ACCESS TO SCIENCE CHEMISTRY PERIODICITY Prepare for learning









- Slides: 20

ACCESS TO SCIENCE – CHEMISTRY PERIODICITY Prepare for learning: pens, notebooks bags and stuff to side benches Periodic Table

üReflective Learner üEffective Participator üTeam worker Learning aims for the session: • Describe the variation of atomic radius, first ionisation energy and electronegativity of group 1 elements. • Describe the reactions of group 1 elements with oxygen, chlorine and water. • Carry out qualitative analysis of common cations and anions. News! Liteion racy and Numeracy Periodic Table

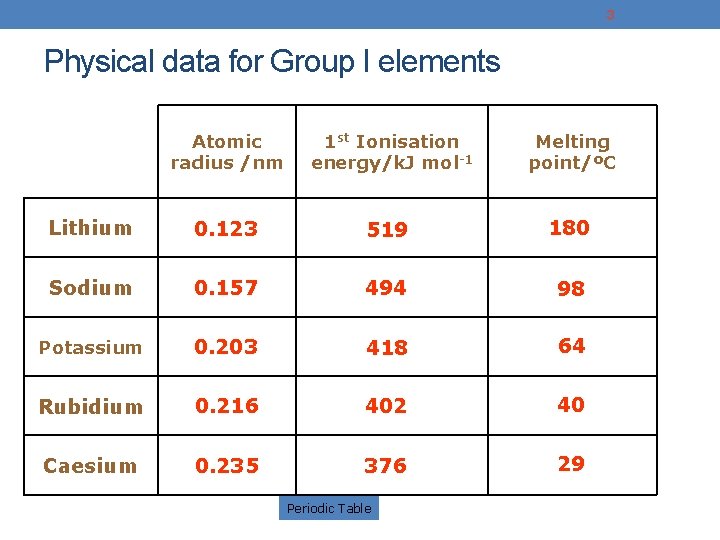
3 Physical data for Group I elements Atomic radius /nm 1 st Ionisation energy/k. J mol-1 Melting point/ºC Lithium 0. 123 519 180 Sodium 0. 157 494 98 Potassium 0. 203 418 64 Rubidium 0. 216 402 40 Caesium 0. 235 376 29 Periodic Table

4 Changes down Group 1 Atomic radius increases because of the increasing number of energy levels 1 st Ionisation energy decreases because of increasing size and shielding Melting point decreases Because of increasing size between adjacent atoms and increased nuclear shielding resulting in longer, weaker metallic bonds. Periodic Table

5 Group 1 elements They are typical metals – forming largely ionic compounds with other elements • They release electrons to form positive ions Na Na+ + e. So, will form ionic bonds with other elements such as oxygen, water and chlorine for example. • They are reducing agents i. e. They donate electrons to oxidising agents Periodic Table

6 OILRIG • They release electrons to form positive ions • Na Na+ + e • They are reducing agents • i. e. They donate electrons to oxidising agents • Oxidation Is Loss; Reduction Is Gain • When a substance is oxidised it loses electrons, so becomes more positively charged • The substance that is oxidised is known as a reducing agent because in the process of being oxidised / losing electrons it causes reduction or the giving electrons to another substance. 4 Na + O 2 2 Na 2 O • Here When substance is it’s reduced it gains electrons, becomes more sodium has lost outer electron to oxygen, so isso oxidised. negatively charged Sodium is acting as a reducing agent because it is giving its electrons to oxygen. • Oxygen The substance that isasreduced is known as process an oxidising agent can be defined being reduced in the because it has gained because in the process of being reduced / gaining electrons it is electrons. causingcan oxidation or taking electrons from another Oxygen also be described as an oxidising agent as substance. it has caused reduction / loss of electrons from sodium in the process. Periodic Table

7 Reactivity of group 1 elements Reactivity increases down the Group because… the outer electron becomes easier to remove as the atomic size increases and the nuclear shielding increases. Periodic Table

8 Reactivity of group 1 Demo • http: //www. youtube. com/watch? v=m 55 kgy. Ap. Yr. Y Activity • Individuals - Describe what you witnessed for the reactivity of the group 1 metals during the demo and video. • Pair – describe to a partner • Share - describe to the group. • Make your notes Periodic Table

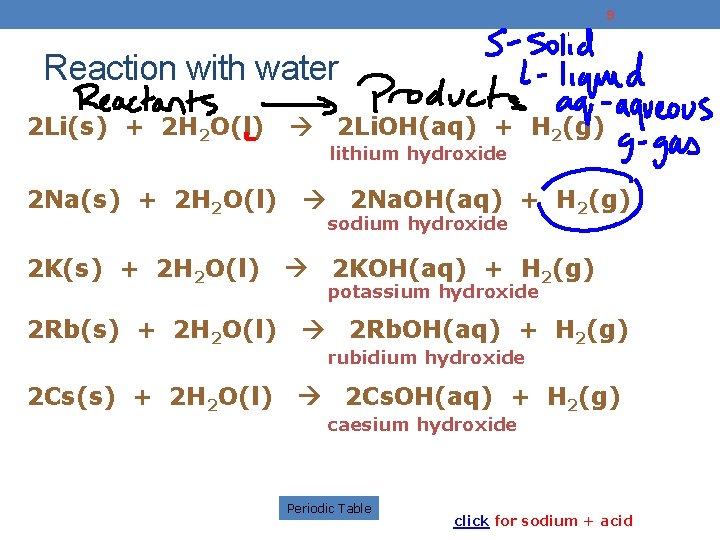
9 Reaction with water 2 Li(s) + 2 H 2 O(l) 2 Na(s) + 2 H 2 O(l) 2 K(s) + 2 H 2 O(l) 2 Li. OH(aq) + H 2(g) lithium hydroxide 2 Rb(s) + 2 H 2 O(l) 2 Cs(s) + 2 H 2 O(l) 2 Na. OH(aq) + H 2(g) sodium hydroxide 2 KOH(aq) + H 2(g) potassium hydroxide 2 Rb. OH(aq) + H 2(g) rubidium hydroxide 2 Cs. OH(aq) + H 2(g) caesium hydroxide Periodic Table click for sodium + acid

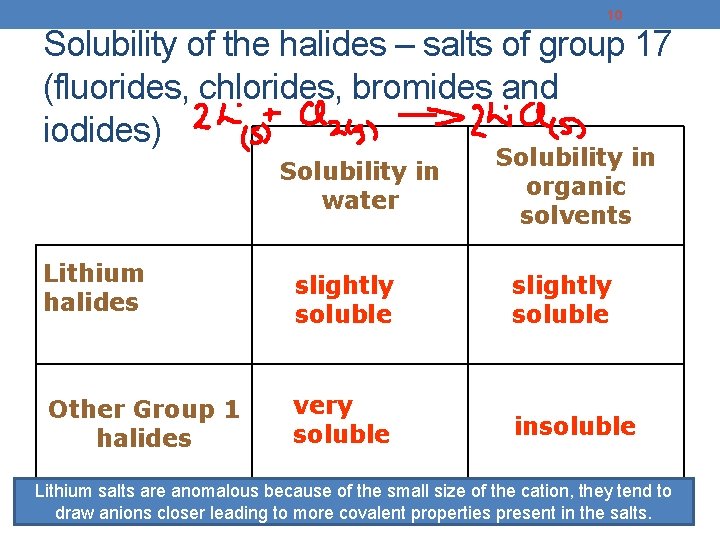
10 Solubility of the halides – salts of group 17 (fluorides, chlorides, bromides and iodides) Solubility in water Lithium halides Other Group 1 halides Solubility in organic solvents slightly soluble very soluble insoluble Lithium salts are anomalous because of the small size of the cation, they tend to draw anions closer leading to. Periodic more Table covalent properties present in the salts.

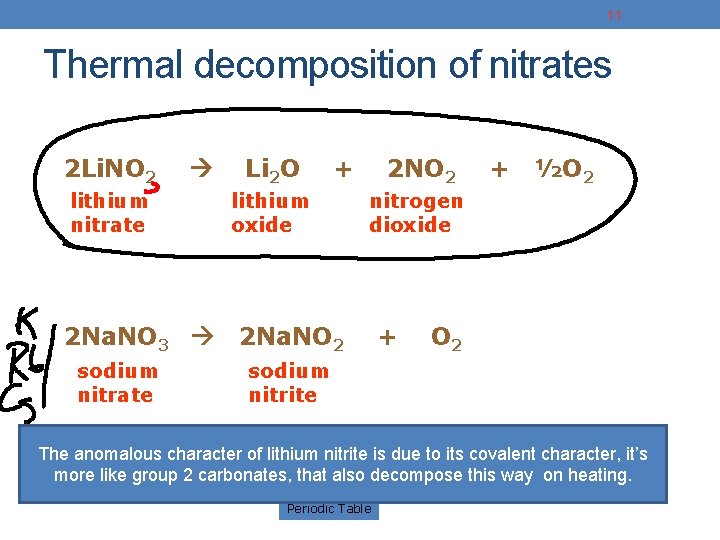
11 Thermal decomposition of nitrates 2 Li. NO 2 lithium nitrate 2 Na. NO 3 sodium nitrate Li 2 O + lithium oxide 2 NO 2 + ½O 2 nitrogen dioxide 2 Na. NO 2 + O 2 sodium nitrite The anomalous character of lithium nitrite is due to its covalent character, it’s more like group 2 carbonates, that also decompose this way on heating. Periodic Table

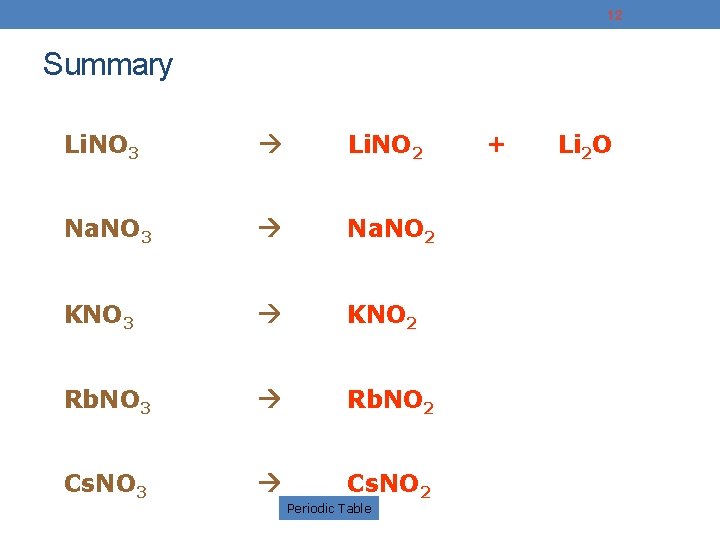
12 Summary Li. NO 3 Li. NO 2 Na. NO 3 Na. NO 2 KNO 3 KNO 2 Rb. NO 3 Rb. NO 2 Cs. NO 3 Cs. NO 2 Periodic Table + Li 2 O

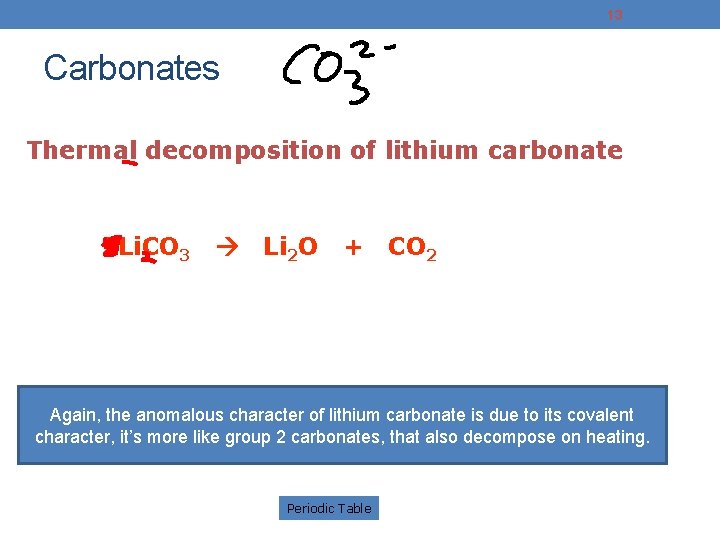
13 Carbonates Thermal decomposition of lithium carbonate 2 Li. CO 3 Li 2 O + CO 2 Again, the anomalous character of lithium carbonate is due to its covalent character, it’s more like group 2 carbonates, that also decompose on heating. Periodic Table

14 Summary • The Group 1 metals are reactive and form ionic salts. • Compounds are generally soluble in water, insoluble in organic solvents and are thermally stable. • Lithium compounds exhibit anomalous properties. Periodic Table

15 • • • • • • Siobhan Naomi Leyton Kevin Sean Andrew Nicola Laura Hannah Rebekha Jacob Anthony Maria Joe Gemma Sarah Glen Amanda Abigail Hasib Eamon Stephen Inna Periodic Table

16 Qualitative analysis of salts • What’s in a salt? • An anion and a cation e. g. Na+ Cl • We have looked at a way of finding out what metal cation is present – flame tests • However, this doesn’t work for all cations – some do not give coloured flames, or give similar colours so it is difficult to distinguish between them. • We are now going to look at some other ways of telling what is in a substance. Periodic Table

17 Tests can be grouped • For cations: often we can distinguish between metal cations by adding a alkali such as sodium hydroxide or ammonium hydroxide (ammonia in solution). • For anions: we can test for carbonates, sulphates, nitrates and halides by using simple tests too. demo Periodic Table

18 Read first! Pairs! • There are lots of tests to analyse compound to identify what is present, here a few of them. . • Work slowly through the analysis and record what you find… • You will do this again in a few weeks with some unknowns as part of your assignment, so make clear notes of results and you might want to take photographs too. Periodic Table

19 Results Periodic Table

Periodic Table click Periodic to return Table 20