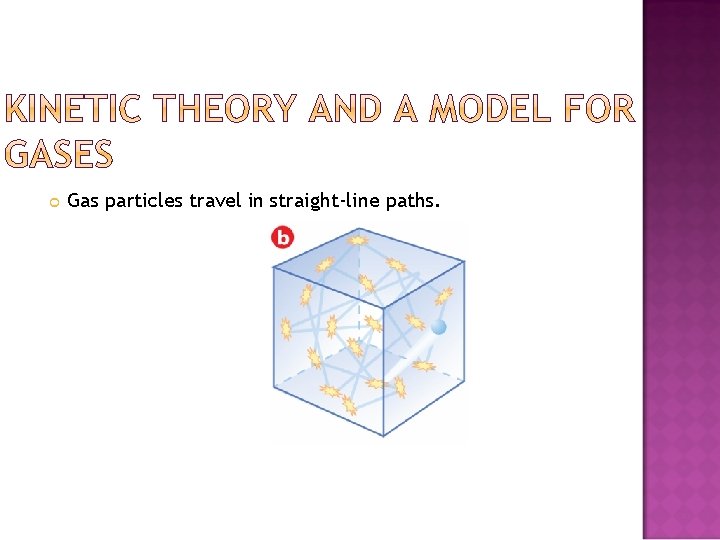
Gas particles travel in straightline paths Gas pressure























- Slides: 35


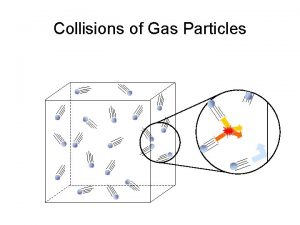
Gas particles travel in straight-line paths.

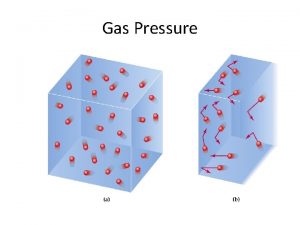
Gas pressure results from the force exerted by a gas per unit surface area of an object. An empty space with no particles and no pressure is called a vacuum. Atmospheric pressure results from the collisions of atoms and molecules in air with objects.

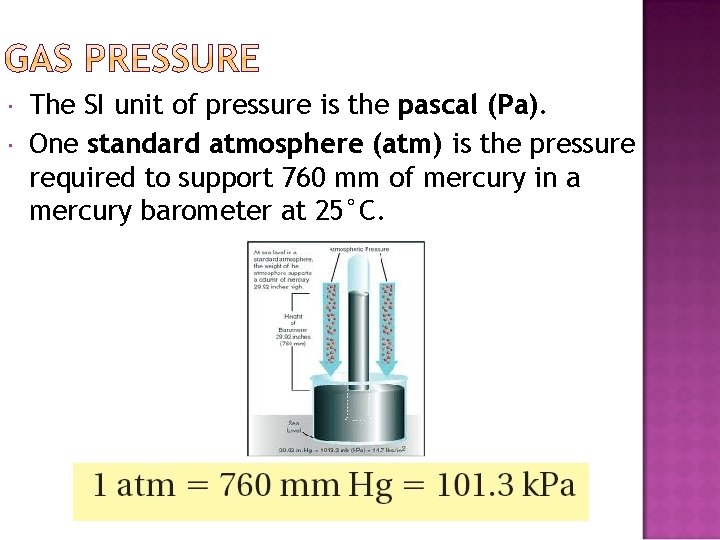
The SI unit of pressure is the pascal (Pa). One standard atmosphere (atm) is the pressure required to support 760 mm of mercury in a mercury barometer at 25°C.

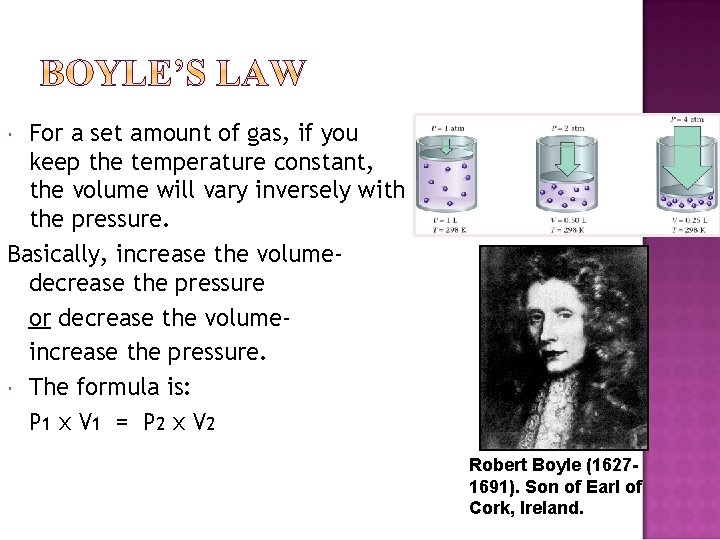
For a set amount of gas, if you keep the temperature constant, the volume will vary inversely with the pressure. Basically, increase the volumedecrease the pressure or decrease the volumeincrease the pressure. The formula is: P 1 x V 1 = P 2 x V 2 Robert Boyle (16271691). Son of Earl of Cork, Ireland.

Knowns V 1=30 L He P 1=103 KPA P 2=25 KPA Unknowns V 2=? * Hint- the originals are always paired together V 2= (P 1) (V 1) P 2

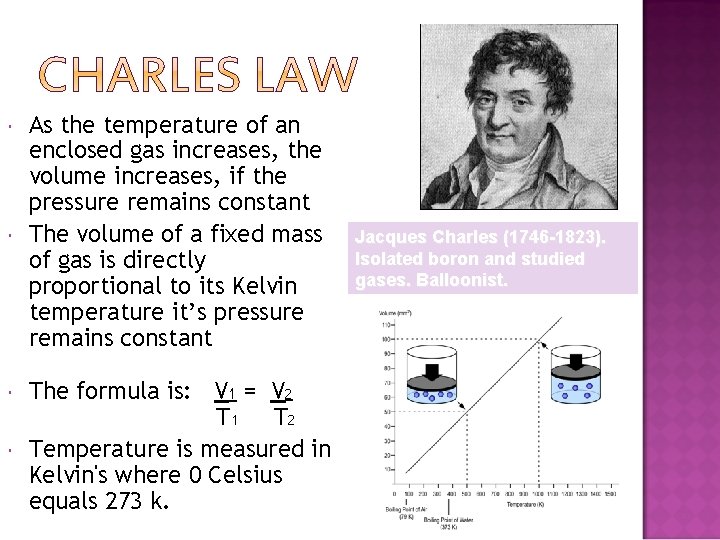
As the temperature of an enclosed gas increases, the volume increases, if the pressure remains constant The volume of a fixed mass of gas is directly proportional to its Kelvin temperature it’s pressure remains constant The formula is: V 1 = V 2 T 1 T 2 Temperature is measured in Kelvin's where 0 Celsius equals 273 k. Jacques Charles (1746 -1823). Isolated boron and studied gases. Balloonist.

Knowns V 1= 4. 00 L T 1= 24 C T 2= 58 C 1 st: need to convert temperature to kelvin! C + 273 = K V 2= (V 1) (T 2) T 1 Unknowns V 2= ?

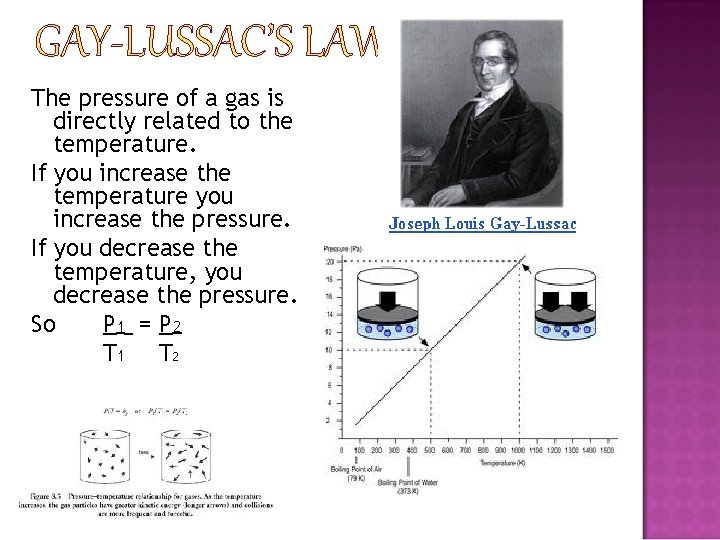
GAY-LUSSAC’S LAW The pressure of a gas is directly related to the temperature. If you increase the temperature you increase the pressure. If you decrease the temperature, you decrease the pressure. So P 1 = P 2 T 1 T 2

Knowns P 1= 103 KPA T 1= 25 C T 2= 928 C 1 st- convert temperatures to kelvin! C + 273 = K P 2= (P 1) (T 2) T 1 Unknowns P 2= ?

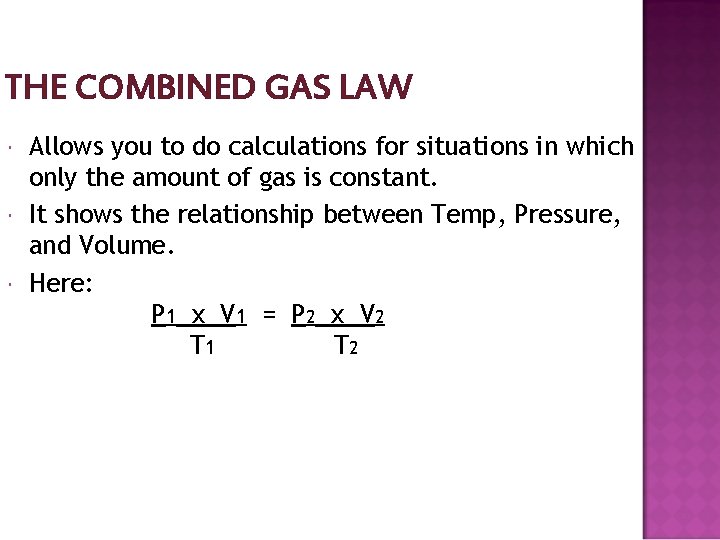
THE COMBINED GAS LAW Allows you to do calculations for situations in which only the amount of gas is constant. It shows the relationship between Temp, Pressure, and Volume. Here: P 1 x V 1 = P 2 x V 2 T 1 T 2

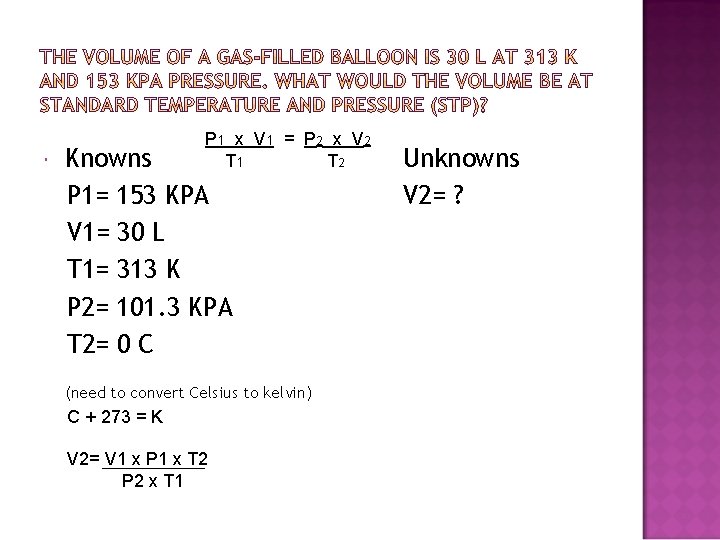
P 1 x V 1 = P 2 x V 2 T 1 T 2 Knowns P 1= 153 KPA V 1= 30 L T 1= 313 K P 2= 101. 3 KPA T 2= 0 C (need to convert Celsius to kelvin) C + 273 = K V 2= V 1 x P 1 x T 2 P 2 x T 1 Unknowns V 2= ?

IDEAL GAS LAW Ideal vs. Real No volume some volume No Inter-molecular forces some Inter-molecular forces Up to this point the amount of gas has been held constant. The Ideal Gas Law allows us to consider varying the amount of gas while also messing with the temp, pressure, and volume.

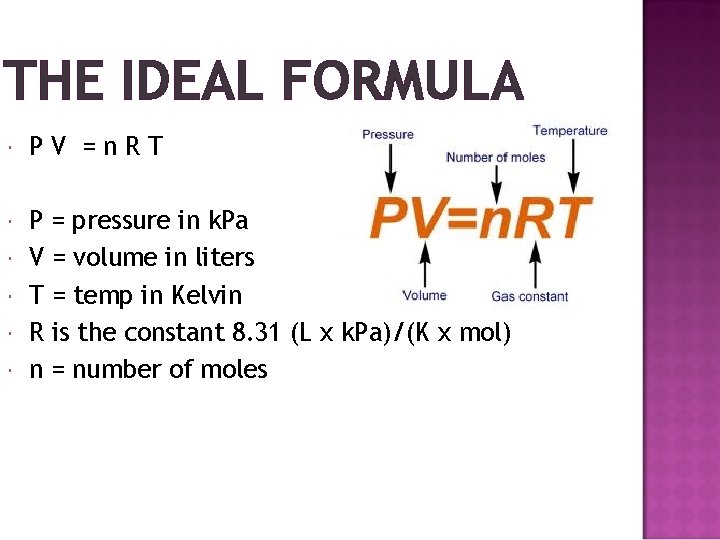
THE IDEAL FORMULA PV =n. RT P = pressure in k. Pa V = volume in liters T = temp in Kelvin R is the constant 8. 31 (L x k. Pa)/(K x mol) n = number of moles

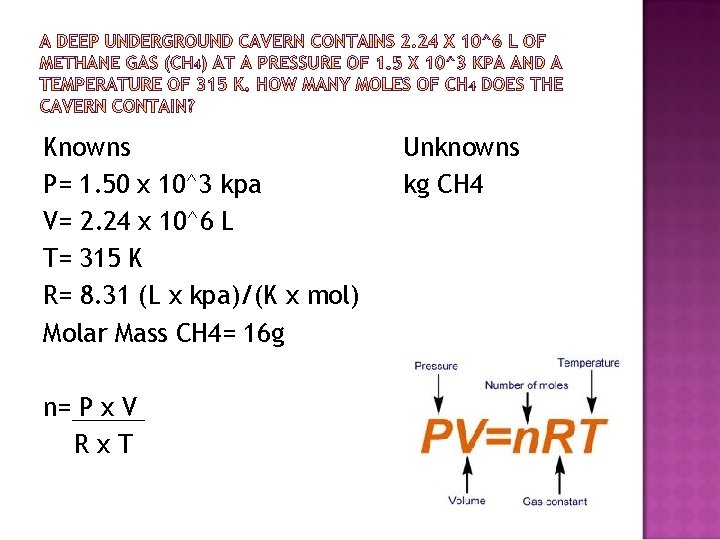
Knowns P= 1. 50 x 10^3 kpa V= 2. 24 x 10^6 L T= 315 K R= 8. 31 (L x kpa)/(K x mol) Molar Mass CH 4= 16 g n= P x V Rx. T Unknowns kg CH 4

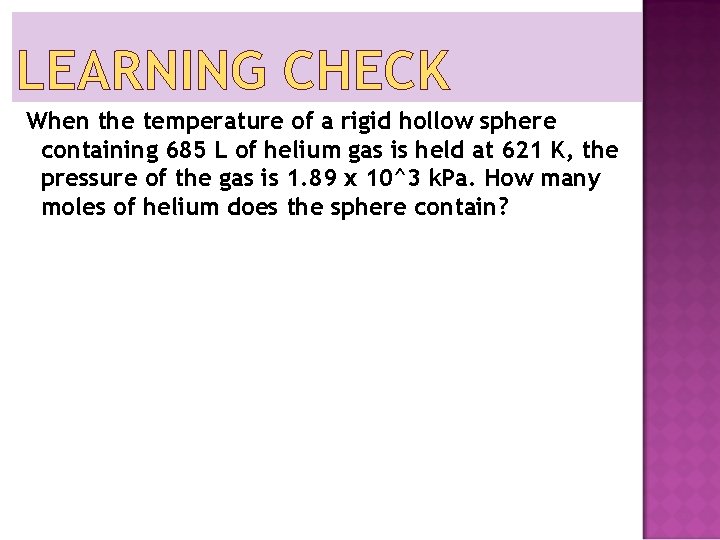
LEARNING CHECK When the temperature of a rigid hollow sphere containing 685 L of helium gas is held at 621 K, the pressure of the gas is 1. 89 x 10^3 k. Pa. How many moles of helium does the sphere contain?

ONE MORE PRACTICE PROBLEM A newborn lamb’s lungs can hold 2. 20 L. How many grams of air do her lungs hold at a pressure of 102 k. Pa and a body temperature of 37 degrees C? Use a molar mass of 29 g for air, which is about 20% O 2 (32 g/mol) and 80% N 2 (28 g/mol)

A. are perfectly elastic B. are inelastic C. never occur D. cause a loss of total kinetic energy

A. thermometer B. Barometer C. Vacuum D. manometer

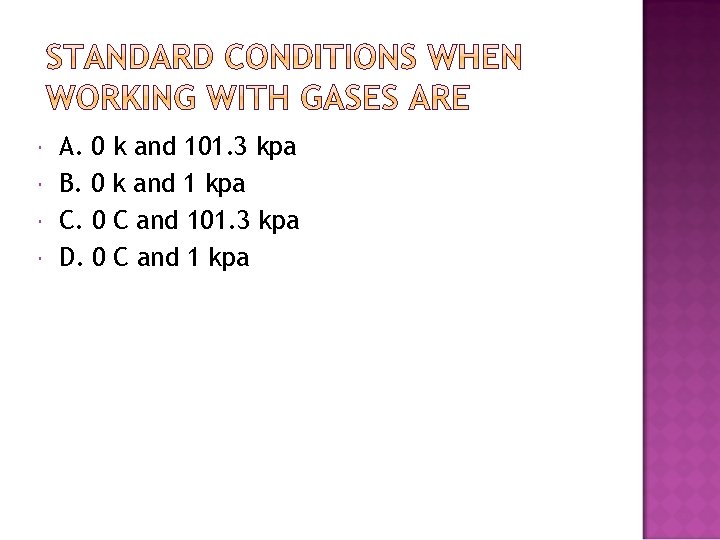
A. 0 k and 101. 3 kpa B. 0 k and 1 kpa C. 0 C and 101. 3 kpa D. 0 C and 1 kpa

A. The atmospheric pressure below sea level is higher B. the atmospheric pressure below sea level is lower C. the pressures are the same D. differences in pressure cannot be determined

A. the walls of a container B. the vacuum maintained in the container C. the simultaneous collisions of fast-moving particles in the container Atmospheric pressure acting on the outside walls of the container

A. -273 K B. 0 k C. 0 degrees celcius D. 273 degrees celcius

A. it increases B. it decreases C. it does not change D. the change cannot be determined

A. it is greater than the rate of condensation B. it is less than the rate of condensation C. it is equal to the rate of condensation D. the rate of evaporation cannot be determined

A. its volume increases more under pressure than an equal volume of liquid does B. its volume increases more under pressure than an equal volume of solid does C. the space between gas particles is much less than the space between liquid or solid particles D. the volume of a gas’s particles is small compared to the overall volume of a gas

A. from a region of high pressure to a region of low pressure B. from a region of high pressure to a region of equally high pressure C. from a region of low pressure to a region of higher pressure D. from a region of low pressure to a region of equally lower pressure

A. It increases by a factor of 4 B. it decreases by a factor of 8 C. it increase by a factor of 8 D. It decreases by a factor of 2

A. it increase B. it stays the same C. it decreases D. The change cannot be predicted

A. vary inversely B. decrease C. not change D. increase

A. 497 L B. 2. 5 L C. 14 L D. . 40 L

A. 10. 6 ml B. 27 ml C. 36 ml D. 8. 0 ml

A. the pressure of the has is inversely proportional to its temperature in kelvins B. the volume of the gas is inversely proportional to its temperature in kelvins C. the volume of the gas is directly proportional to its temperature in kelvins D. the pressure of the gas is directly proportional to its temperature in kelvins

A. pressure and volume only B. temperature and pressure only C. volume and temperature only D. temperature, pressure, and volume

 Postulate 2 states that gas particles are:
Postulate 2 states that gas particles are: Gas like mixture of charged particles
Gas like mixture of charged particles Solid liquid gas particles
Solid liquid gas particles Site:slidetodoc.com
Site:slidetodoc.com Gas particles are separated by relatively large distances
Gas particles are separated by relatively large distances Properties of solids and liquids
Properties of solids and liquids Chapter 21: temperature, heat, and expansion answer key
Chapter 21: temperature, heat, and expansion answer key Dynamothermal
Dynamothermal Where is intrapleural pressure
Where is intrapleural pressure Bevel of et tube
Bevel of et tube Oncotic pressure
Oncotic pressure Intrapleural pressure
Intrapleural pressure Blood pressure regulation
Blood pressure regulation Pressure support vs pressure control
Pressure support vs pressure control Confining pressure vs directed pressure
Confining pressure vs directed pressure Pressure tolerant vs pressure sensitive
Pressure tolerant vs pressure sensitive High pressure area
High pressure area Oncotic vs osmotic pressure
Oncotic vs osmotic pressure Starling forces equation
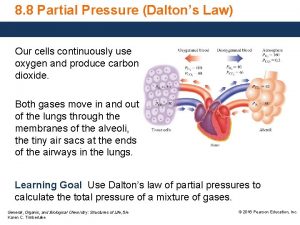
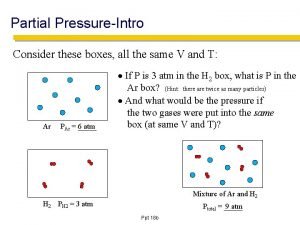
Starling forces equation How to find partial pressure from total pressure
How to find partial pressure from total pressure Bernoulli equation pipe flow
Bernoulli equation pipe flow Pressure support vs pressure control
Pressure support vs pressure control Hydrostatic oncotic pressure
Hydrostatic oncotic pressure Partial pressure
Partial pressure Pressure support ventilation
Pressure support ventilation Factors affecting gfr
Factors affecting gfr Pressure mapping for pressure ulcers
Pressure mapping for pressure ulcers Eightfold paths of buddhism
Eightfold paths of buddhism Load paths
Load paths Shortest path problem linear programming
Shortest path problem linear programming Dd-paths in software testing
Dd-paths in software testing Paths, trees, and flowers
Paths, trees, and flowers Euler path and circuit
Euler path and circuit Paths start and stop at
Paths start and stop at Cyclomatic complexity example
Cyclomatic complexity example Yes there are two paths
Yes there are two paths