Single Replacement Reactions Silver replacing Copper in reaction

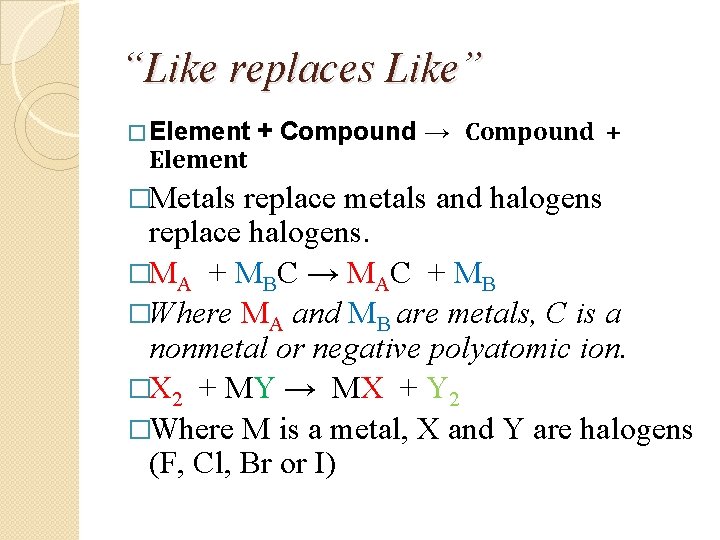

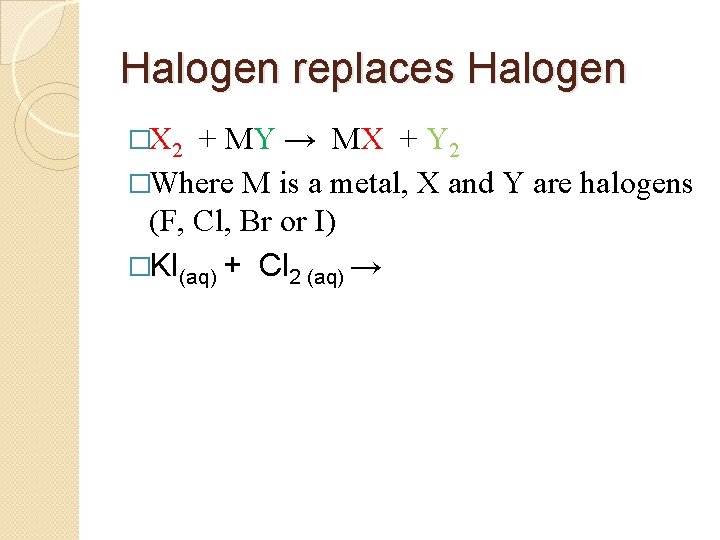
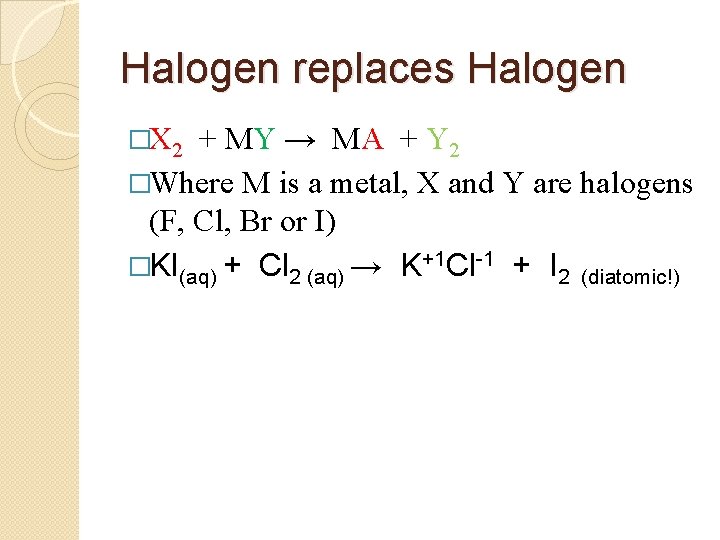
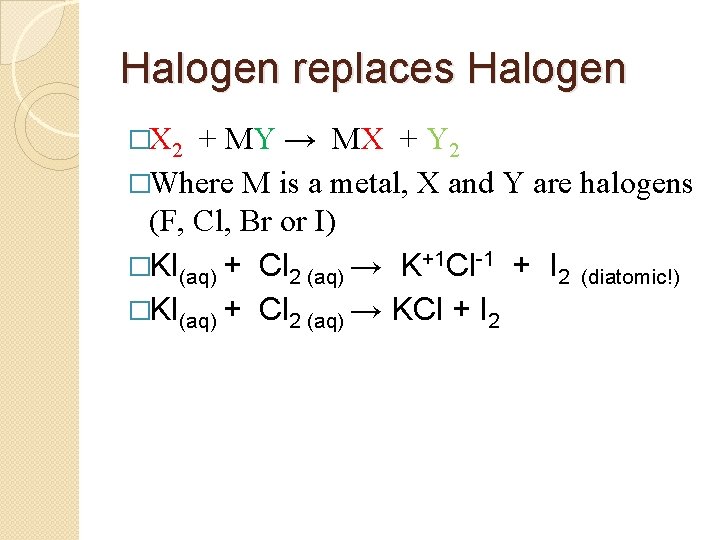
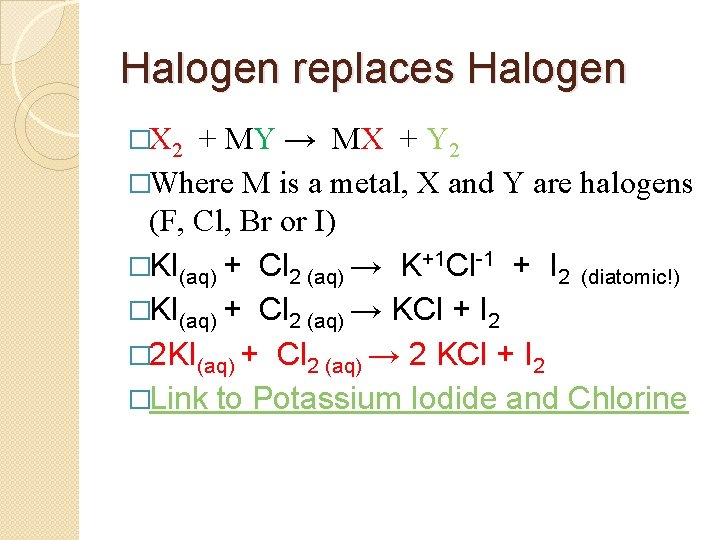




- Slides: 35

Single Replacement Reactions Silver replacing Copper in reaction of silver nitrate and copper Link to silver nitrate and copper

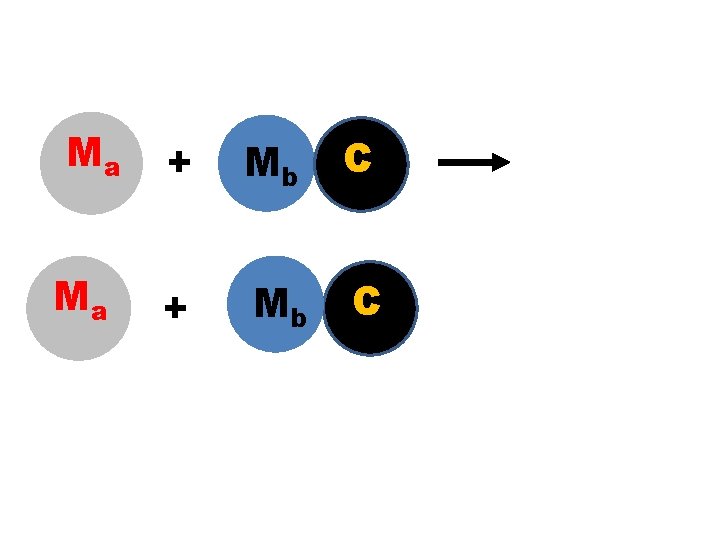
Ma Ma + Mb C
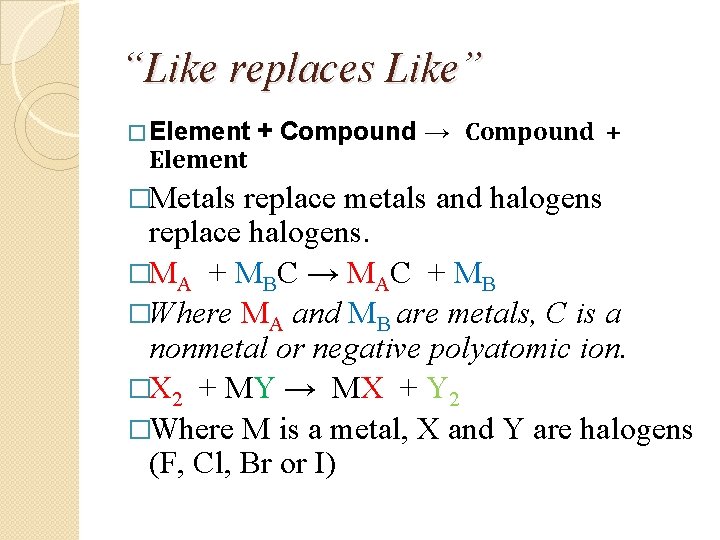
“Like replaces Like” � Element �Metals + Compound → Compound + replace metals and halogens replace halogens. �MA + MBC → MAC + MB �Where MA and MB are metals, C is a nonmetal or negative polyatomic ion. �X 2 + MY → MX + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I)

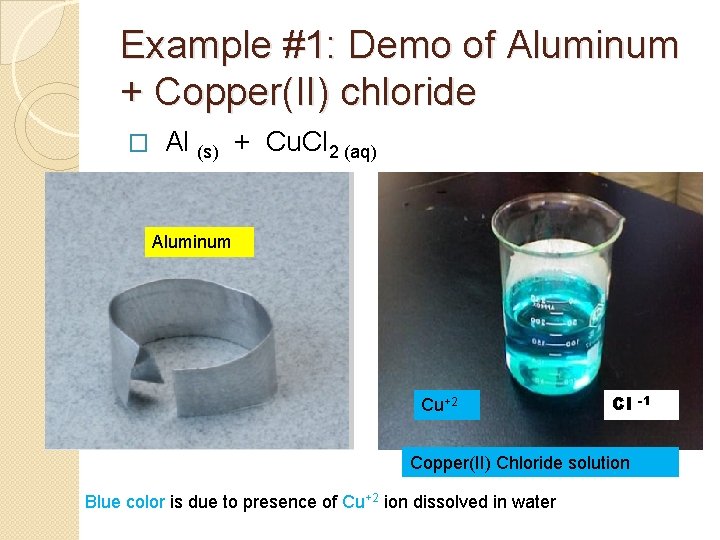
Example #1: Demo of Aluminum + Copper(II) chloride Al (s) + Cu. Cl 2 (aq) → � Aluminum Cu+2 Cl Copper(II) Chloride solution Blue color is due to presence of Cu+2 ion dissolved in water -1

What is the reddish solid that formed? Why has the solution turned from blue to colorless? Immediately after mixing Final appearance after 30 minutes

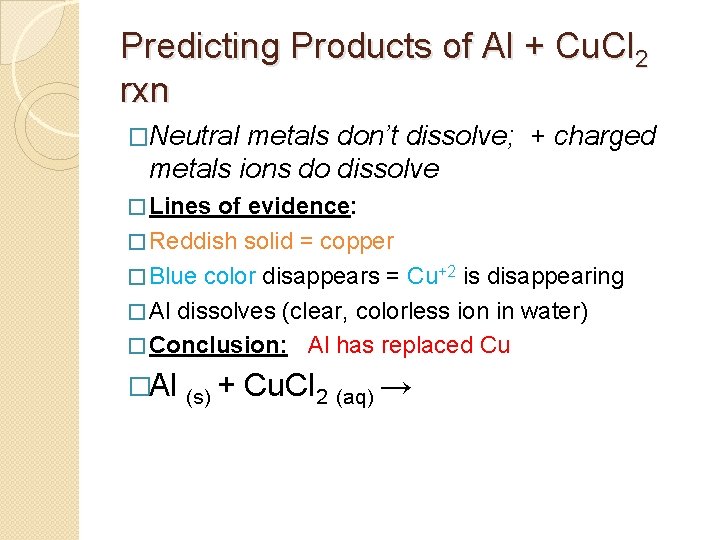
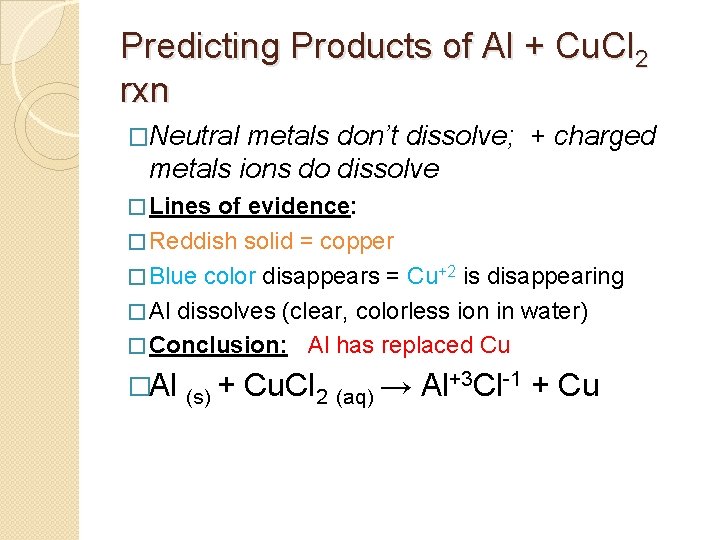
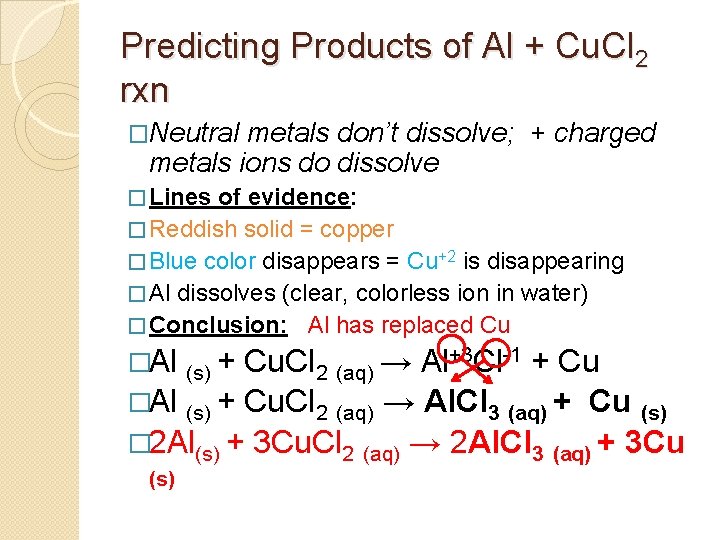
Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) →

Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) → Al+3 Cl-1 + Cu

Predicting Products of Al + Cu. Cl 2 rxn �Neutral metals don’t dissolve; + charged metals ions do dissolve � Lines of evidence: � Reddish solid = copper � Blue color disappears = Cu+2 is disappearing � Al dissolves (clear, colorless ion in water) � Conclusion: Al has replaced Cu �Al (s) + Cu. Cl 2 (aq) → Al+3 Cl-1 + Cu Cu. Cl 2 (aq) → Al. Cl 3 (aq) + Cu (s) � 2 Al(s) + 3 Cu. Cl 2 (aq) → 2 Al. Cl 3 (aq) + 3 Cu (s)
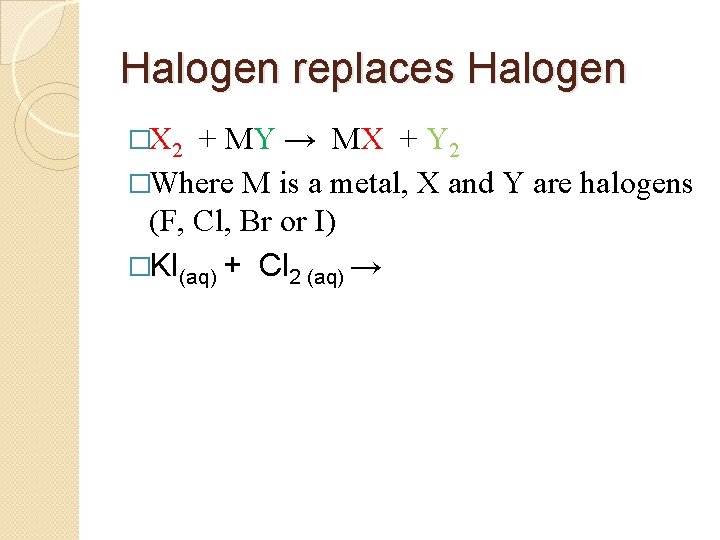
Halogen replaces Halogen �X 2 + MY → MX + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) →
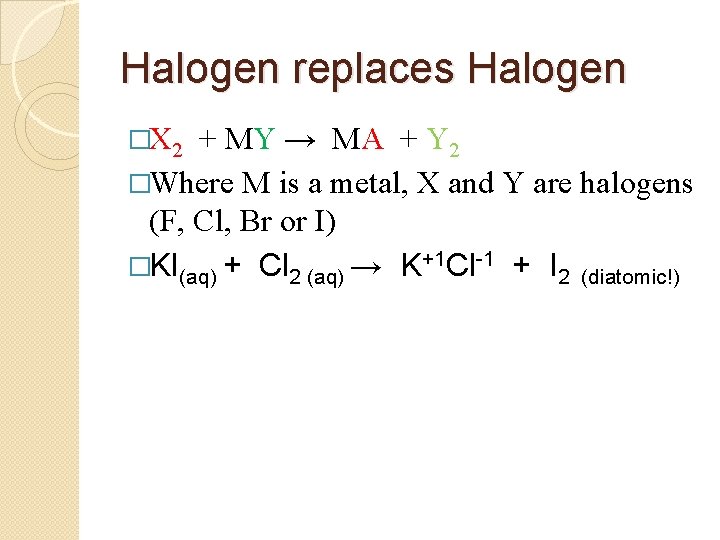
Halogen replaces Halogen �X 2 + MY → MA + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!)
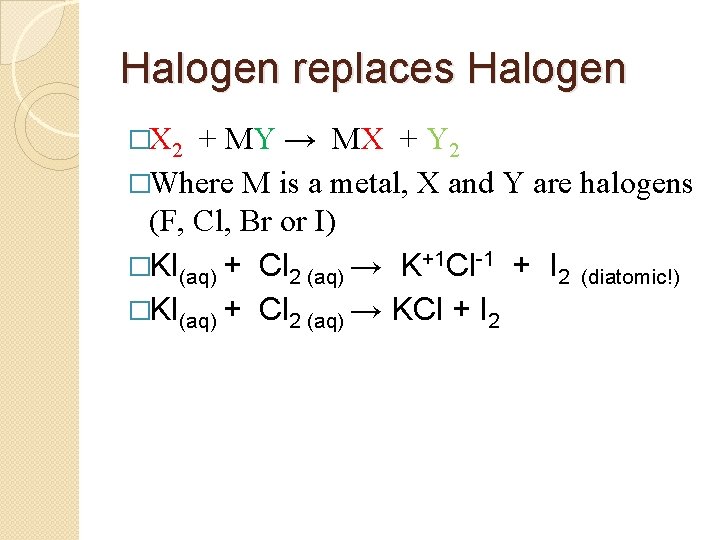
Halogen replaces Halogen �X 2 + MY → MX + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!) �KI(aq) + Cl 2 (aq) → KCl + I 2
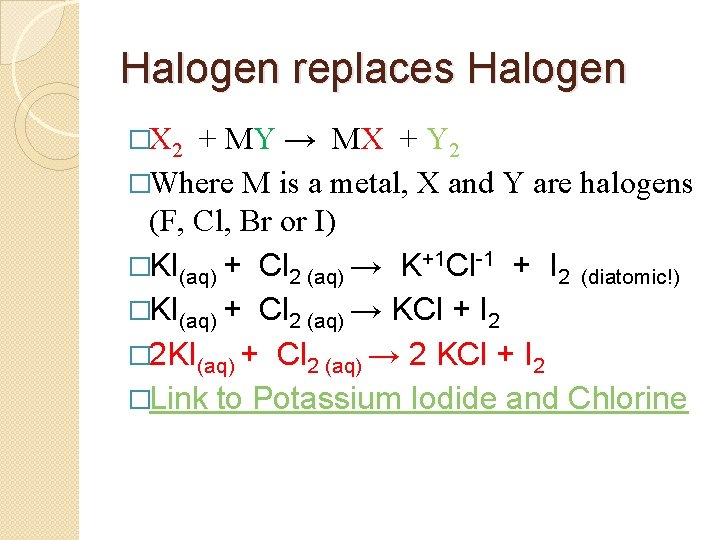
Halogen replaces Halogen �X 2 + MY → MX + Y 2 �Where M is a metal, X and Y are halogens (F, Cl, Br or I) �KI(aq) + Cl 2 (aq) → K+1 Cl-1 + I 2 (diatomic!) �KI(aq) + Cl 2 (aq) → KCl + I 2 � 2 KI(aq) + Cl 2 (aq) → 2 KCl + I 2 �Link to Potassium Iodide and Chlorine

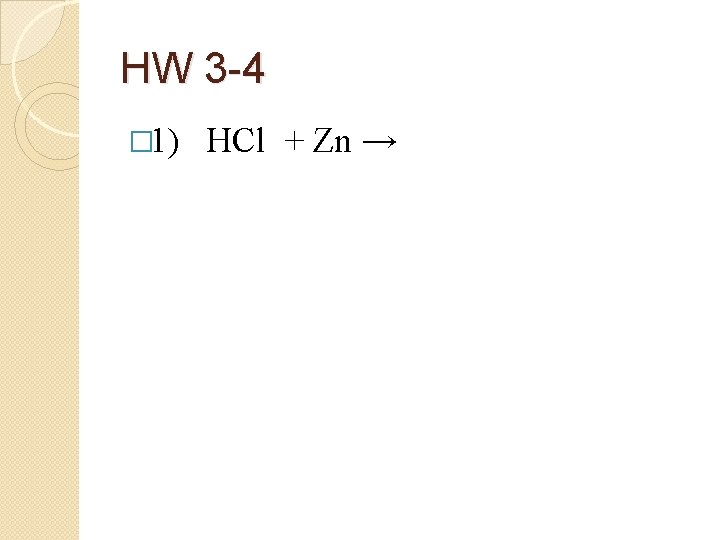
HW 3 -4 � 1) HCl + Zn →

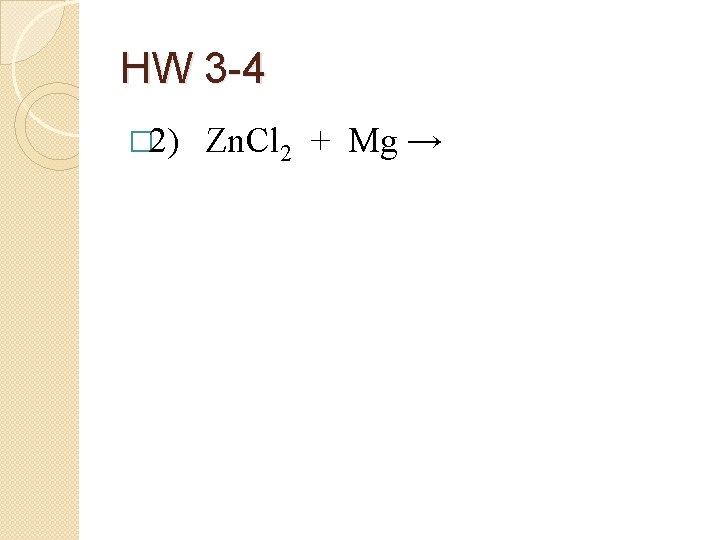
HW 3 -4 � 2) Zn. Cl 2 + Mg →

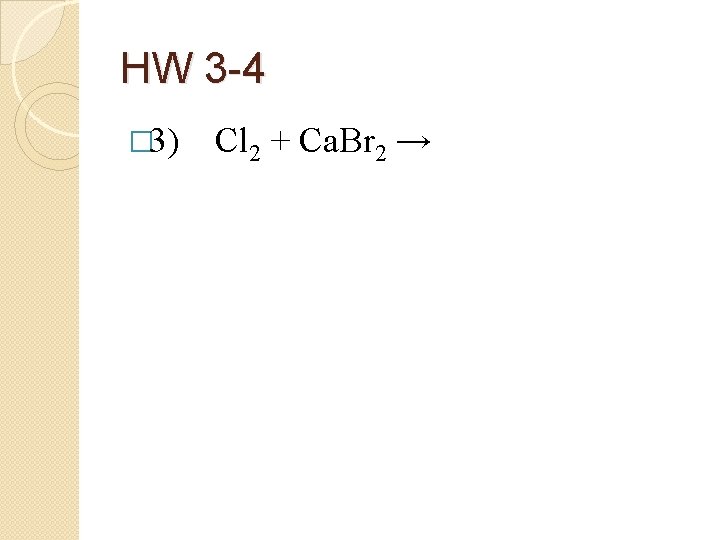
HW 3 -4 � 3) Cl 2 + Ca. Br 2 →

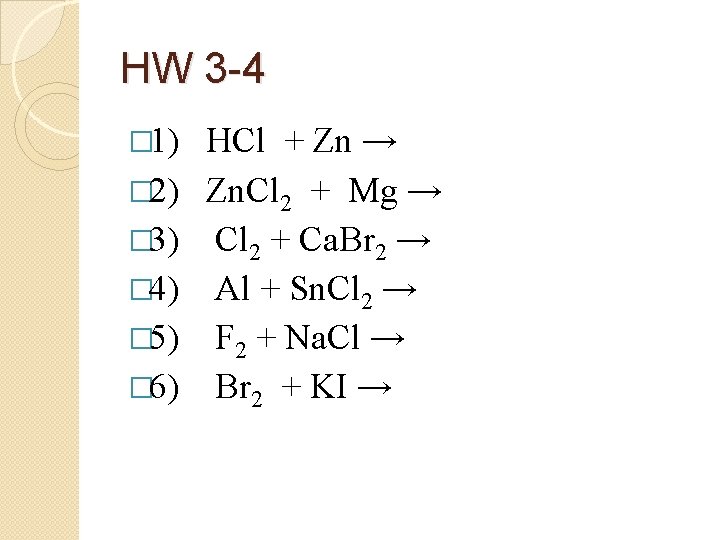
HW 3 -4 � 1) � 2) � 3) � 4) � 5) � 6) HCl + Zn → Zn. Cl 2 + Mg → Cl 2 + Ca. Br 2 → Al + Sn. Cl 2 → F 2 + Na. Cl → Br 2 + KI →

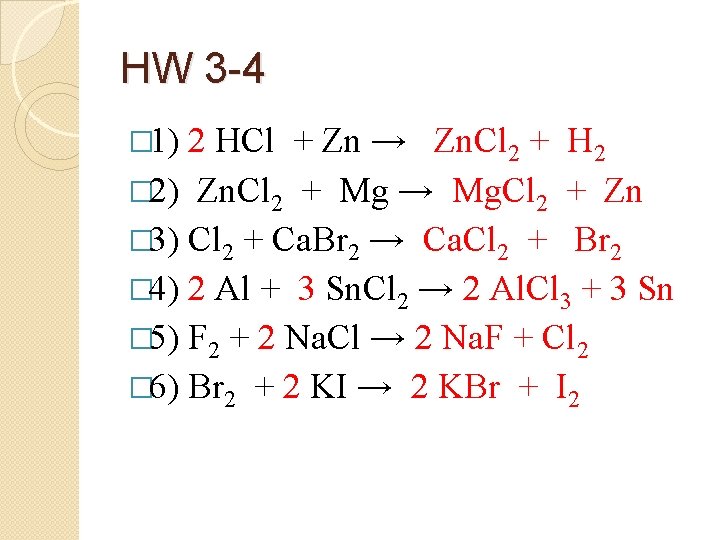
HW 3 -4 � 1) 2 HCl + Zn → Zn. Cl 2 + H 2 � 2) Zn. Cl 2 + Mg → Mg. Cl 2 + Zn � 3) Cl 2 + Ca. Br 2 → Ca. Cl 2 + Br 2 � 4) 2 Al + 3 Sn. Cl 2 → 2 Al. Cl 3 + 3 Sn � 5) F 2 + 2 Na. Cl → 2 Na. F + Cl 2 � 6) Br 2 + 2 KI → 2 KBr + I 2

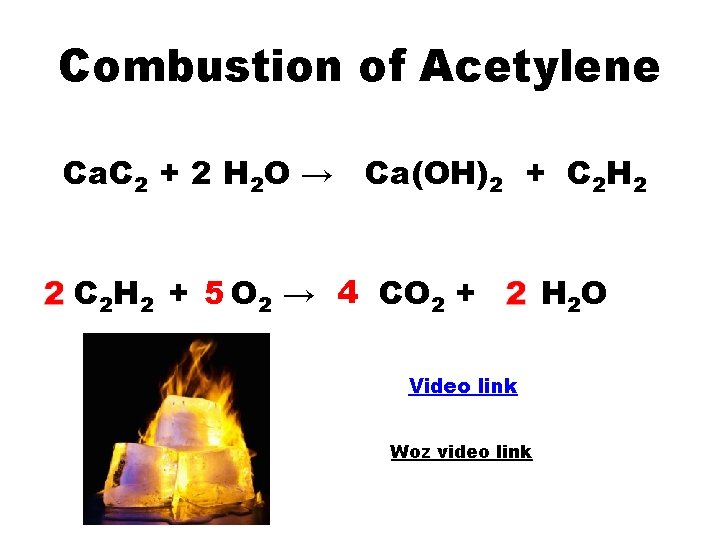
Combustion of Acetylene Ca. C 2 + 2 H 2 O → Ca(OH)2 + C 2 H 2 + 5 O 2 → 4 CO 2 + Video link Woz video link H 2 O

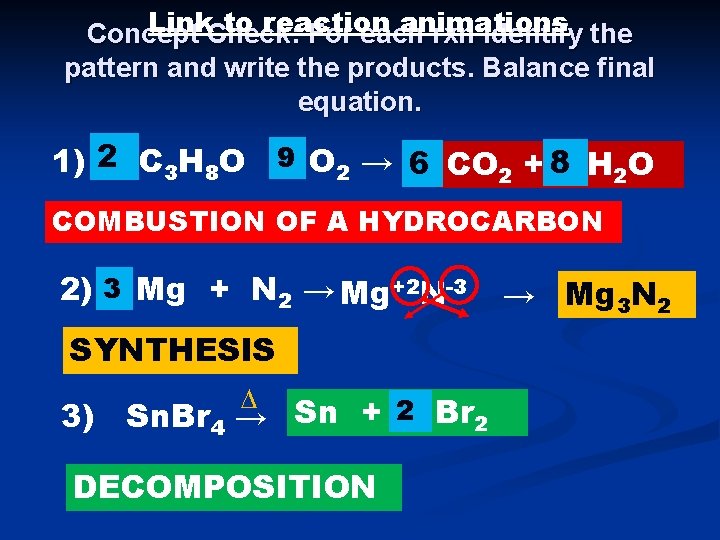
Link. Check: to reaction animations Concept For each rxn identify the pattern and write the products. Balance final equation. 1) 2 C 3 H 8 O +9 O 2 → 63 CO 2 + 8 4 H 2 O COMBUSTION OF A HYDROCARBON 2) 3 Mg + N 2 → Mg+2 N-3 SYNTHESIS ∆ 3) Sn. Br 4 → Sn + 2 Br 2 DECOMPOSITION → Mg 3 N 2

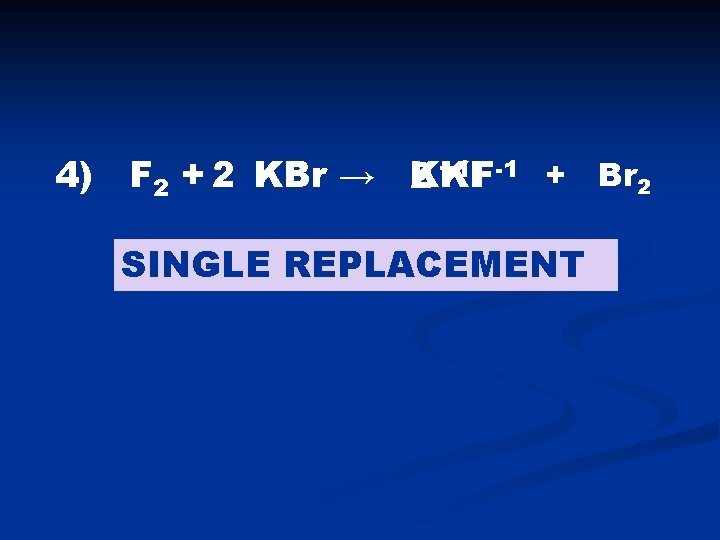
4) F 2 + 2 KBr → K KF F-1 + Br 2 2 +1 SINGLE REPLACEMENT

Activity Series �Some processes are spontaneous in one direction but not the other � Example: Rock can fall from a cliff to the ground, but a rock on the ground can’t climb the cliff by itself.

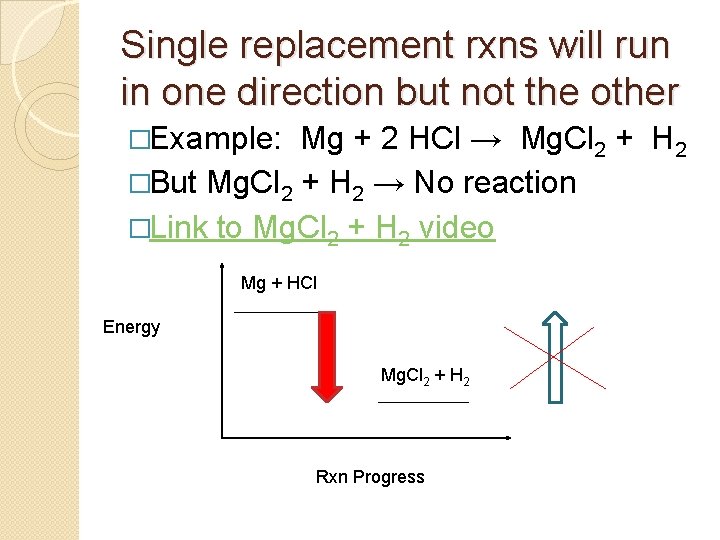
Single replacement rxns will run in one direction but not the other �Example: Mg + 2 HCl → Mg. Cl 2 + H 2 �But Mg. Cl 2 + H 2 → No reaction �Link to Mg. Cl 2 + H 2 video Mg + HCl Energy Mg. Cl 2 + H 2 Rxn Progress

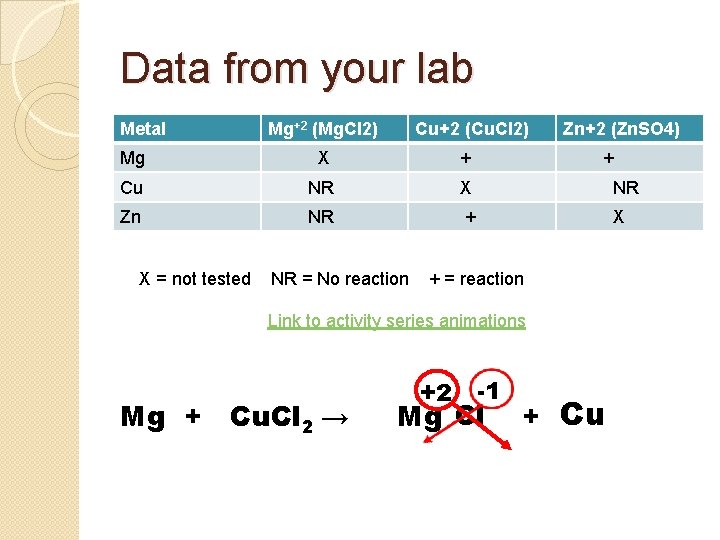
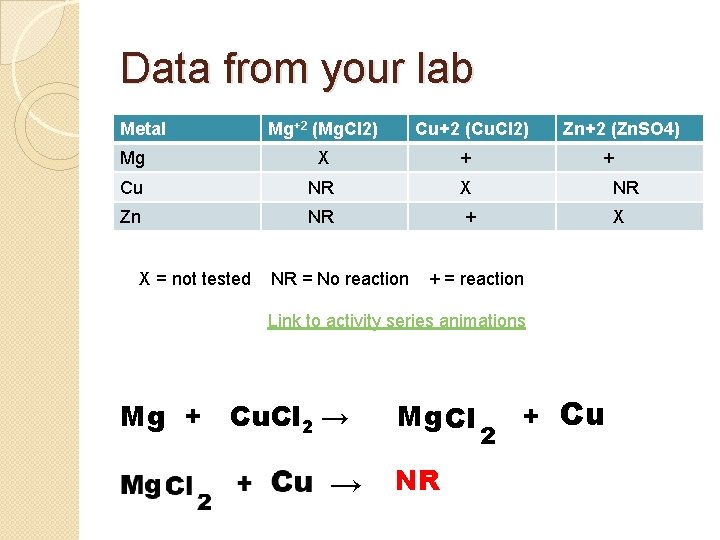
Data from your lab Metal Mg+2 (Mg. Cl 2) Cu+2 (Cu. Cl 2) Zn+2 (Zn. SO 4) Mg X + Cu NR X NR Zn NR + X X = not tested NR = No reaction + + = reaction Link to activity series animations Mg + Cu. Cl 2 → +2 -1 Mg Cl + Cu

Data from your lab Metal Mg+2 (Mg. Cl 2) Cu+2 (Cu. Cl 2) Zn+2 (Zn. SO 4) Mg X + Cu NR X NR Zn NR + X X = not tested NR = No reaction + + = reaction Link to activity series animations Mg + Cu. Cl 2 → → Mg Cl NR 2 + Cu

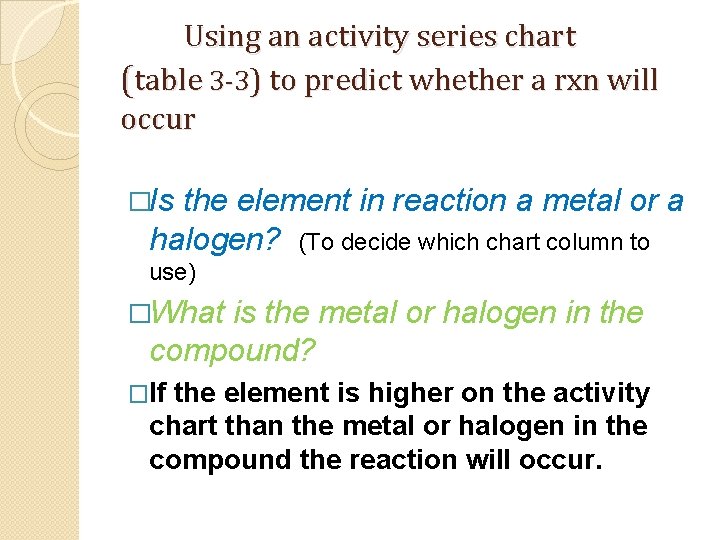
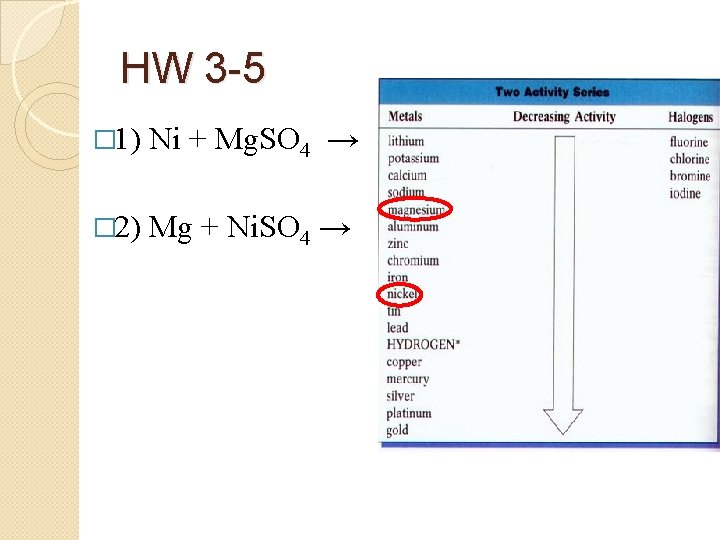
Using an activity series chart (table 3 -3) to predict whether a rxn will occur �Is the element in reaction a metal or a halogen? (To decide which chart column to use) �What is the metal or halogen in the compound? �If the element is higher on the activity chart than the metal or halogen in the compound the reaction will occur.

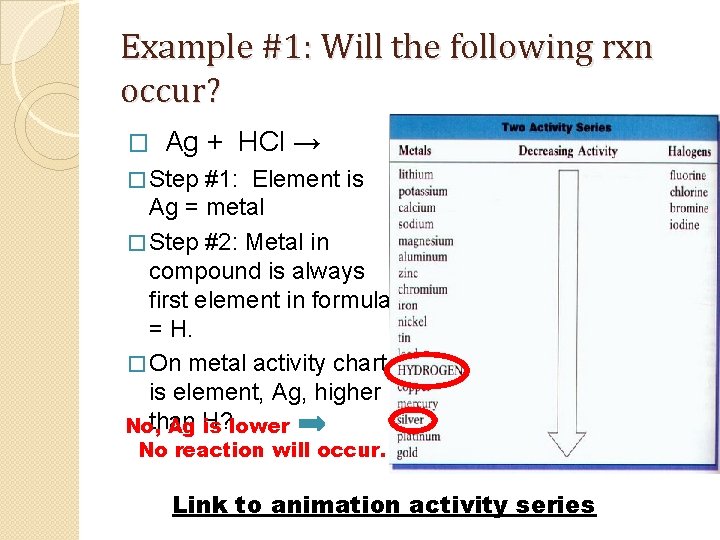
Example #1: Will the following rxn occur? � Ag + HCl → � Step #1: Element is Ag = metal � Step #2: Metal in compound is always first element in formula = H. � On metal activity chart, is element, Ag, higher than No, Ag H? is lower No reaction will occur. Link to animation activity series

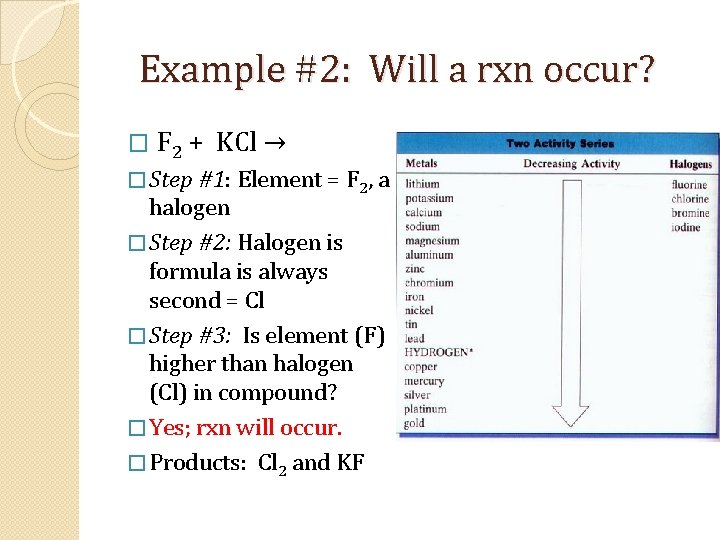
Example #2: Will a rxn occur? � F 2 + KCl → � Step #1: Element = F 2, a halogen � Step #2: Halogen is formula is always second = Cl � Step #3: Is element (F) higher than halogen (Cl) in compound? � Yes; rxn will occur. � Products: Cl 2 and KF

Applications of Activity Series: A more active metal being used to protect a metal supporting a structure � Using Zinc (more active) to protect iron (less chemically active but physically stronger); the iron inside the concrete is helping to support the weight of the bridge

HW 3 -5 � 1) Ni + Mg. SO 4 → � 2) Mg + Ni. SO 4 →

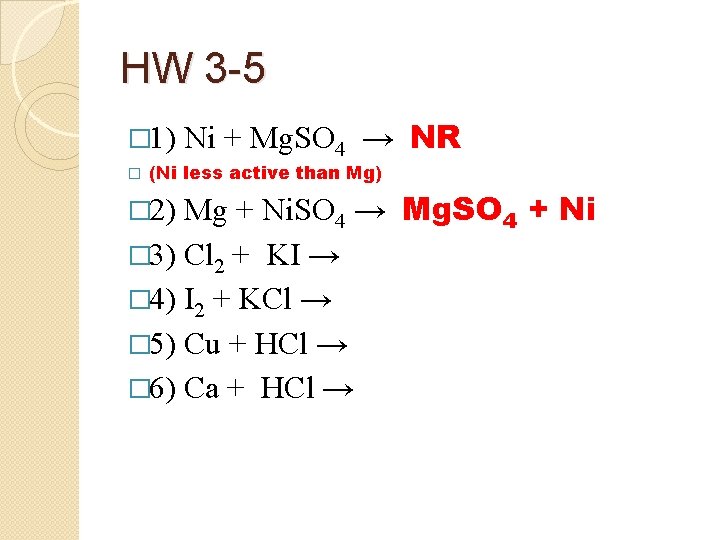
HW 3 -5 � 1) � Ni + Mg. SO 4 → NR (Ni less active than Mg) Mg + Ni. SO 4 → Mg. SO 4 + Ni � 3) Cl 2 + KI → � 4) I 2 + KCl → � 5) Cu + HCl → � 6) Ca + HCl → � 2)

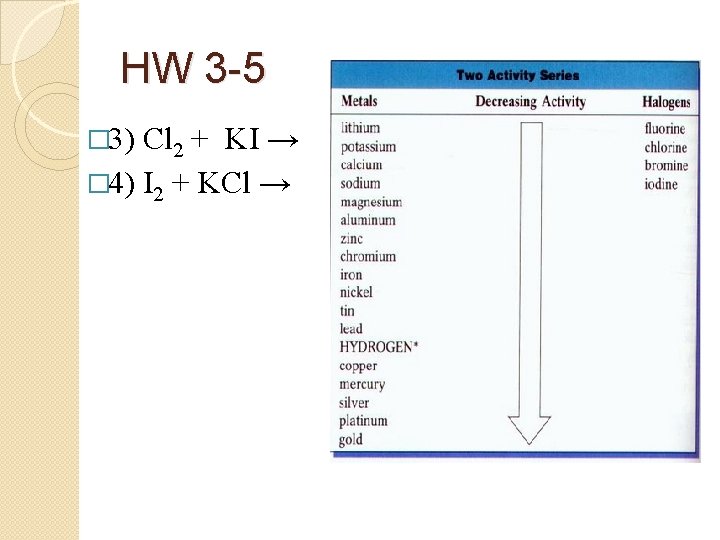
HW 3 -5 � 3) Cl 2 + KI → � 4) I 2 + KCl →

HW 3 -5 � 3) Cl 2 + 2 KI → 2 KCl + I 2 � (Cl is more active than I) � 4) I 2 + KCl → NR

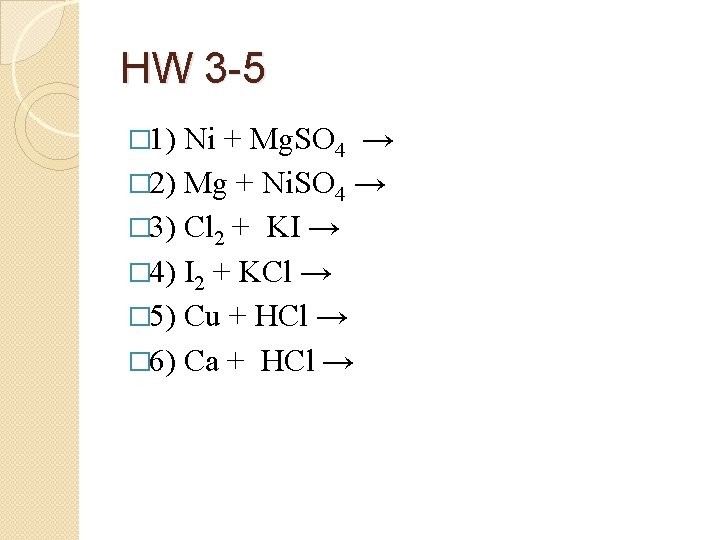
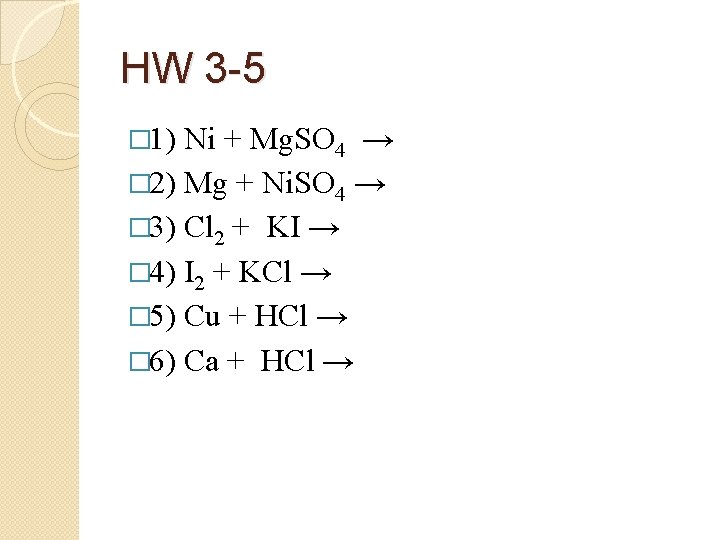
HW 3 -5 � 1) Ni + Mg. SO 4 → � 2) Mg + Ni. SO 4 → � 3) Cl 2 + KI → � 4) I 2 + KCl → � 5) Cu + HCl → � 6) Ca + HCl →

HW 3 -5 � 1) Ni + Mg. SO 4 → � 2) Mg + Ni. SO 4 → � 3) Cl 2 + KI → � 4) I 2 + KCl → � 5) Cu + HCl → � 6) Ca + HCl →

HW 3 -5 � 1) � Ni + Mg. SO 4 → NR (Ni less active than Mg) Mg + Ni. SO 4 → Mg. SO 4 + Ni � 3) Cl 2 + 2 KI → 2 KCl + I 2 � 2) � (Cl is more active then I) � 4) I 2 + KCl → NR � 5) Cu + HCl → NR � 6) Ca + 2 HCl → Ca. Cl 2 + H 2