Chemical tests to identify anions Connector 1 What











- Slides: 14

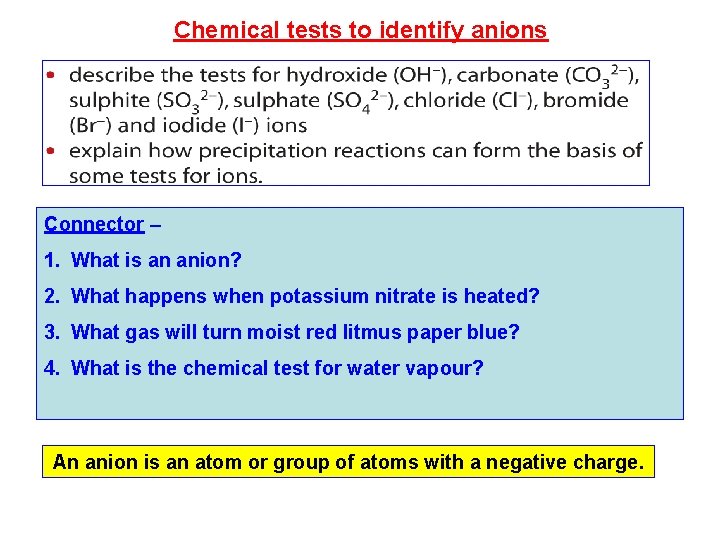
Chemical tests to identify anions Connector – 1. What is an anion? 2. What happens when potassium nitrate is heated? 3. What gas will turn moist red litmus paper blue? 4. What is the chemical test for water vapour? An anion is an atom or group of atoms with a negative charge.

Experiment – Testing for anions. 1 Carry out the following tests and record the test and your observations: 1. Testing for hydroxides Use a glass rod to put a spot of sodium hydroxide on red litmus paper and on universal indicator paper. Repeat using ammonia soln. 2. Testing for carbonates and sulphites a. Put a sample of sodium carbonate in a boiling tube, then carefully add an equal dilute HCl. Pour the invisible gas from the boiling tube into another boiling tube that contains 2 cm 3 of limewater and gently shake. b. Repeat the experiment using sodium sulphite, but this time test the gas using i. a piece of filter paper soaked in acidified Na 2 Cr 2 O 7; ii. moist blue litmus paper, over the mouth of the boiling tube. Write word and balanced symbol equations for the two decomposition reactions.

Hydroxides OH-

Carbonates, sulphites Write the balanced symbol equation

Carbonates

Sulphite ion; SO 32 -

Questions

Experiment – Testing for anions. 2 Carry out the following tests and record the test and your observations: 3. Testing for sulphates Put 2 cm 3 of sodium sulphate in a test tube add the same volume of dilute HCl, and barium chloride soln. 4. Testing for halide ions In separate test tubes put 2 cm 3 of potassium chloride, potassium bromide and potassium iodide soln. , i. to each one add 2 cm 3 of dilute nitric acid followed by 2 cm 3 of silver nitrate soln. – record your observations ii. Now add 2 cm 3 of dil. ammonia soln. iii. Then add 2 cm 3 of conc. ammonia soln. Write word and balanced symbol equations for the reactions that occurred in the precipitation reactions. (Note: Ignore the acids in tests 3 & 4. )

Sulphate Write the balanced symbol equation


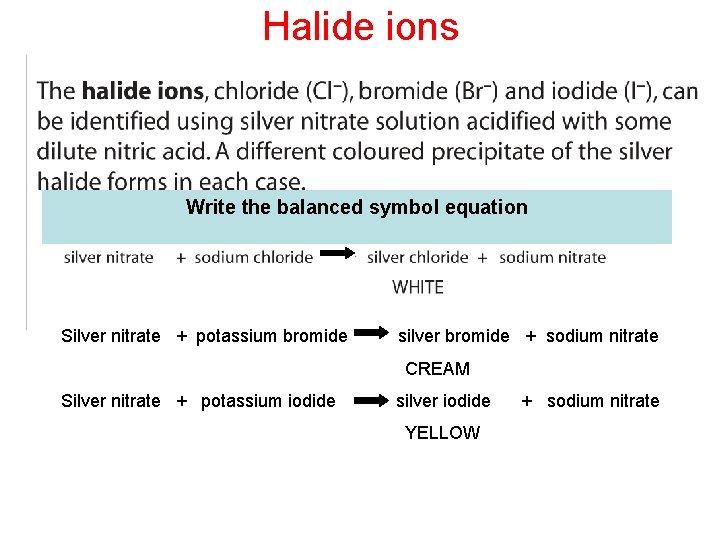
Halide ions Write the balanced symbol equation Silver nitrate + potassium bromide silver bromide + sodium nitrate CREAM Silver nitrate + potassium iodide silver iodide YELLOW + sodium nitrate

Halide ions

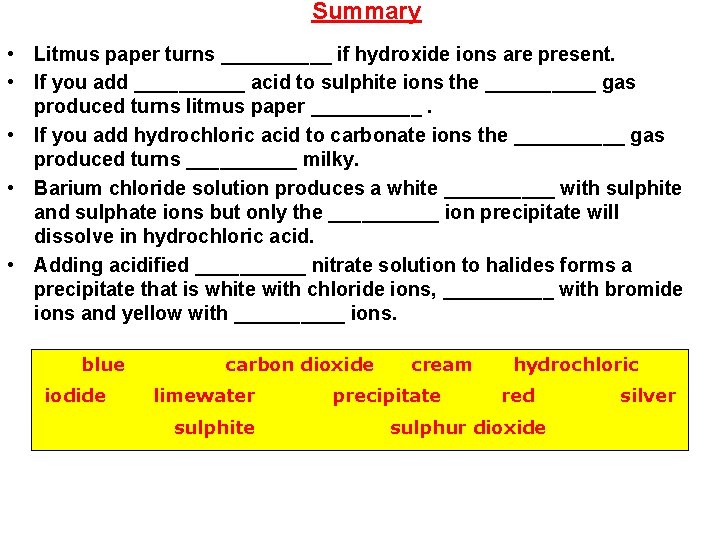
Summary • Litmus paper turns _____ if hydroxide ions are present. • If you add _____ acid to sulphite ions the _____ gas produced turns litmus paper _____. • If you add hydrochloric acid to carbonate ions the _____ gas produced turns _____ milky. • Barium chloride solution produces a white _____ with sulphite and sulphate ions but only the _____ ion precipitate will dissolve in hydrochloric acid. • Adding acidified _____ nitrate solution to halides forms a precipitate that is white with chloride ions, _____ with bromide ions and yellow with _____ ions. blue iodide carbon dioxide limewater sulphite cream precipitate hydrochloric red sulphur dioxide silver

Testing for anions 1 Dil. HCl Sodium hydroxide soln. . Solid Sodium carbonate Solid Sodium sulphite limewater Red litmus paper Blue litmus paper Solution of acidified potassium (or sodium) dichromate Strips of filter paper (to be dipped into acidified K 2 Cr 2 O 7) UI paper Test tubes Boiling tubes Test tube racks Testing for anions 2 Dil. HCl Dil. HNO 3 Sodium sulphate soln Na. Cl soln. Na. Br soln Na. I soln Barium chloride soln. Silver nitrate soln Dilute Ammonia soln. Concentrated ammonia soln Test tubes Boiling tubes Test tube racks
 Ace different tests help iq tests
Ace different tests help iq tests Lingual bar connector
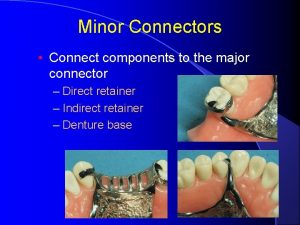
Lingual bar connector Minor connector denture
Minor connector denture Major physiological anions
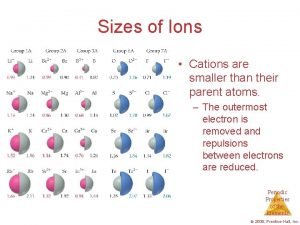
Major physiological anions Sizes of ions
Sizes of ions Three ionic compounds
Three ionic compounds Common ions
Common ions What is the brown ring in the brown ring test
What is the brown ring in the brown ring test Which is an example of a polyatomic ion?h2co3-mg+ne+ -
Which is an example of a polyatomic ion?h2co3-mg+ne+ - Cell chapter 20
Cell chapter 20 Polyatomic ions game
Polyatomic ions game Dicarbon tetraoxide
Dicarbon tetraoxide Classification of anions
Classification of anions Anions
Anions Hfacid or base
Hfacid or base