Chemical Reactions Chemical Physical Changes In a physical



- Slides: 14

Chemical Reactions

Chemical & Physical Changes • In a physical change, the chemical composition of the substance remains constant. • Examples of physical changes are the melting of ice or the boiling of water. • In a chemical change, the chemical composition of the substance changes; a chemical reaction occurs. • During a chemical reaction, a new substance is formed.

Evidence for Chemical Reactions • There are four observations which indicate a chemical reaction is taking place. 1. A gas is released. • Gas may be observed in many ways in a reaction from light fizzing to heavy bubbling. • Shown here is the release of hydrogen gas from the reaction of magnesium metal with acid.

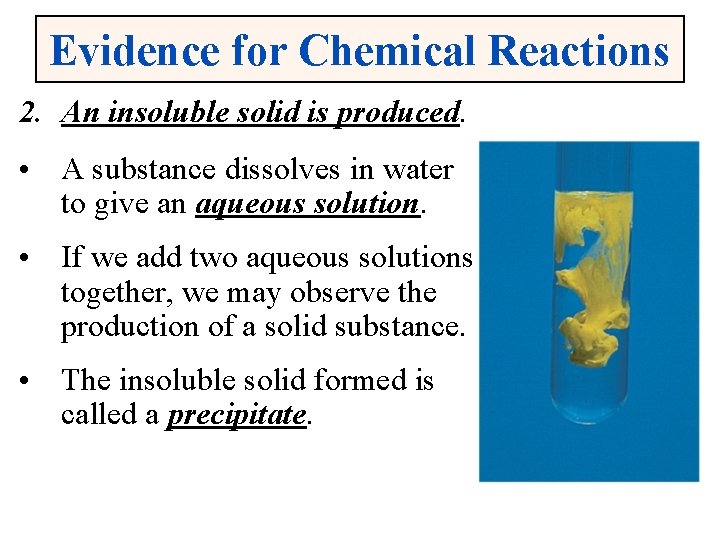
Evidence for Chemical Reactions 2. An insoluble solid is produced. • A substance dissolves in water to give an aqueous solution. • If we add two aqueous solutions together, we may observe the production of a solid substance. • The insoluble solid formed is called a precipitate.

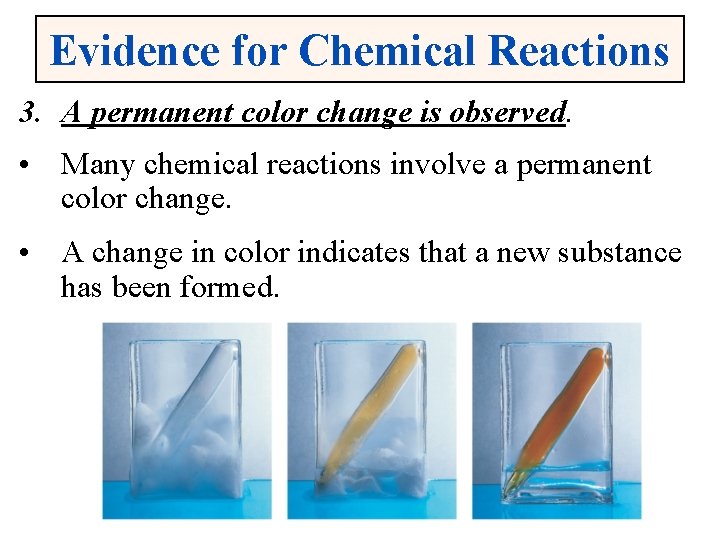
Evidence for Chemical Reactions 3. A permanent color change is observed. • Many chemical reactions involve a permanent color change. • A change in color indicates that a new substance has been formed.

Evidence for Chemical Reactions 4. An energy change is observed. • Examples of a heat energy change in a chemical reaction are heat and light given off.

Writing Chemical Equations • A chemical equation describes a chemical reaction using formulas and symbols. A general chemical equation is: A+B → C+D • In this equation, A and B are reactants and C and D are products.

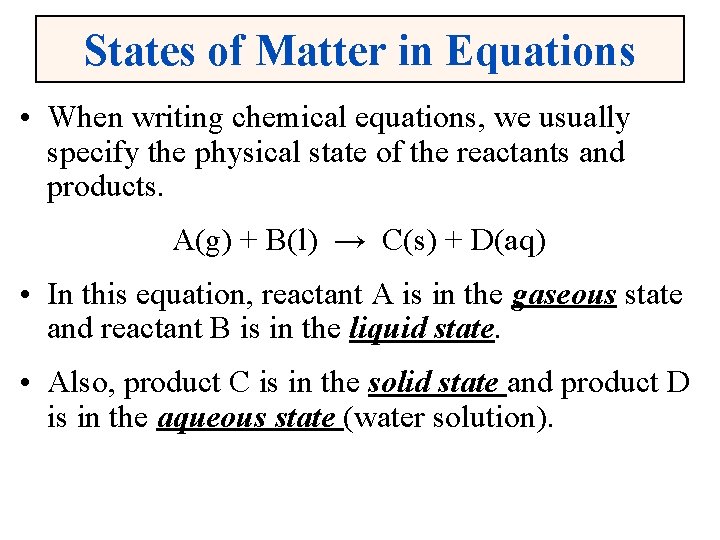
States of Matter in Equations • When writing chemical equations, we usually specify the physical state of the reactants and products. A(g) + B(l) → C(s) + D(aq) • In this equation, reactant A is in the gaseous state and reactant B is in the liquid state. • Also, product C is in the solid state and product D is in the aqueous state (water solution).

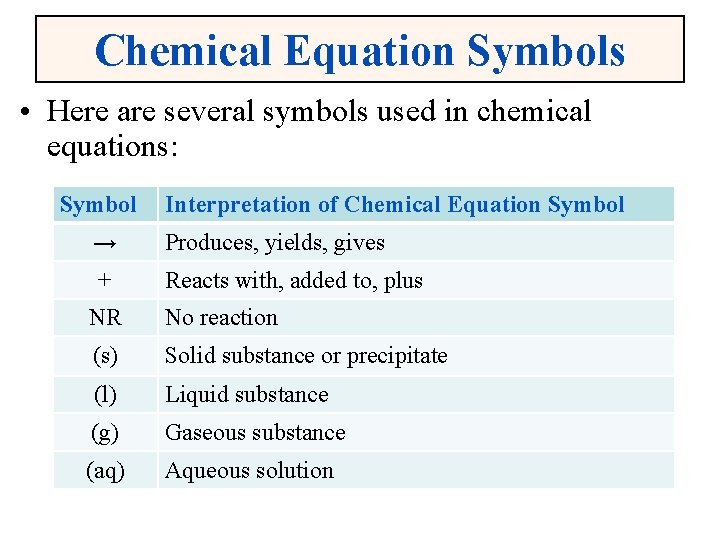
Chemical Equation Symbols • Here are several symbols used in chemical equations: Symbol Interpretation of Chemical Equation Symbol → Produces, yields, gives + Reacts with, added to, plus NR No reaction (s) Solid substance or precipitate (l) Liquid substance (g) Gaseous substance (aq) Aqueous solution

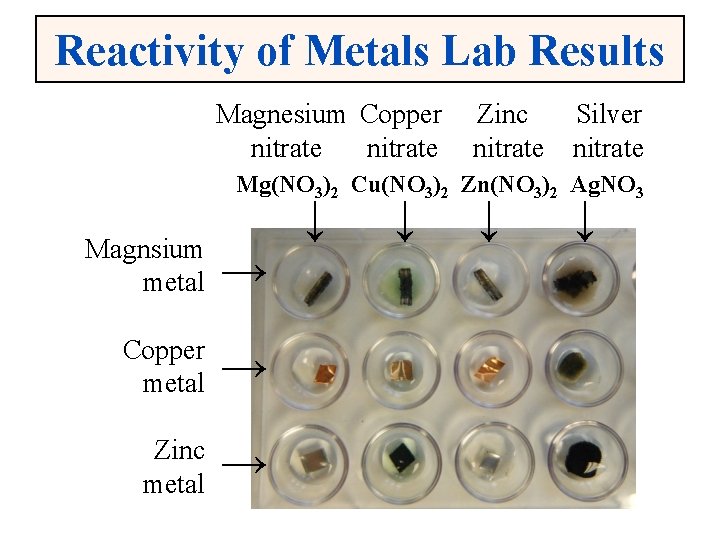
Reactivity of Metals Lab Results Magnesium Copper nitrate Zinc nitrate Silver nitrate Mg(NO 3)2 Cu(NO 3)2 Zn(NO 3)2 Ag. NO 3 ↓ Magnsium metal → Copper metal → Zinc metal → ↓ ↓ ↓

A Chemical Reaction • Lets look at a chemical reaction that occurred in the metal reactivity lab: Cu(NO 3)2(aq) + Zn(s) →Zn(NO 3)2(aq) + Cu(s) • The equation can be read as follows: – Aqueous copper nitrate is added to solid zinc and yields aqueous zinc nitrate and solid copper. – Notice zinc replaced copper in the nitrate solution and solid copper formed as a precipitate.

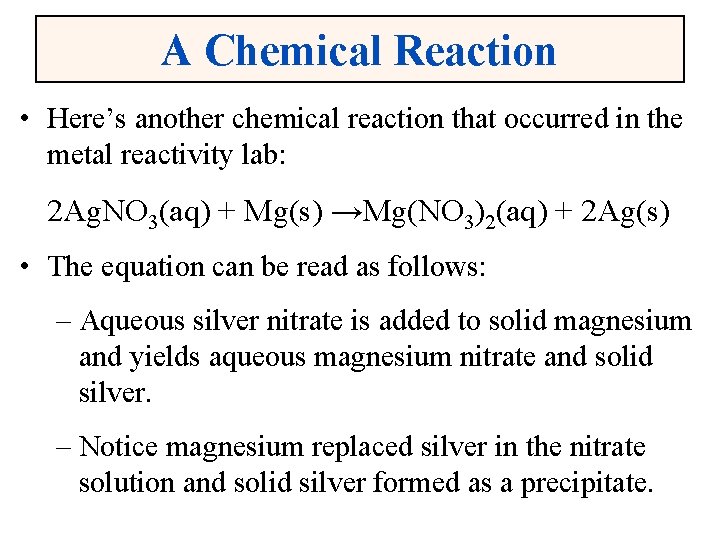
A Chemical Reaction • Here’s another chemical reaction that occurred in the metal reactivity lab: 2 Ag. NO 3(aq) + Mg(s) →Mg(NO 3)2(aq) + 2 Ag(s) • The equation can be read as follows: – Aqueous silver nitrate is added to solid magnesium and yields aqueous magnesium nitrate and solid silver. – Notice magnesium replaced silver in the nitrate solution and solid silver formed as a precipitate.

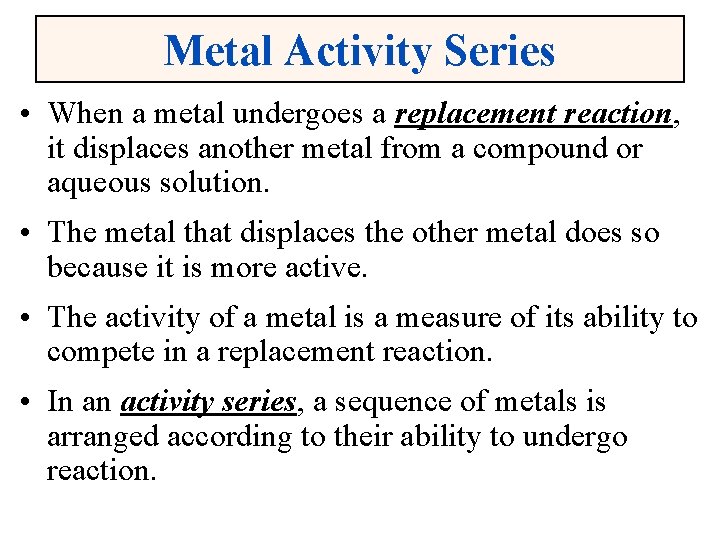
Metal Activity Series • When a metal undergoes a replacement reaction, it displaces another metal from a compound or aqueous solution. • The metal that displaces the other metal does so because it is more active. • The activity of a metal is a measure of its ability to compete in a replacement reaction. • In an activity series, a sequence of metals is arranged according to their ability to undergo reaction.

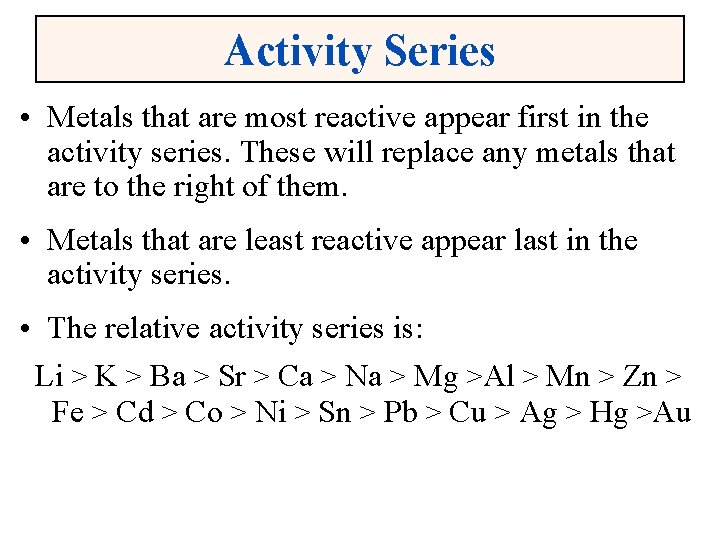
Activity Series • Metals that are most reactive appear first in the activity series. These will replace any metals that are to the right of them. • Metals that are least reactive appear last in the activity series. • The relative activity series is: Li > K > Ba > Sr > Ca > Na > Mg >Al > Mn > Zn > Fe > Cd > Co > Ni > Sn > Pb > Cu > Ag > Hg >Au
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Examples of chemical change
Examples of chemical change Types of reactions
Types of reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Physical change
Physical change Compare and contrast chemical and physical changes
Compare and contrast chemical and physical changes Physical and chemical changes
Physical and chemical changes Is rocket fuel burning a physical change
Is rocket fuel burning a physical change Physical and chemical changes generation genius
Physical and chemical changes generation genius Physical l change
Physical l change What is an example of chemical and physical change
What is an example of chemical and physical change Difference between chemical and physical change
Difference between chemical and physical change Which is true about chemical change
Which is true about chemical change



























