (mg/L)

Nitrate is a Pervasive Challenge • ≈ 69% of systems observe nitrate in finished water • ≈ 91% of US population are served by water systems with detectable nitrate levels • ≈ 4% of systems have observed nitrate levels above the MCL • 555 systems violated the nitrate MCL in 2014 • 4, 203 systems experienced nitrate monitoring / reporting violations in 2014

Introduction to sources of elevated nitrate levels

What is Source of Concern? • Acute – Blue baby syndrome (metheglobinemia) – Low frequency but fatal risk for infants (10 mg/L) • Chronic – Linked to miscarriages, lymphoma, gastric cancer, hypertension, thyroid disorder • Co‐contaminants – Viruses, bacteria, toxins, pesticides

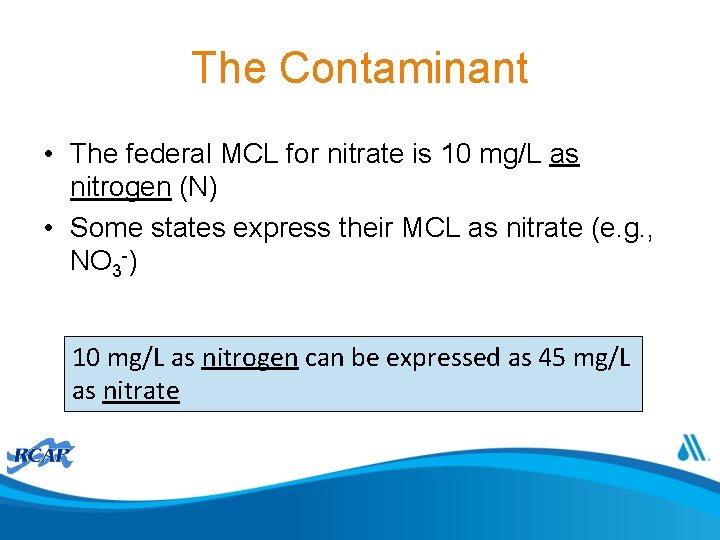
Sources of Nitrate • Addition of Nutrients • Critical nutrient sources are location specific • Both direct and indirect nutrient inputs need to be considered Addressing Nitrate in California’s Drinking Water, Tulare Lake Basin and Salinas Valley (Harter et al, 2012)

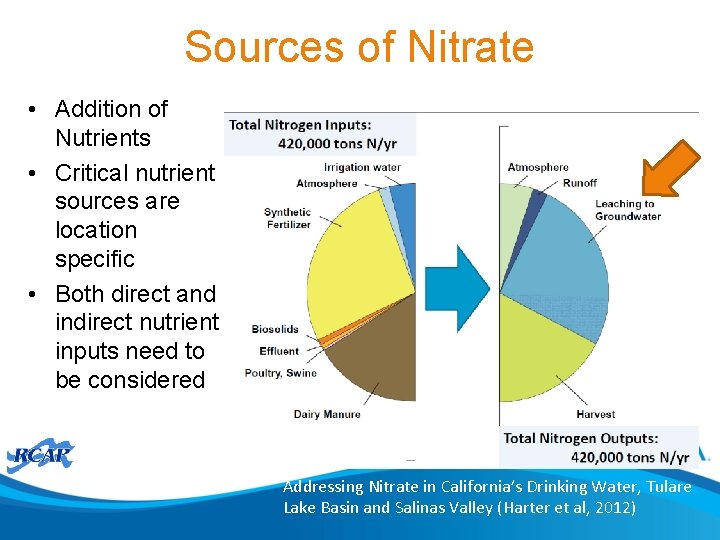
Risk of Nitrate Occurrence Nolan et al. (2002)

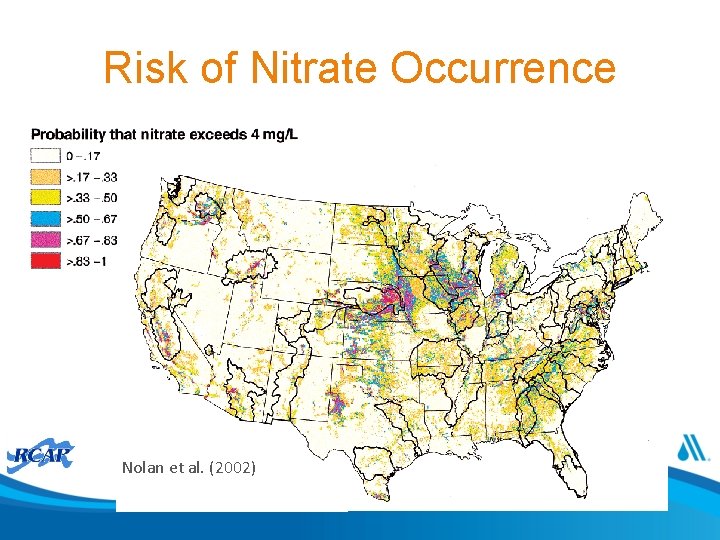
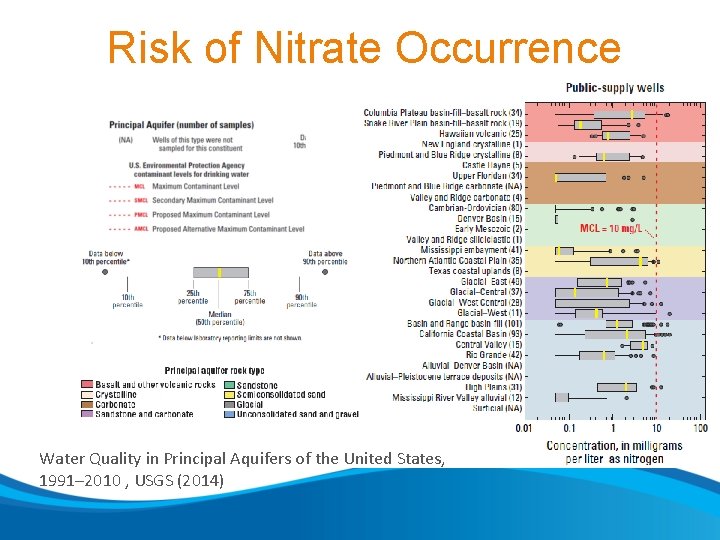
Risk of Nitrate Occurrence Water Quality in Principal Aquifers of the United States, 1991– 2010 , USGS (2014)

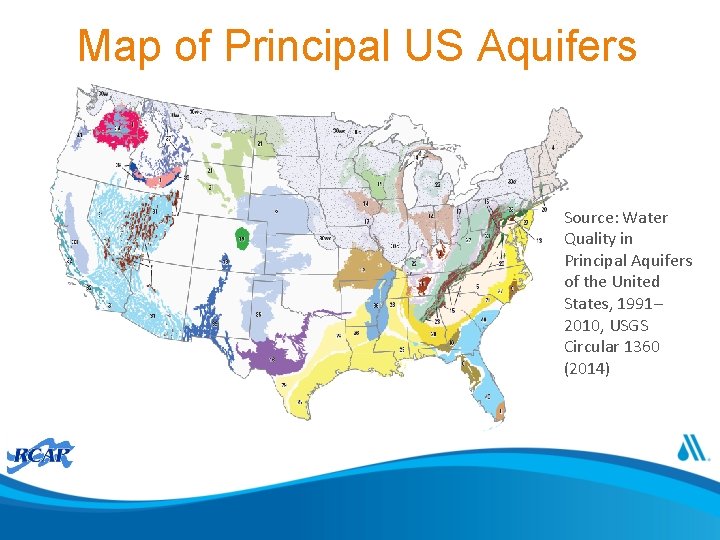
Map of Principal US Aquifers Source: Water Quality in Principal Aquifers of the United States, 1991– 2010, USGS Circular 1360 (2014)

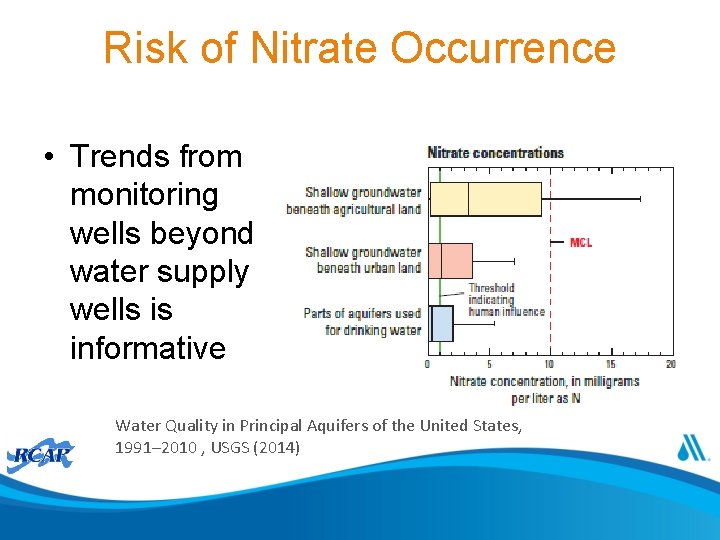
Risk of Nitrate Occurrence • Trends from monitoring wells beyond water supply wells is informative Water Quality in Principal Aquifers of the United States, 1991– 2010 , USGS (2014)

Risk of Nitrate Occurrence • Redox conditions • Presence of an aquitard • Depth Water Quality in Principal Aquifers of the United States, 1991– 2010 , USGS (2014)

Groundwater Utilization • The rate water is pumped from a well • The number of nearby wells and their groundwater withdrawal • Trends in groundwater withdrawal – Over time – Seasonal

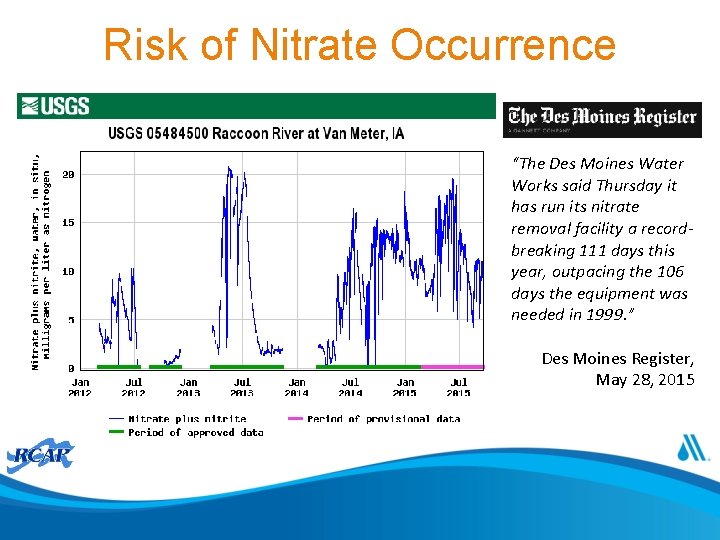
Risk of Nitrate Occurrence “The Des Moines Water Works said Thursday it has run its nitrate removal facility a recordbreaking 111 days this year, outpacing the 106 days the equipment was needed in 1999. ” Des Moines Register, May 28, 2015

Knowledge Checkpoint • Who is at greatest risk from nitrate? • What aquifers are at greater risk of elevated nitrate levels? • Is depth of well important to evaluating the risk of nitrate contamination?

Group Discussion • Has anyone here identified a source with high nitrate? • What did you do as a result of the elevated nitrate?

Monitoring for nitrate and regulatory limits

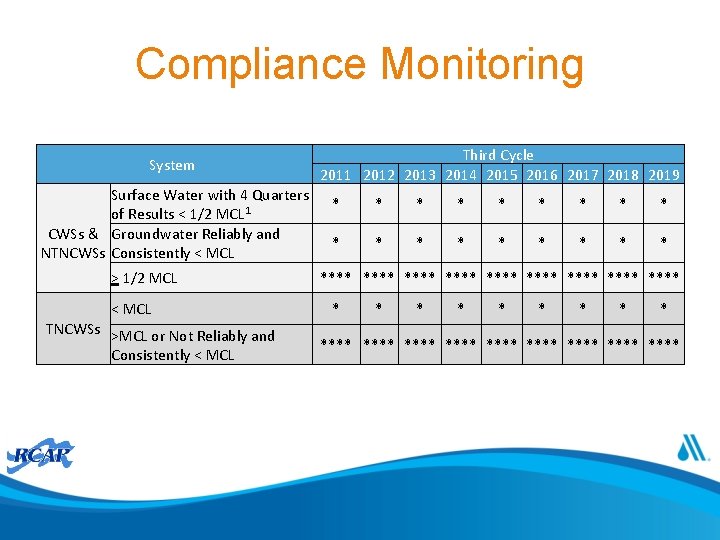
Compliance Monitoring System Surface Water with 4 Quarters of Results < 1/2 MCL 1 CWSs & Groundwater Reliably and NTNCWSs Consistently < MCL > 1/2 MCL < MCL TNCWSs >MCL or Not Reliably and Consistently < MCL Third Cycle 2011 2012 2013 2014 2015 2016 2017 2018 2019 * * * * **** **** **** * * * * **** **** ****

Compliance Monitoring

What is a Violation? • Exceeding 10 mg/L • Follow significant digit and rounding guidance • Take a confirmation sample

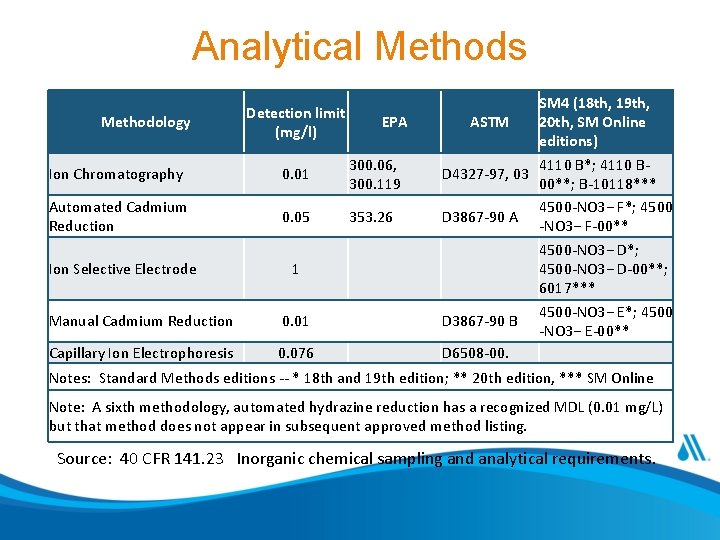
Analytical Methods Methodology Detection limit (mg/l) EPA Ion Chromatography 0. 01 300. 06, 300. 119 Automated Cadmium Reduction 0. 05 353. 26 Ion Selective Electrode 1 Manual Cadmium Reduction 0. 01 Capillary Ion Electrophoresis 0. 076 SM 4 (18 th, 19 th, ASTM 20 th, SM Online editions) 4110 B*; 4110 BD 4327 -97, 03 00**; B-10118*** 4500 -NO 3− F*; 4500 D 3867 -90 A -NO 3− F-00** 4500 -NO 3− D*; 4500 -NO 3− D-00**; 6017*** 4500 -NO 3− E*; 4500 D 3867 -90 B -NO 3− E-00** D 6508 -00. Notes: Standard Methods editions -- * 18 th and 19 th edition; ** 20 th edition, *** SM Online Note: A sixth methodology, automated hydrazine reduction has a recognized MDL (0. 01 mg/L) but that method does not appear in subsequent approved method listing. Source: 40 CFR 141. 23 Inorganic chemical sampling and analytical requirements.

Analytical Methods Source: www. nemi. gov.

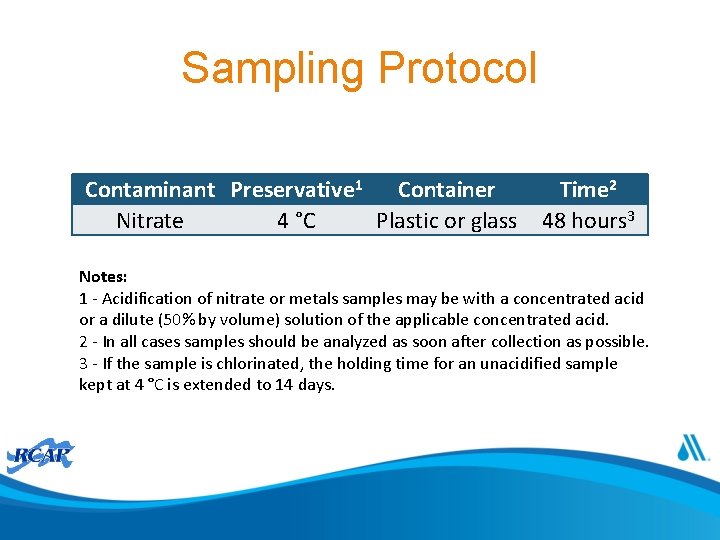
Sampling Protocol Contaminant Preservative 1 Container Nitrate 4 °C Plastic or glass Time 2 48 hours 3 Notes: 1 - Acidification of nitrate or metals samples may be with a concentrated acid or a dilute (50% by volume) solution of the applicable concentrated acid. 2 - In all cases samples should be analyzed as soon after collection as possible. 3 - If the sample is chlorinated, the holding time for an unacidified sample kept at 4 °C is extended to 14 days.

Knowledge Checkpoint • What conditions must be met to get a waiver from nitrate monitoring? • Determine if the following utilities are in compliance: – Utility A – one annual observation of 10. 4 mg/L – Utility B – Initial observation 10. 5 mg/L and confirmation sample is 9. 5 mg/L • How frequently must a utility monitor if it observed nitrate at 6 mg/L

Alternative water supply options

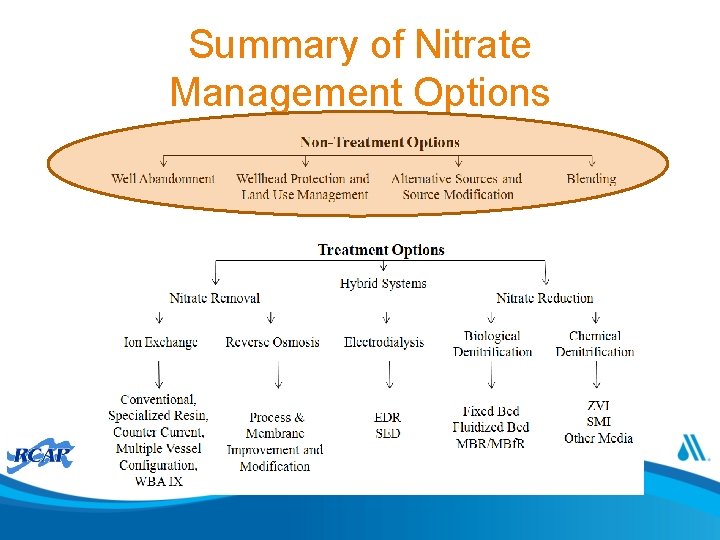
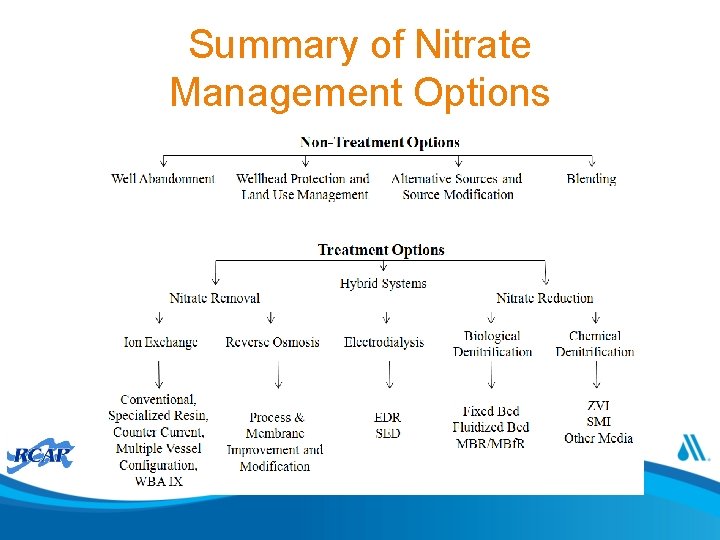
Summary of Nitrate Management Options

Non-Treatment Options • • Well abandonment Developing a new well Re-drilling or modifying an existing well Improving source protection Connecting to a nearby system Blending with a low nitrate source Bottled Water

Well Abandonment • Key considerations: – Adequate capacity from other wells – Appropriate abandonment procedures

Drilling a Replacement Well or Re. Drilling a Well • Adequate information about subsurface conditions is key limiting factor

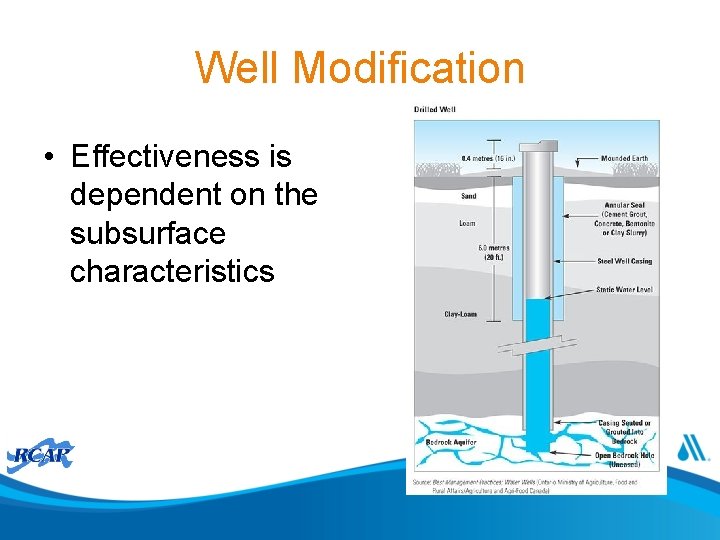
Well Modification • Effectiveness is dependent on the subsurface characteristics

Wellhead Protection and Land Use Management • Agricultural practices, • Management of animal waste disposal (e. g. , poultry, swine, dairy) • Control of wastewater treatment plant discharge, and • Monitoring and remediation of septic tank discharges

Connect to Nearby System • Regional solutions can take a number of forms: – – – Mutual Aid Arrangements Sharing Arrangements Water Purchase Arrangements Collaborative Water Resource Development Contract Services Arrangements Consolidation • Depending on the severity of a system’s nitrate challenge and local circumstances any one of these solutions may be appropriate. • Both RCAP and Water. RF have developed tools to assist water systems evaluate connecting to nearby systems

Blending • Nitrate dilution via an alternate source • Relies on availability of low nitrate sources • Requires capital investment and increased monitoring

Bottled Water • Bottled water is only a temporary solution; it may not be used for long-term compliance

Knowledge Checkpoint • What is key consideration when considering well modification or re-drilling? • What is the key consideration when pursuing blending as a solution? • Who is responsible for maintenance of POU devices if installed for SDWA compliance?

Centralized water treatment technologies

Summary of Nitrate Management Options

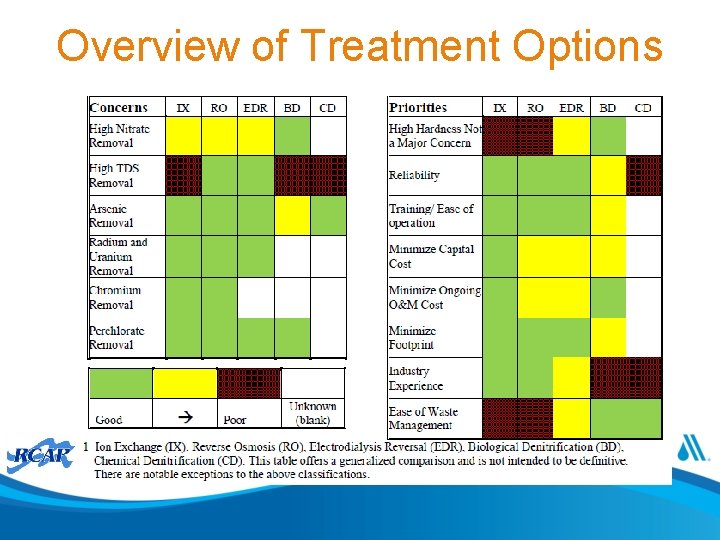
Overview of Treatment Options

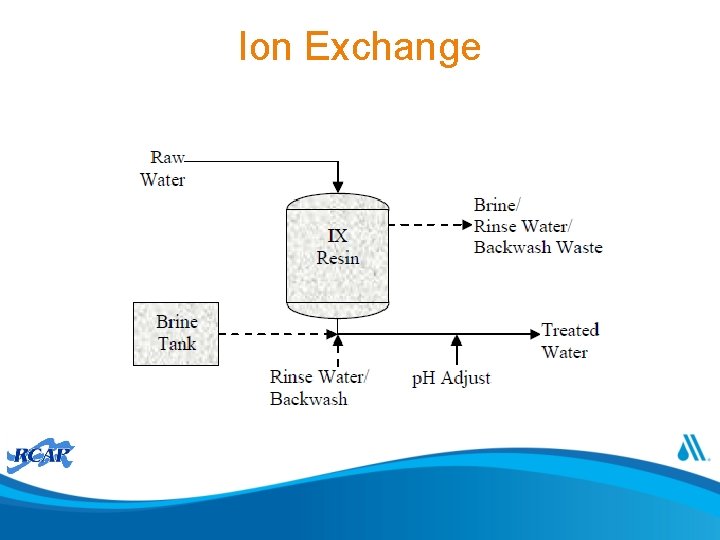
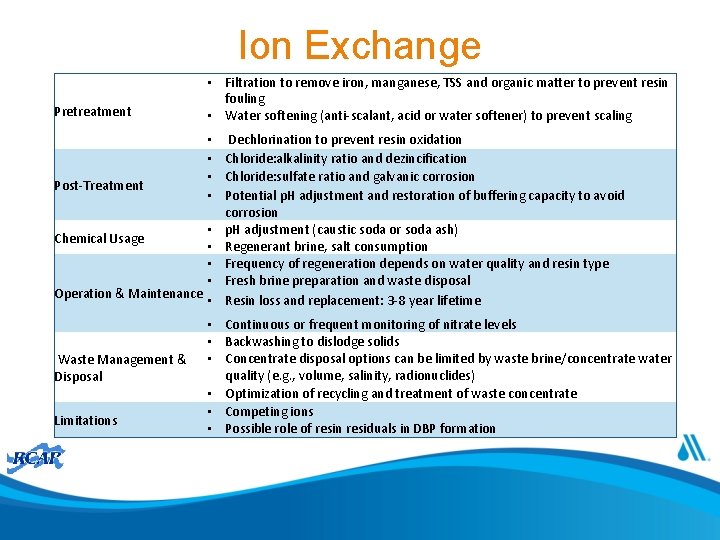
Ion Exchange

Ion Exchange Pretreatment Post-Treatment • Filtration to remove iron, manganese, TSS and organic matter to prevent resin fouling • Water softening (anti-scalant, acid or water softener) to prevent scaling • • Operation & Maintenance • Chemical Usage Waste Management & Disposal Limitations Dechlorination to prevent resin oxidation Chloride: alkalinity ratio and dezincification Chloride: sulfate ratio and galvanic corrosion Potential p. H adjustment and restoration of buffering capacity to avoid corrosion p. H adjustment (caustic soda or soda ash) Regenerant brine, salt consumption Frequency of regeneration depends on water quality and resin type Fresh brine preparation and waste disposal Resin loss and replacement: 3 -8 year lifetime • Continuous or frequent monitoring of nitrate levels • Backwashing to dislodge solids • Concentrate disposal options can be limited by waste brine/concentrate water quality (e. g. , volume, salinity, radionuclides) • Optimization of recycling and treatment of waste concentrate • Competing ions • Possible role of resin residuals in DBP formation

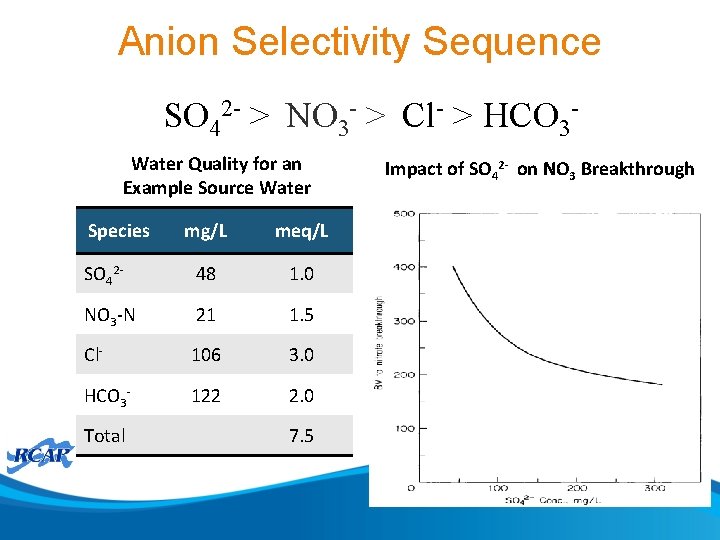
Anion Selectivity Sequence SO 42 - > NO 3 - > Cl- > HCO 3 Water Quality for an Example Source Water Species mg/L meq/L SO 42 - 48 1. 0 NO 3 -N 21 1. 5 Cl- 106 3. 0 HCO 3 - 122 2. 0 Total 7. 5 Impact of SO 42 - on NO 3 Breakthrough

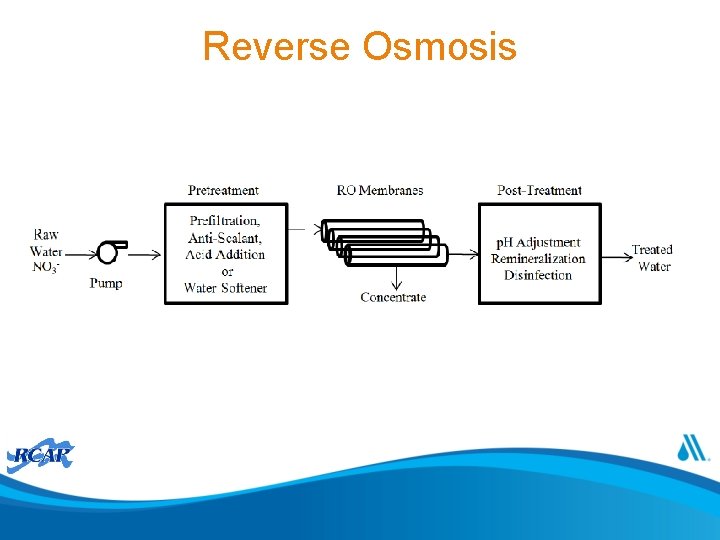
Reverse Osmosis

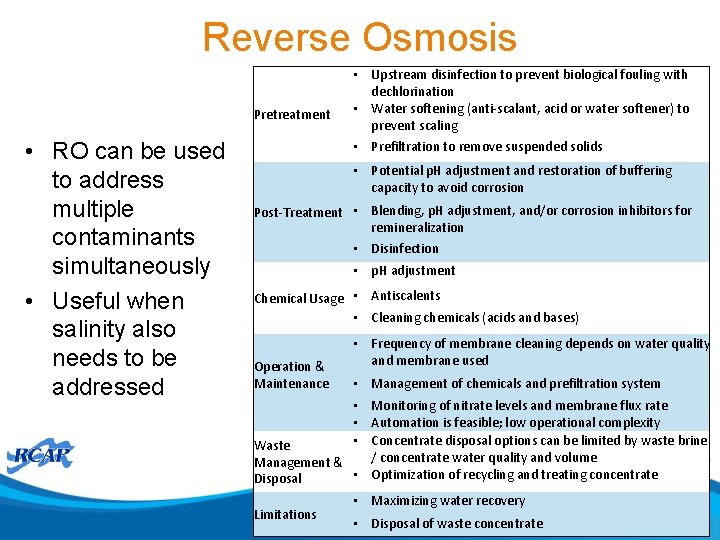
Reverse Osmosis Pretreatment • RO can be used to address multiple contaminants simultaneously • Useful when salinity also needs to be addressed • Upstream disinfection to prevent biological fouling with dechlorination • Water softening (anti-scalant, acid or water softener) to prevent scaling • Prefiltration to remove suspended solids • Potential p. H adjustment and restoration of buffering capacity to avoid corrosion Post-Treatment • Blending, p. H adjustment, and/or corrosion inhibitors for remineralization • Disinfection • p. H adjustment Chemical Usage • Antiscalents • Cleaning chemicals (acids and bases) Operation & Maintenance • Frequency of membrane cleaning depends on water quality and membrane used • Management of chemicals and prefiltration system • Monitoring of nitrate levels and membrane flux rate • Automation is feasible; low operational complexity • Concentrate disposal options can be limited by waste brine Waste / concentrate water quality and volume Management & • Optimization of recycling and treating concentrate Disposal Limitations • Maximizing water recovery • Disposal of waste concentrate

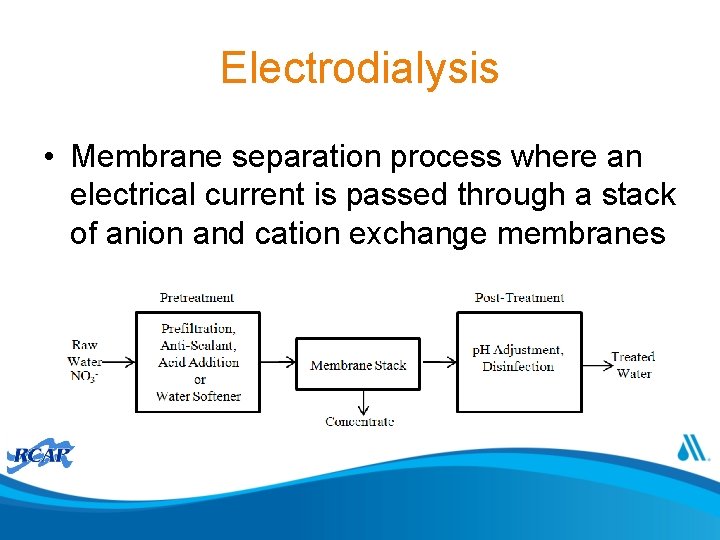
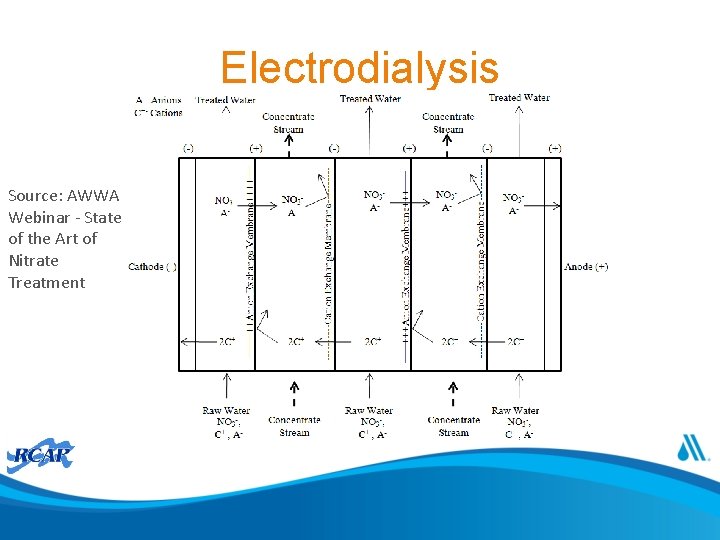
Electrodialysis • Membrane separation process where an electrical current is passed through a stack of anion and cation exchange membranes

Electrodialysis Source: AWWA Webinar - State of the Art of Nitrate Treatment

Review of ED/EDR Design Considerations “Membrane life, cleaning frequency, and pretreatment needs are dependent on feed water quality. ” • Filtration to remove suspended solids • Treatment for iron and manganese removal Pretreatment • Water softening or use of anti-scalants or acid to prevent scaling • p. H adjustment to avoid corrosion (if acid used to prevent scaling) Post-Treatment • Disinfection • Possible p. H adjustment (acids and bases) Chemical Usage • Possible anti-scalants • Possible cleaning chemicals • Highly automated Operation & • Frequency of membrane cleaning depends on Maintenance water quality and membrane used • Management of chemicals and prefiltration system • Concentrate disposal options can be limited by waste brine/concentrate water quality (e. g. , Waste volume, salinity, metals and radionuclides) Management & Disposal • Optimization of recycling and treatment of waste concentrate • Need to prevent membrane scaling and fouling Limitations • Disposal of waste concentrate • High system complexity

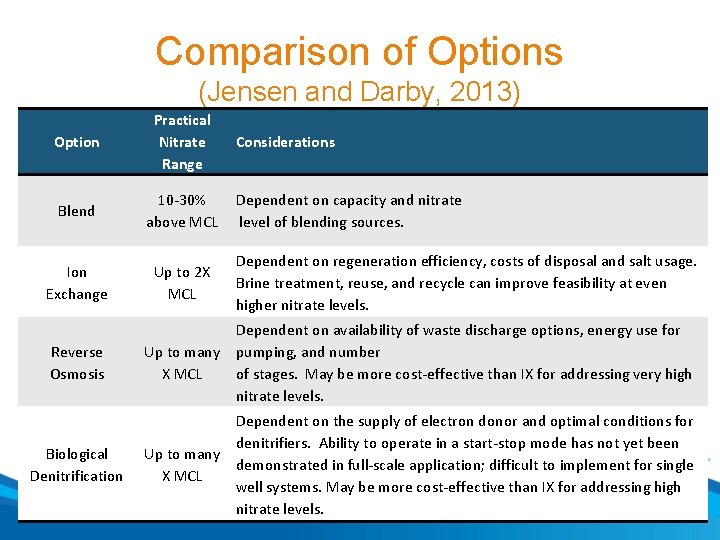
Comparison of Options (Jensen and Darby, 2013) Option Practical Nitrate Range Blend 10 -30% above MCL Ion Exchange Up to 2 X MCL Considerations Dependent on capacity and nitrate level of blending sources. Dependent on regeneration efficiency, costs of disposal and salt usage. Brine treatment, reuse, and recycle can improve feasibility at even higher nitrate levels. Reverse Osmosis Dependent on availability of waste discharge options, energy use for Up to many pumping, and number X MCL of stages. May be more cost-effective than IX for addressing very high nitrate levels. Biological Denitrification Dependent on the supply of electron donor and optimal conditions for denitrifiers. Ability to operate in a start-stop mode has not yet been Up to many demonstrated in full-scale application; difficult to implement for single X MCL well systems. May be more cost-effective than IX for addressing high nitrate levels.

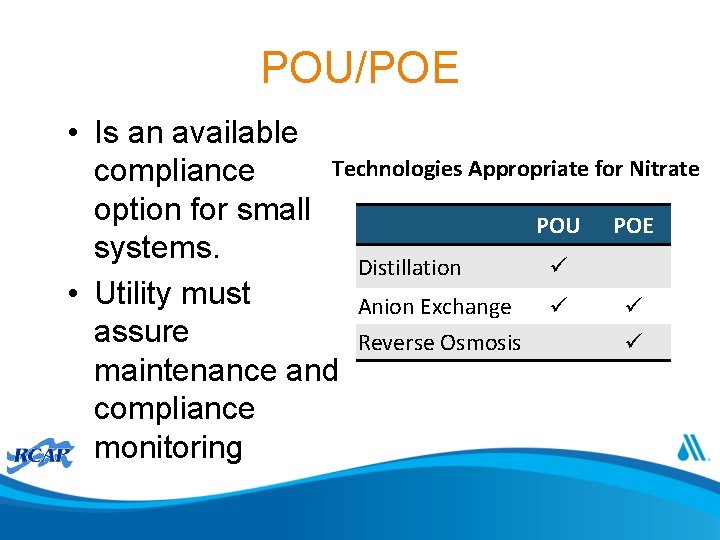
POU/POE • Is an available Technologies Appropriate for Nitrate compliance option for small POU POE systems. Distillation • Utility must Anion Exchange assure Reverse Osmosis maintenance and compliance monitoring

Point-of-Use / Point-of-Entry • “Water systems using POU treatment for nitrate removal should make special efforts to educate customers about the need for using only the tap that is treated, the health risks associated with consuming untreated water, and the need for a proper replacement frequency of the AX resins. ”

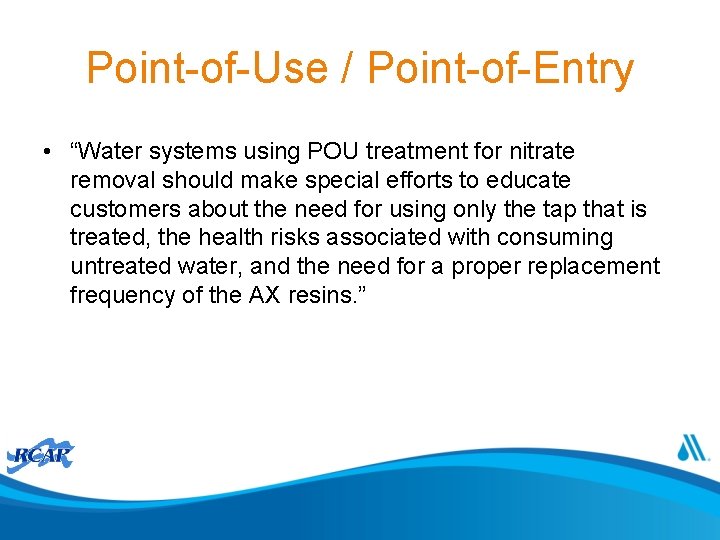
Activity • For well field in following slide describe one or more blending strategies within the following constraints: – All 7 wells are permitted for the same withdrawal and have the same size pump – At least 3 wells running at permitted withdrawal to meet demand – System has a single storage tank

Exercise 1 Maximum Nitrate Concentration (mg/L as Nitrogen) 2. 1 - 5. 0 5. 1 - 10 10. 1 - 20

Knowledge Checkpoint • Can ion exchange reduce 20 mg/L nitrate well water SDWA compliant drinking water? • What is key constraint for ion exchange and reverse osmosis? • Does elevated chloride reduce nitrate removal by ion exchange?

Activity • For the scenario presented in the following slides: 1. What is the system’s compliance status and what actions are required. 2. Identify long-term control measures for detailed evaluation, include at least two treatment options. 3. Prepare a brief summary of why those measures and the pros and cons of each

Activity – Mock Event • System • Community water system serving 6, 000 households • Relies on ground water supply only • Current Treatment • System complies with Ground Water Rule by meeting 4 -log virus inactivation using free chlorine • Water supply • Three wells • Well 1 – 50% of supply through most of year (currently withdrawal is at maximum allowed by state) • Well 2 – 50% of supply during low-demand periods • Well 3 – 25% of supply during peak demand period (July through September); use is limited by arsenic levels in well (typically 8. 9 ug/L) • Wells are approximately the same depth and located in the same aquifer • See Map 1 for relative location of wells • Each well has a unique point of entry to distribution system • Resources • Self sufficient utility with 1 full-time operator in charge and 2 part-time staff • Combined years of service in small ground water systems of 15 years • Event • System is on annual monitoring for nitrate • Laboratory notifies system and state of most recent round of observations on August 15 • Results are: • Well 1 – 10. 40 mg/L as N • Well 2 – 12. 00 mg/L as N • Well 3 – 0. 5 mg/L as N • Follow-up monitoring occurred and results received are: • Well 1 – 10. 20 mg/L as N • Well 2 – 13. 00 mg/L as N • Well 3 – 0. 2 mg/L as N

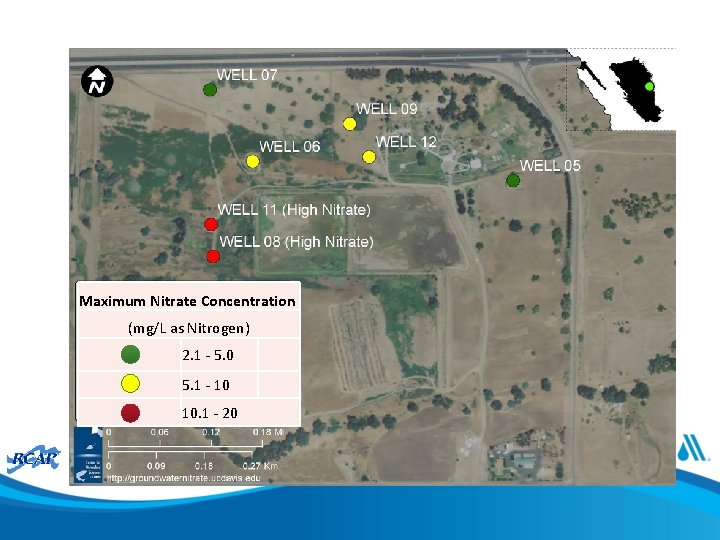
Activity – Available Land Use Information CAFOs (hogs) Abandoned mines Well 3 Residential with sewer Well 2 Residential with septic tanks N Well 1 WWTP (secondary treatment) Residential with septic tanks Residential on sewer

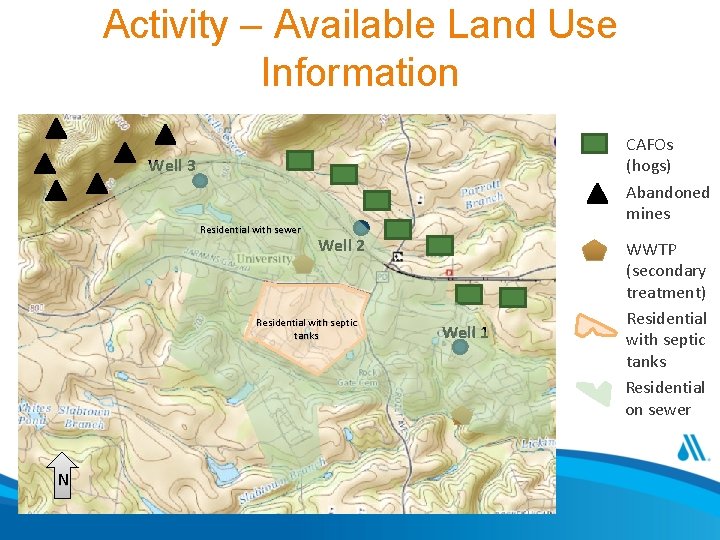
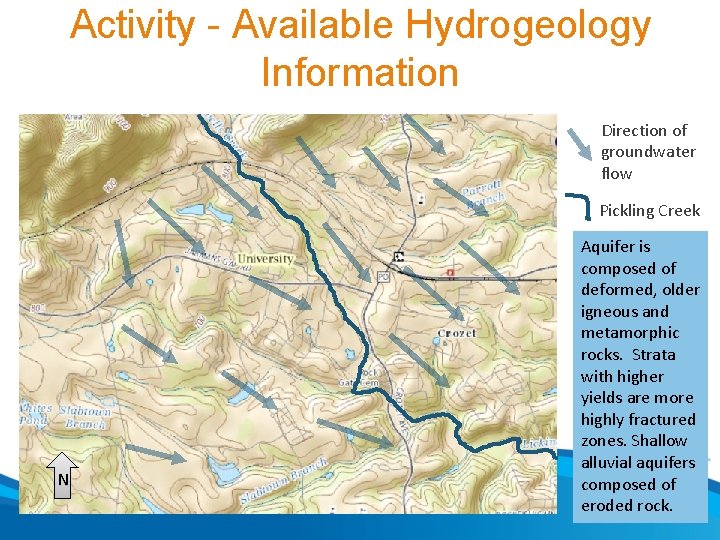
Activity - Available Hydrogeology Information Direction of groundwater flow Pickling Creek N Aquifer is composed of deformed, older igneous and metamorphic rocks. Strata with higher yields are more highly fractured zones. Shallow alluvial aquifers composed of eroded rock.

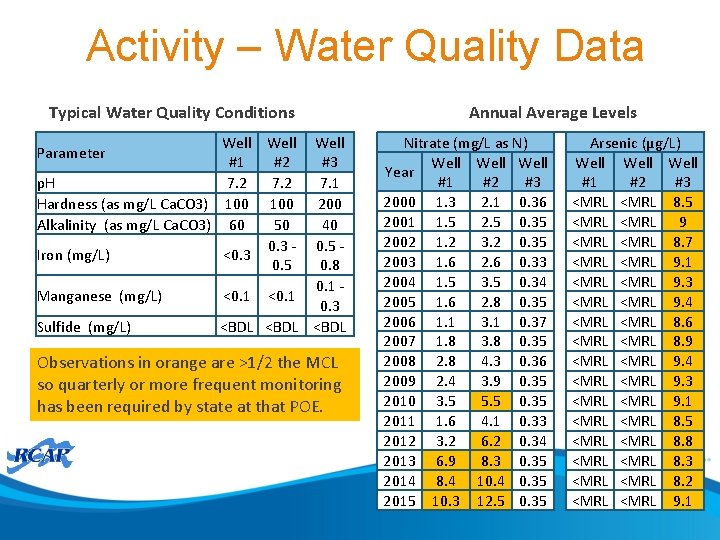
Activity – Water Quality Data Typical Water Quality Conditions Well #1 #2 #3 p. H 7. 2 7. 1 Hardness (as mg/L Ca. CO 3) 100 200 Alkalinity (as mg/L Ca. CO 3) 60 50 40 0. 3 - 0. 5 - Iron (mg/L) <0. 3 0. 5 0. 8 0. 1 - Manganese (mg/L) <0. 1 0. 3 Sulfide (mg/L) <BDL Parameter Observations in orange are >1/2 the MCL so quarterly or more frequent monitoring has been required by state at that POE. Annual Average Levels Nitrate (mg/L as N) Well Year #1 #2 #3 2000 1. 3 2. 1 0. 36 2001 1. 5 2. 5 0. 35 2002 1. 2 3. 2 0. 35 2003 1. 6 2. 6 0. 33 2004 1. 5 3. 5 0. 34 2005 1. 6 2. 8 0. 35 2006 1. 1 3. 1 0. 37 2007 1. 8 3. 8 0. 35 2008 2. 8 4. 3 0. 36 2009 2. 4 3. 9 0. 35 2010 3. 5 5. 5 0. 35 2011 1. 6 4. 1 0. 33 2012 3. 2 6. 2 0. 34 2013 6. 9 8. 3 0. 35 2014 8. 4 10. 4 0. 35 2015 10. 3 12. 5 0. 35 Arsenic (µg/L) Well #1 #2 #3 <MRL 8. 5 <MRL 9 <MRL 8. 7 <MRL 9. 1 <MRL 9. 3 <MRL 9. 4 <MRL 8. 6 <MRL 8. 9 <MRL 9. 4 <MRL 9. 3 <MRL 9. 1 <MRL 8. 5 <MRL 8. 8 <MRL 8. 3 <MRL 8. 2 <MRL 9. 1

Resources • Basic Information about Nitrate – http: //water. epa. gov/drink/contaminants/basicinformation/nitrate. cfm • An Assessment of the State of Nitrate Treatment Alternatives – http: //www. awwa. org/Portals/0/files/resource%20 dev%20 groups/tech%20 an d%20 educ%20 program/documents/TECNitrate. Report. Final. Jan 2012. pdf • National Compliance Costs from a Potential Revision to the Nitrate Regulation – http: //www. awwa. org/publications/journal-awwa/abstract/articleid/27202. aspx • Nitrogen and Phosphorus Pollution Data Access Tool – http: //www 2. epa. gov/nutrient-policy-data/nitrogen-and-phosphorus-pollution-dataaccess-tool • University of California Davis – http: //groundwaternitrate. ucdavis. edu/