Gas Pressure Air Pressure Pressure Units Units of
















- Slides: 55

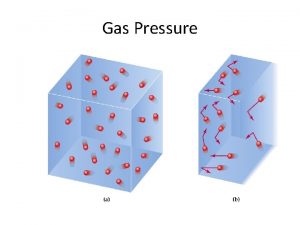
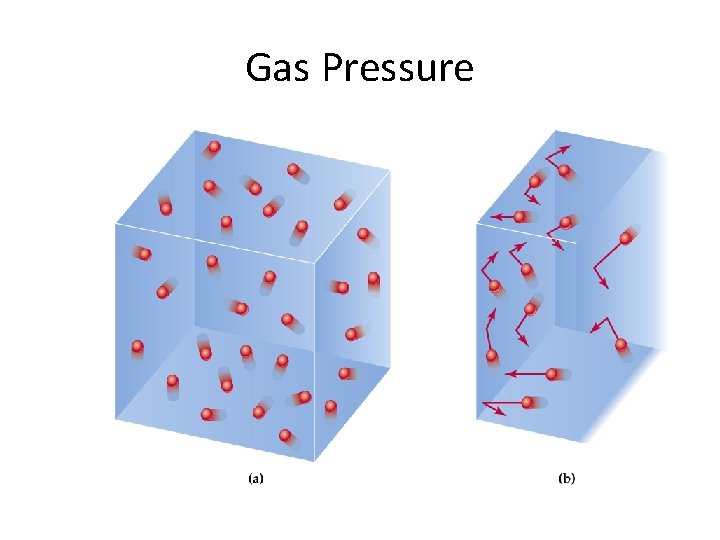
Gas Pressure

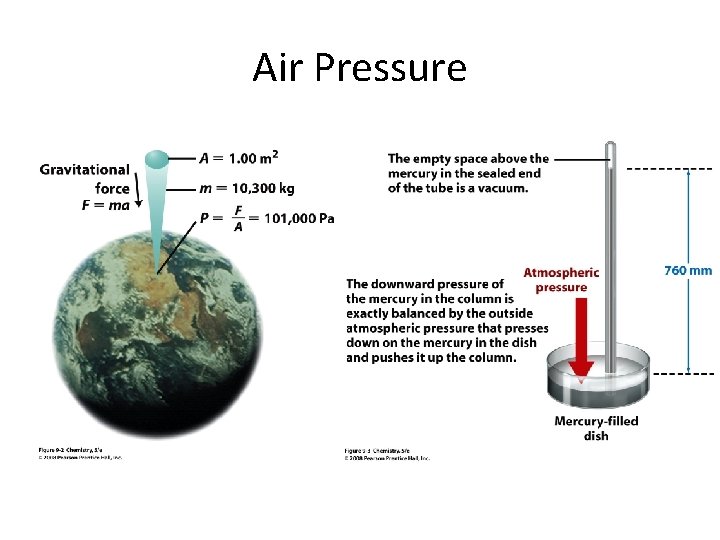
Air Pressure

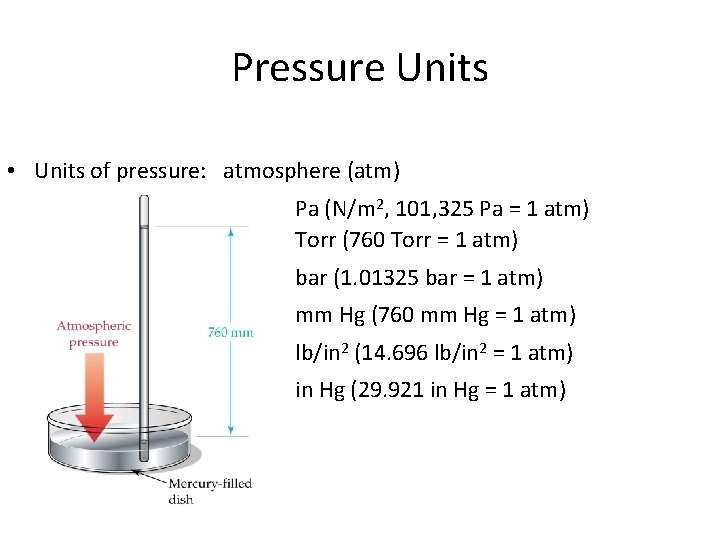
Pressure Units • Units of pressure: atmosphere (atm) Pa (N/m 2, 101, 325 Pa = 1 atm) Torr (760 Torr = 1 atm) bar (1. 01325 bar = 1 atm) mm Hg (760 mm Hg = 1 atm) lb/in 2 (14. 696 lb/in 2 = 1 atm) in Hg (29. 921 in Hg = 1 atm)

Universal Gas Behavior • Unlike solids and liquids, gas behavior is generally independent of chemical identity. • Depends on four things only: – Absolute temperature – Pressure – Volume – Amount (moles)

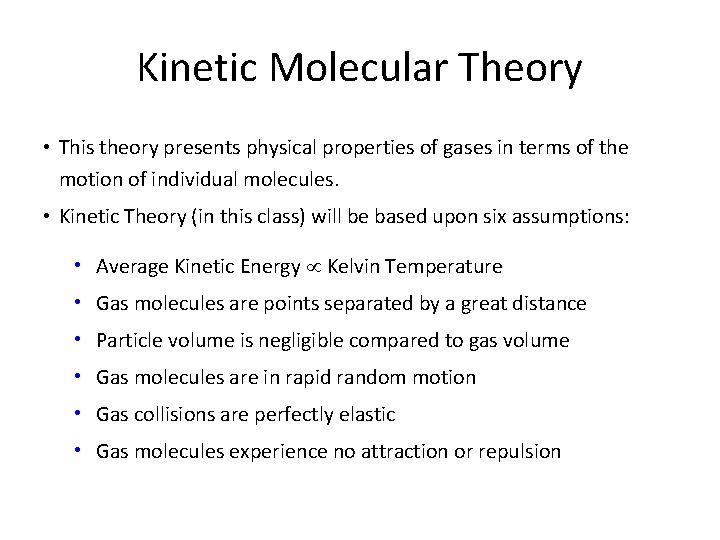
Kinetic Molecular Theory • This theory presents physical properties of gases in terms of the motion of individual molecules. • Kinetic Theory (in this class) will be based upon six assumptions: • Average Kinetic Energy Kelvin Temperature • Gas molecules are points separated by a great distance • Particle volume is negligible compared to gas volume • Gas molecules are in rapid random motion • Gas collisions are perfectly elastic • Gas molecules experience no attraction or repulsion

Gas Behavior: Gases in a Box • Insert 1 mole of gas into a fixed volume container. Then: 1. Gas expands to fill the container. Why? 2. The pressure becomes whatever value the gas laws dictate for that volume, mole, and temperature combination.

Gas Behavior: Gases in a Piston • Insert 1 mole of gas into a piston. Then: 1. Gas fills the piston. Why? 2. The piston changes volume until the pressure inside is equal to the pressure outside. Why?

Understanding the Gas Laws • Two keys to understanding the gas laws: – Understand which parameters are changing – Understand which are NOT changing

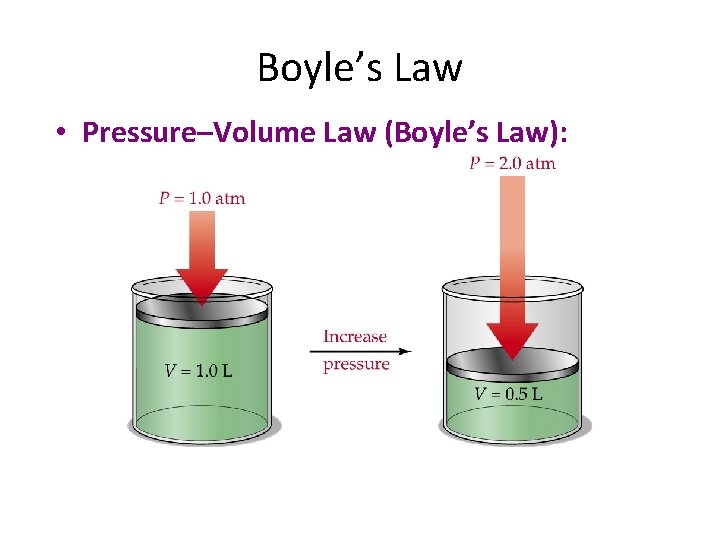
Boyle’s Law • Pressure–Volume Law (Boyle’s Law):

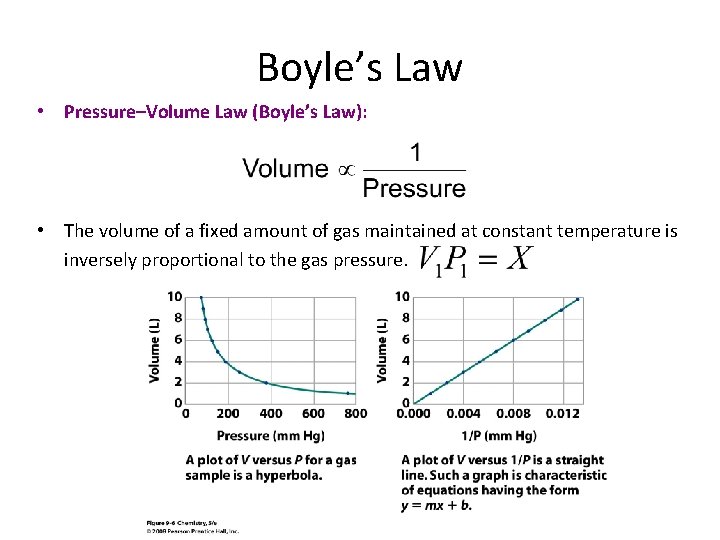
Boyle’s Law • Pressure–Volume Law (Boyle’s Law): • The volume of a fixed amount of gas maintained at constant temperature is inversely proportional to the gas pressure.

Charles’ Law • Temperature–Volume Law (Charles’ Law):

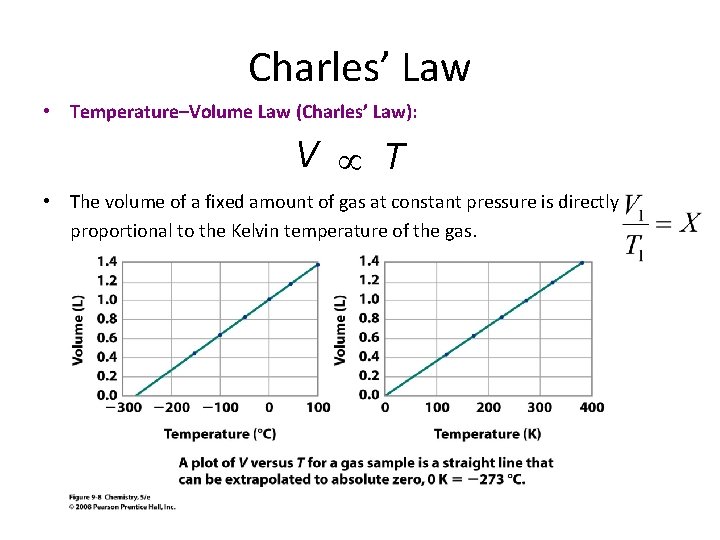
Charles’ Law • Temperature–Volume Law (Charles’ Law): V T • The volume of a fixed amount of gas at constant pressure is directly proportional to the Kelvin temperature of the gas.

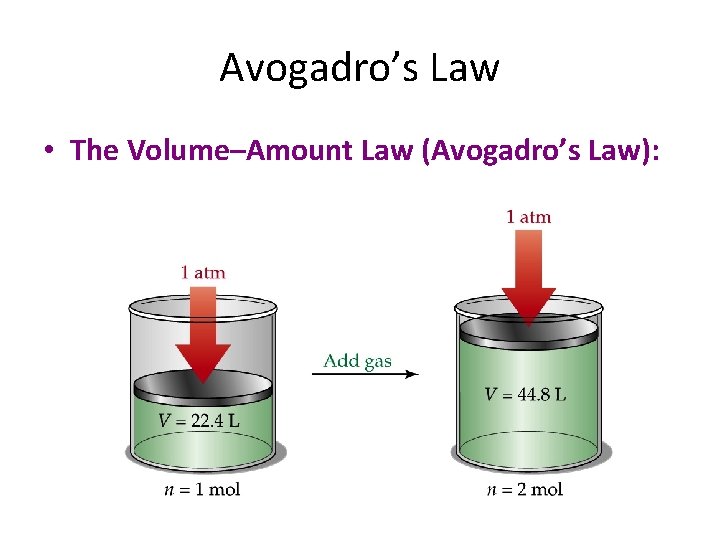
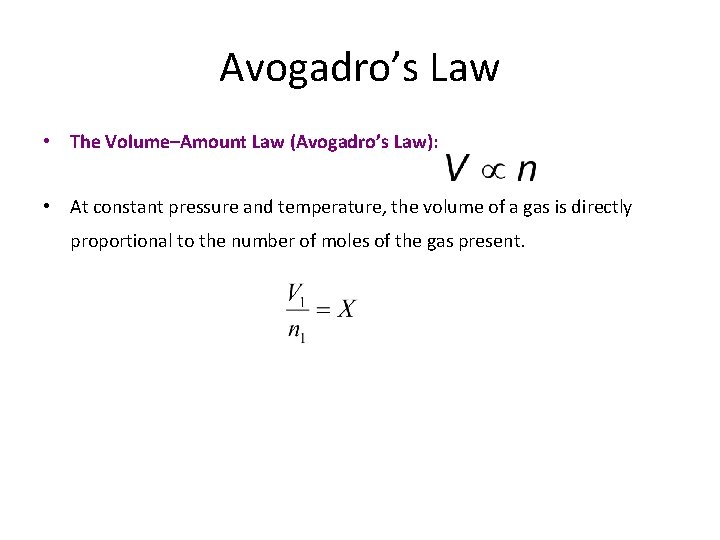
Avogadro’s Law • The Volume–Amount Law (Avogadro’s Law):

Avogadro’s Law • The Volume–Amount Law (Avogadro’s Law): • At constant pressure and temperature, the volume of a gas is directly proportional to the number of moles of the gas present.

Collecting the Gas Laws • Mathematically one can combine all of the statements we’ve made about gases. • Two equivalent equations come from this: – Combined gas law – Ideal gas law

Combined Gas Law • Combining the law gives: • But if it equals a constant, then after any change it will still be equal to the constant: • We write it this way: • Nothing needs to be held constant now • Remember that anything that does stay constant can be cancelled.

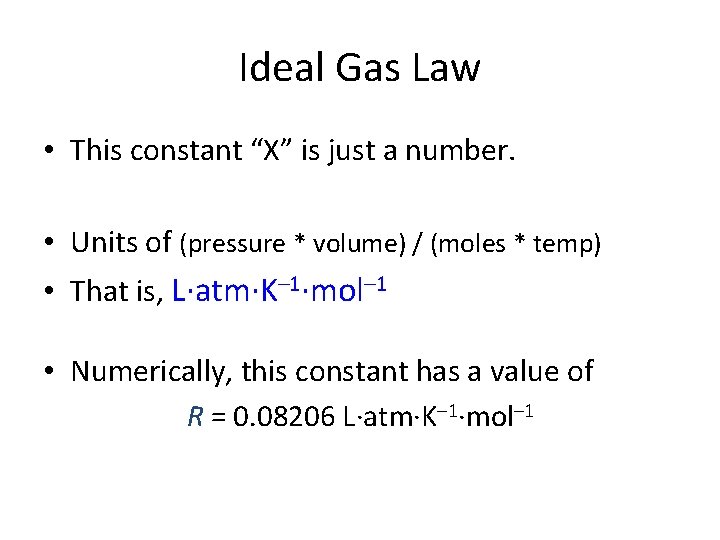
Ideal Gas Law • This constant “X” is just a number. • Units of (pressure * volume) / (moles * temp) • That is, L·atm·K– 1·mol– 1 • Numerically, this constant has a value of R = 0. 08206 L·atm·K– 1·mol– 1

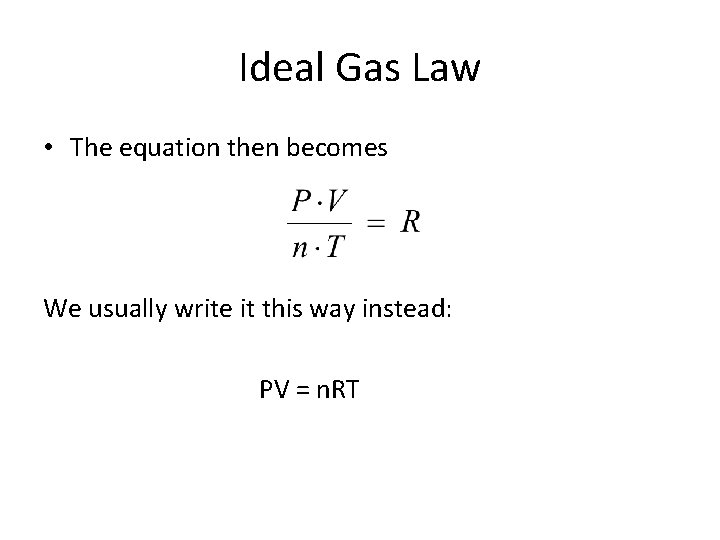
Ideal Gas Law • The equation then becomes We usually write it this way instead: PV = n. RT

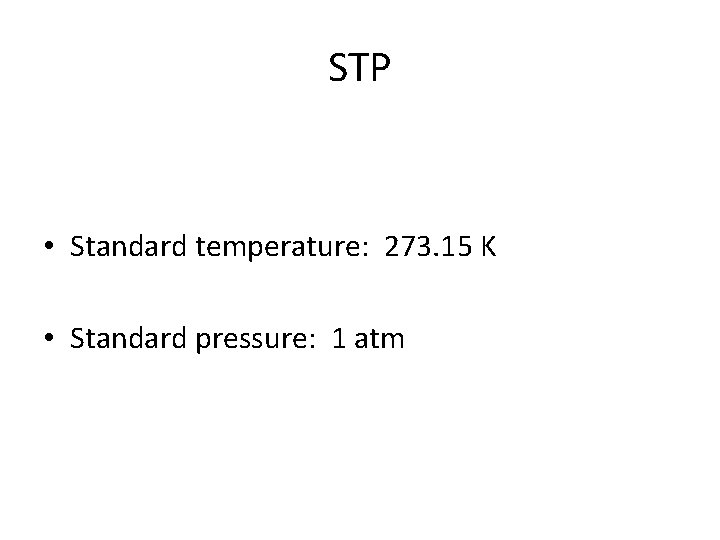
STP • Standard temperature: 273. 15 K • Standard pressure: 1 atm

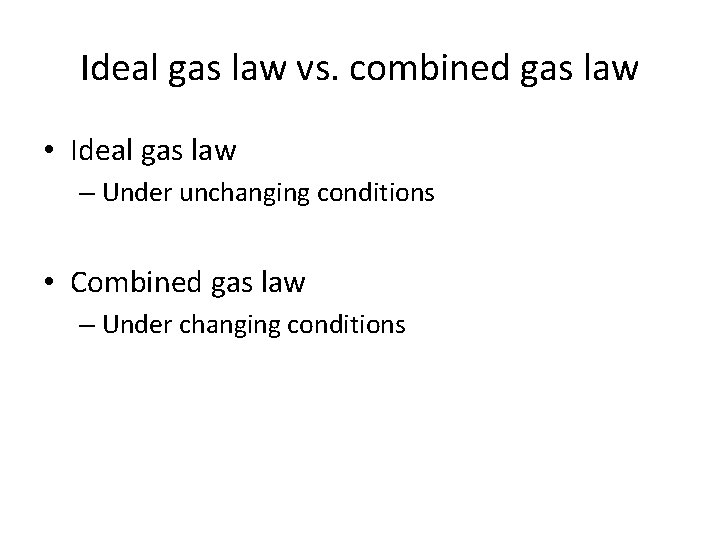
Ideal gas law vs. combined gas law • Ideal gas law – Under unchanging conditions • Combined gas law – Under changing conditions

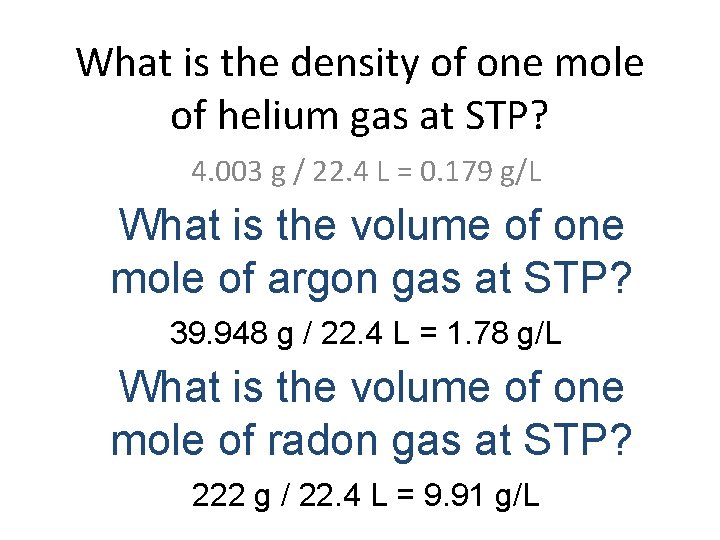
What is the volume of one mole of helium gas at STP? 22. 4 L What is the volume of one mole of argon gas at STP? 22. 4 L What is the volume of one mole of radon gas at STP? 22. 4 L

What is the density of one mole of helium gas at STP? 4. 003 g / 22. 4 L = 0. 179 g/L What is the volume of one mole of argon gas at STP? 39. 948 g / 22. 4 L = 1. 78 g/L What is the volume of one mole of radon gas at STP? 222 g / 22. 4 L = 9. 91 g/L

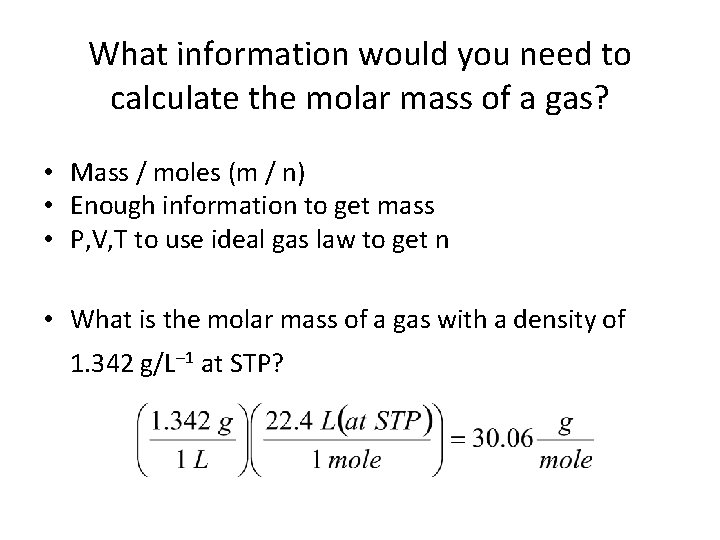
What information would you need to calculate the molar mass of a gas? • Mass / moles (m / n) • Enough information to get mass • P, V, T to use ideal gas law to get n • What is the molar mass of a gas with a density of 1. 342 g/L– 1 at STP?

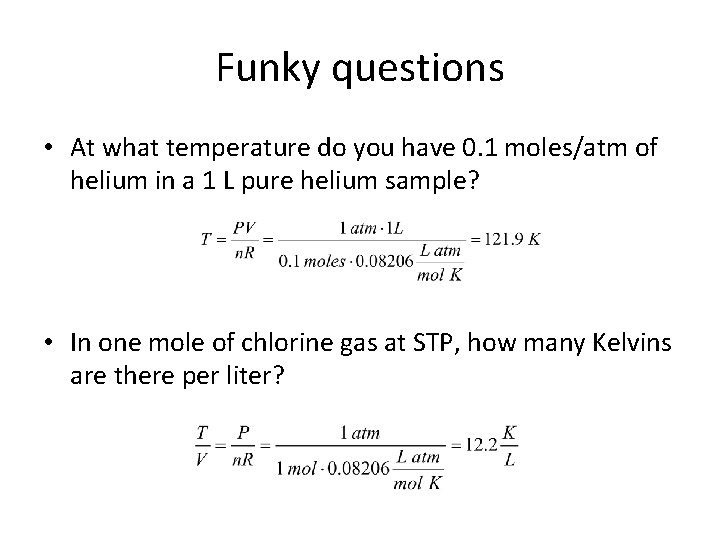
Funky questions • At what temperature do you have 0. 1 moles/atm of helium in a 1 L pure helium sample? • In one mole of chlorine gas at STP, how many Kelvins are there per liter?

Gas-phase stoichiometry • We have a new route to moles PV=n. RT • But we need to know first how two different gases behave when in the same space

Gas Mixtures • Two gases in the same container have the same volume—whatever the volume of the container is. • Two gases in the same container have the same temperature—whatever the temperature is inside the container.

Gas Mixtures • Two gases in the same container do NOT have the same pressure. • They have whatever pressure they would have if they were in the container alone. • That is, solve PV=n. RT for each gas in the mixture separately.

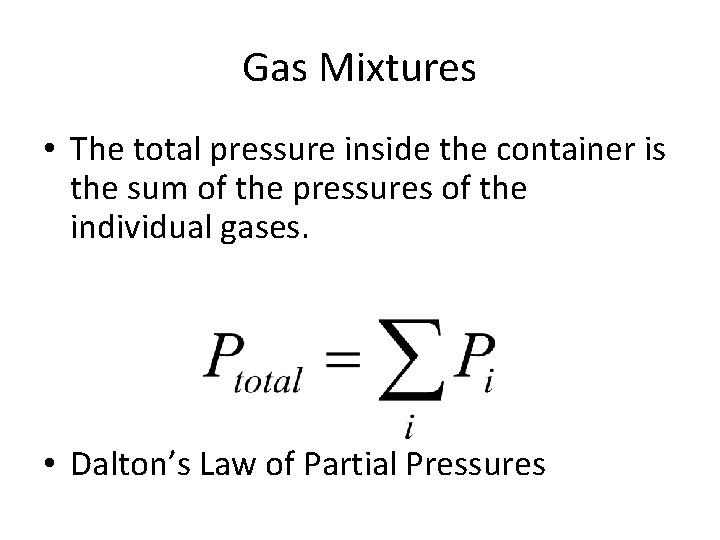
Gas Mixtures • The total pressure inside the container is the sum of the pressures of the individual gases. • Dalton’s Law of Partial Pressures

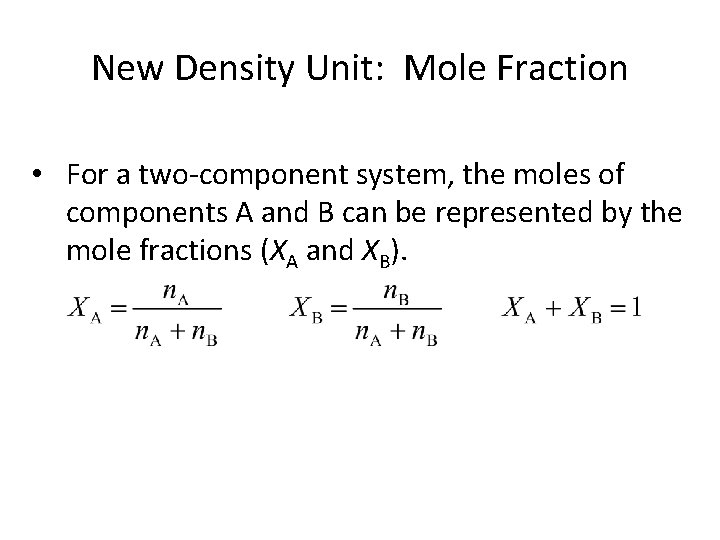
New Density Unit: Mole Fraction • For a two-component system, the moles of components A and B can be represented by the mole fractions (XA and XB).

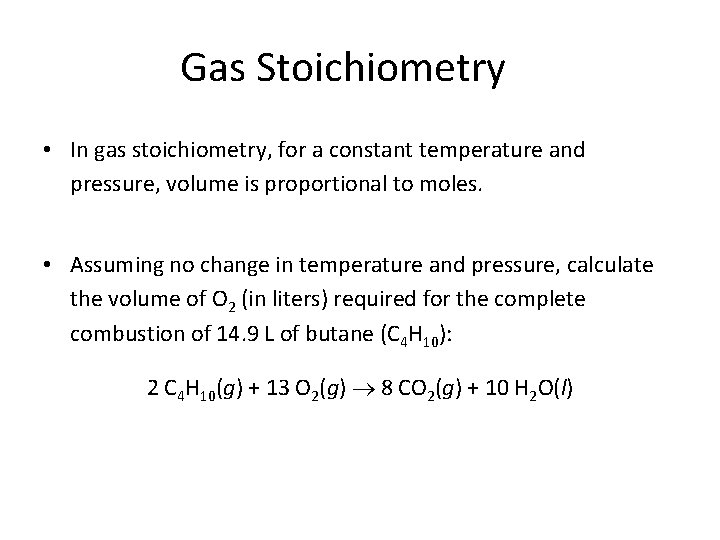
Gas Stoichiometry • In gas stoichiometry, for a constant temperature and pressure, volume is proportional to moles. • Assuming no change in temperature and pressure, calculate the volume of O 2 (in liters) required for the complete combustion of 14. 9 L of butane (C 4 H 10): 2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g) + 10 H 2 O(l)

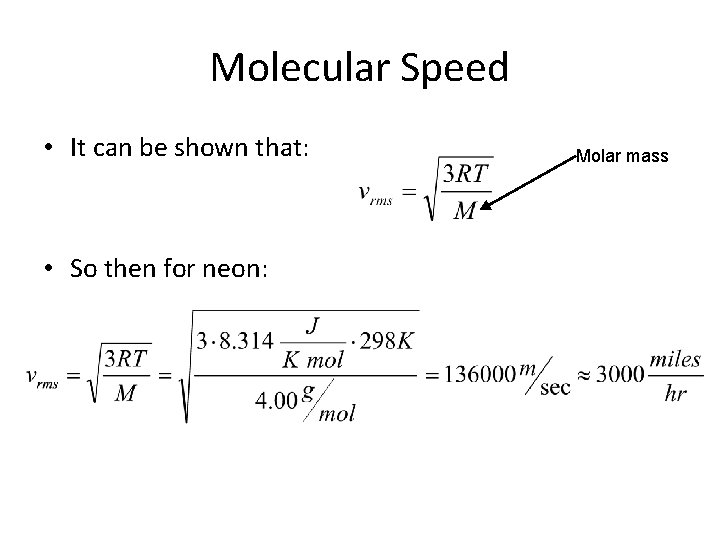
Molecular Speed • It can be shown that: • So then for neon: Molar mass

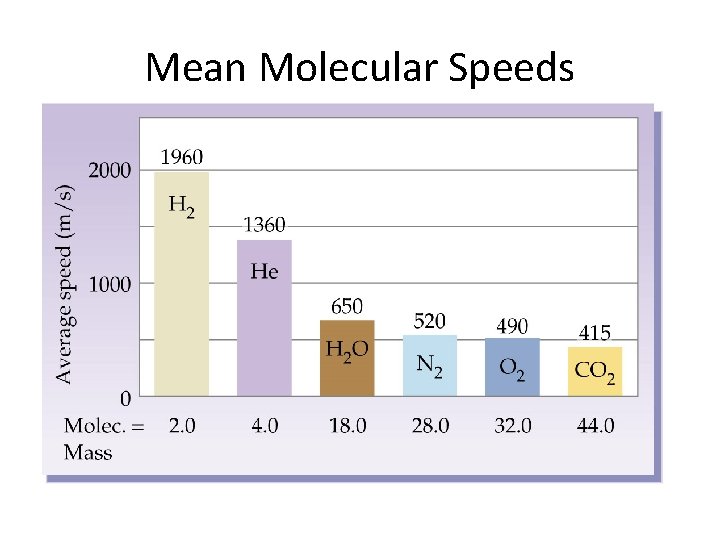
Mean Molecular Speeds

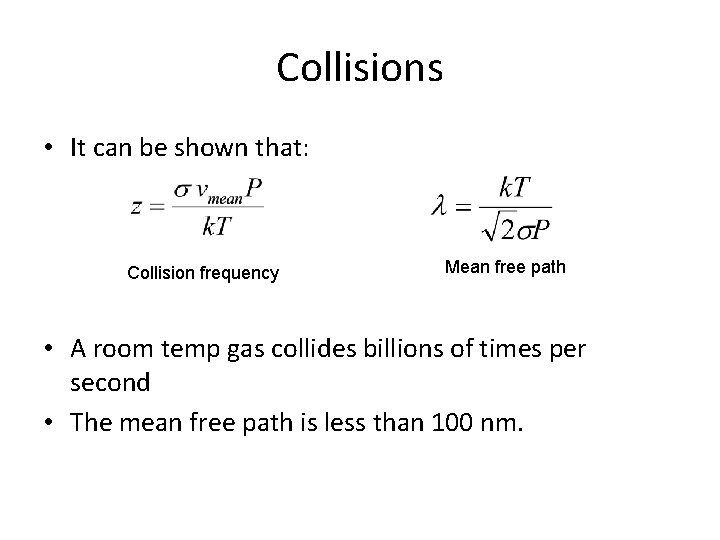
Collisions • It can be shown that: Collision frequency Mean free path • A room temp gas collides billions of times per second • The mean free path is less than 100 nm.

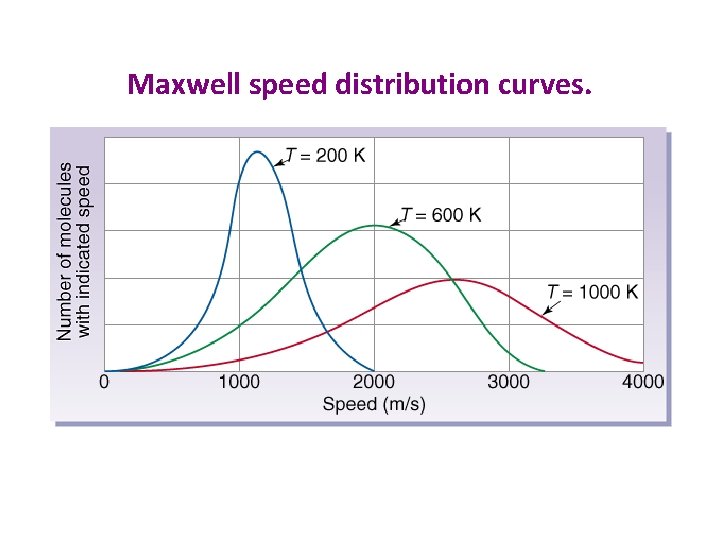
Maxwell speed distribution curves.

Same Behavior vs. Different Behavior • Most gas behaviors are based upon comparisons of their relative energies (temperatures) – Same temperature = same behavior • Some gas behaviors are based upon comparisons of their relative speeds – Same speed = same behavior

Graham’s Law • Diffusion is the mixing of different gases by random molecular motion and collision.

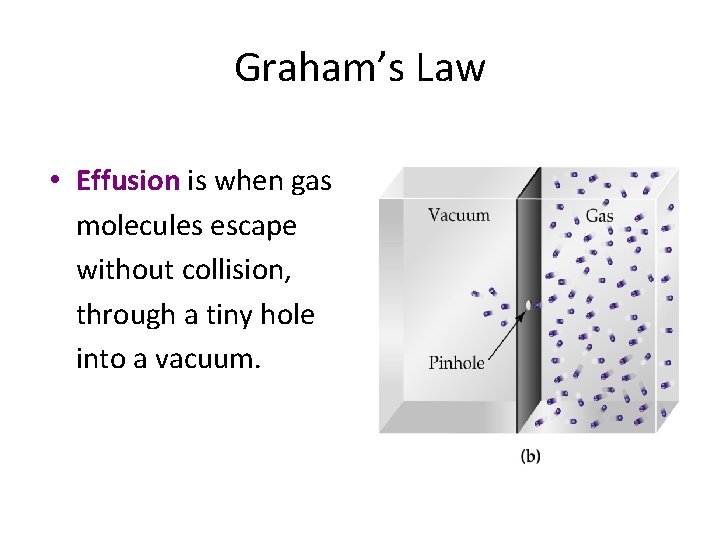
Graham’s Law • Effusion is when gas molecules escape without collision, through a tiny hole into a vacuum.

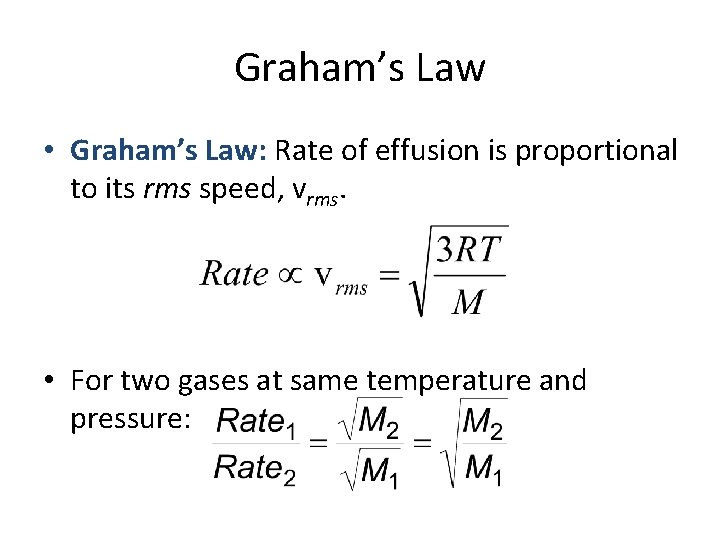
Graham’s Law • Graham’s Law: Rate of effusion is proportional to its rms speed, vrms. • For two gases at same temperature and pressure:

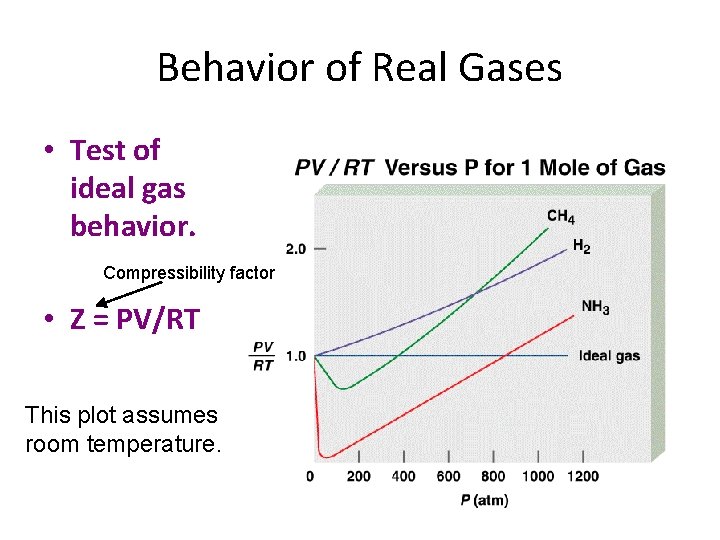
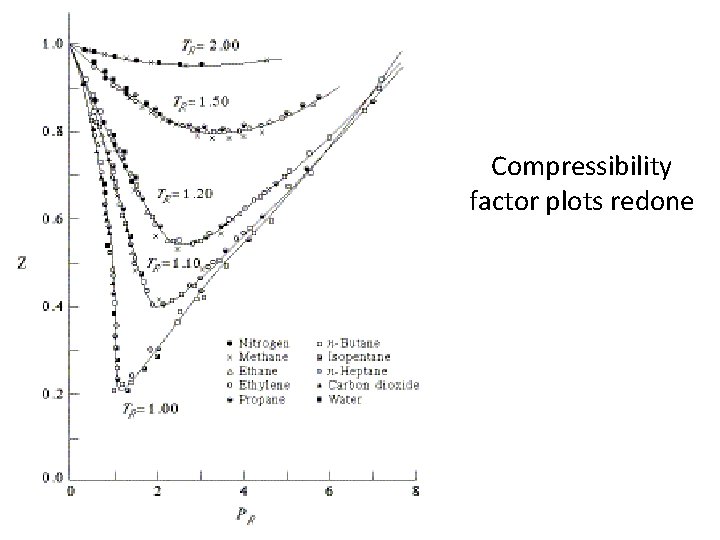
Behavior of Real Gases • Test of ideal gas behavior. Compressibility factor • Z = PV/RT This plot assumes room temperature.

Real Gases • All the assumptions of kinetic molecular theory break down when explored in sufficient detail. • Two assumptions break down first: – The volume of gas molecules is negligible – There are no attractive or repulsive forces between molecules

Non-negligible volumes • The volume of molecules affects pressurevolume behavior more than temperaturepressure behavior. • For a given small volume, the pressure will be higher than the ideal gas suggests. .

Behavior of Real Gases • Test of ideal gas behavior. Volume non-idealities seen here!

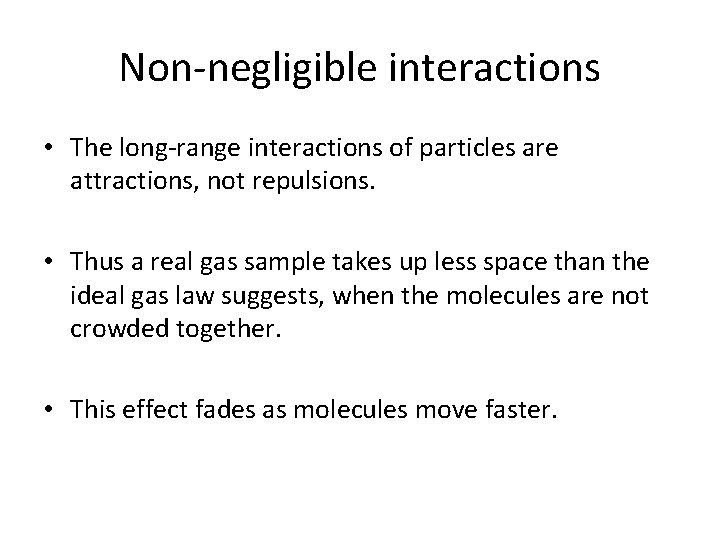
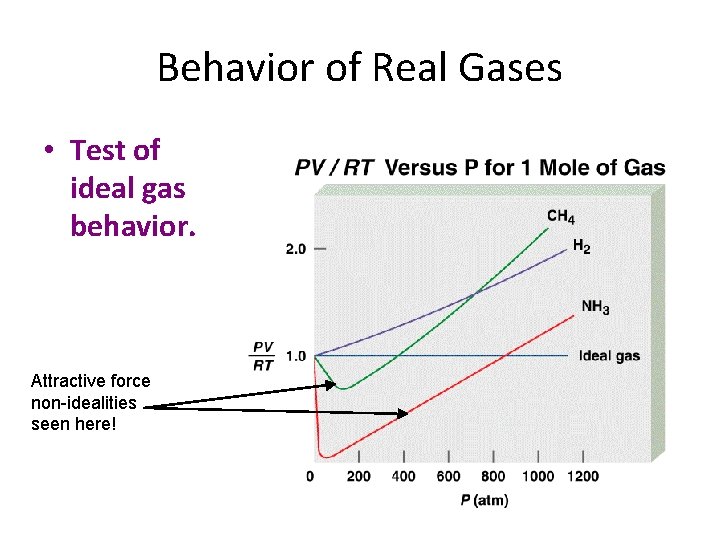
Non-negligible interactions • The long-range interactions of particles are attractions, not repulsions. • Thus a real gas sample takes up less space than the ideal gas law suggests, when the molecules are not crowded together. • This effect fades as molecules move faster.

Behavior of Real Gases • Test of ideal gas behavior. Attractive force non-idealities seen here!

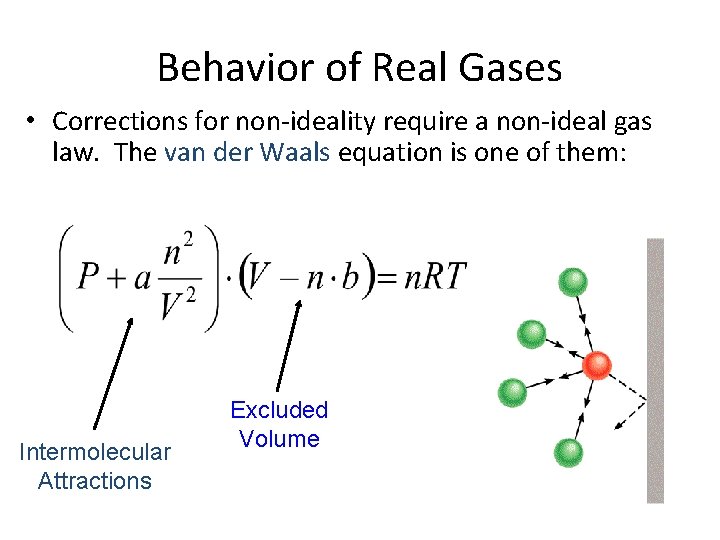
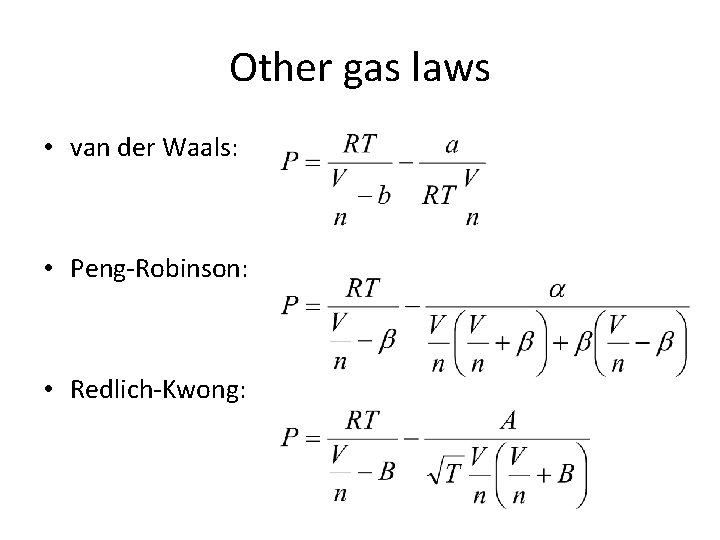
Behavior of Real Gases • Corrections for non-ideality require a non-ideal gas law. The van der Waals equation is one of them: Intermolecular Attractions Excluded Volume

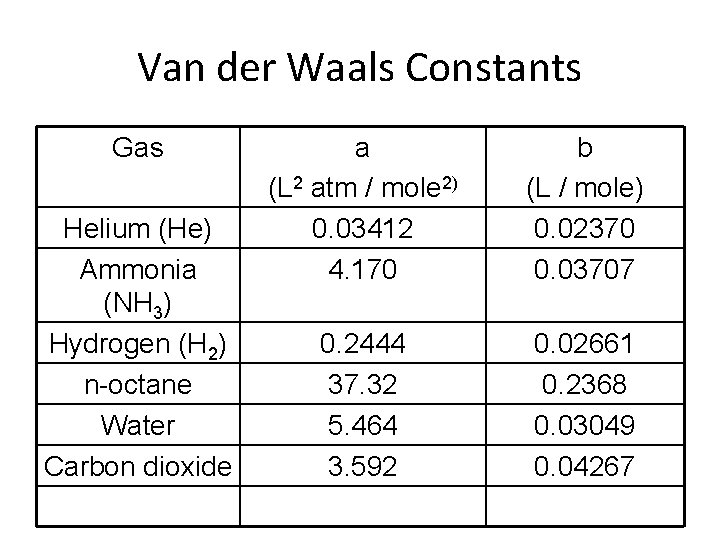
Van der Waals Constants Gas Helium (He) Ammonia (NH 3) Hydrogen (H 2) n-octane Water Carbon dioxide a (L 2 atm / mole 2) 0. 03412 4. 170 b (L / mole) 0. 02370 0. 03707 0. 2444 37. 32 5. 464 3. 592 0. 02661 0. 2368 0. 03049 0. 04267

Other gas laws • van der Waals: • Peng-Robinson: • Redlich-Kwong:

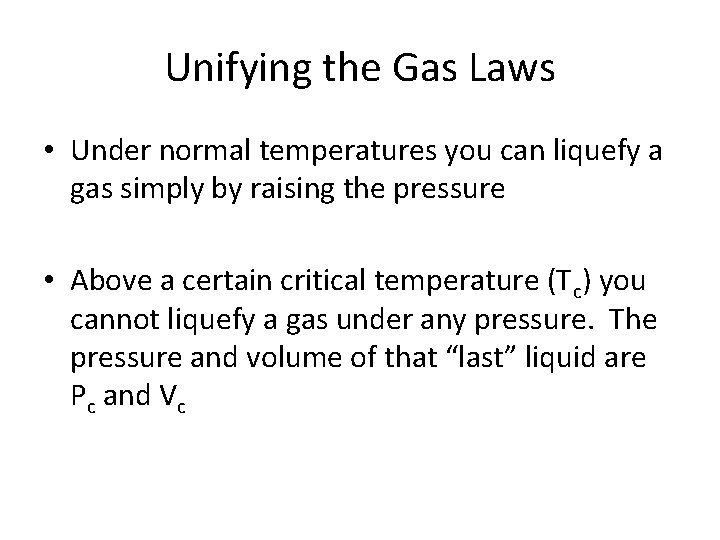
Unifying the Gas Laws • Under normal temperatures you can liquefy a gas simply by raising the pressure • Above a certain critical temperature (Tc) you cannot liquefy a gas under any pressure. The pressure and volume of that “last” liquid are Pc and Vc

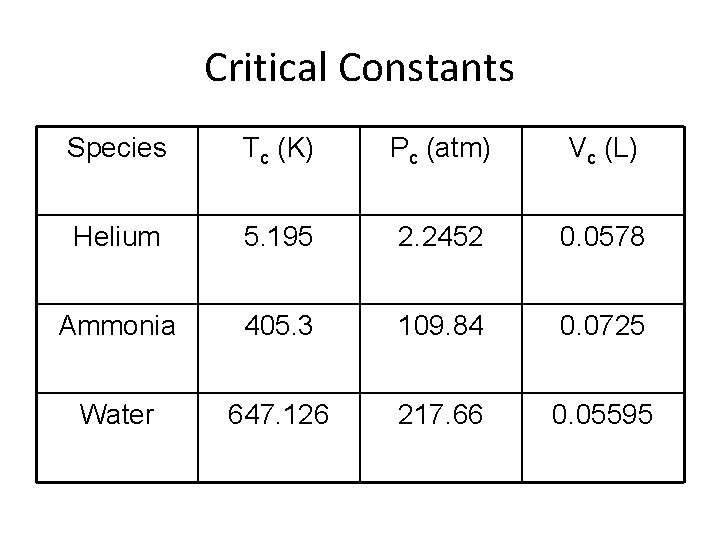
Critical Constants Species Tc (K) Pc (atm) Vc (L) Helium 5. 195 2. 2452 0. 0578 Ammonia 405. 3 109. 84 0. 0725 Water 647. 126 217. 66 0. 05595

“Critical” adjustments • Now we stop using temperature (and pressure and volume) in the gas laws. • Instead we write the reduced temperature (TR) as a fraction of the critical temperature (Tc). • That is TR = T / Tc

Compressibility factor plots redone

Atmosphere

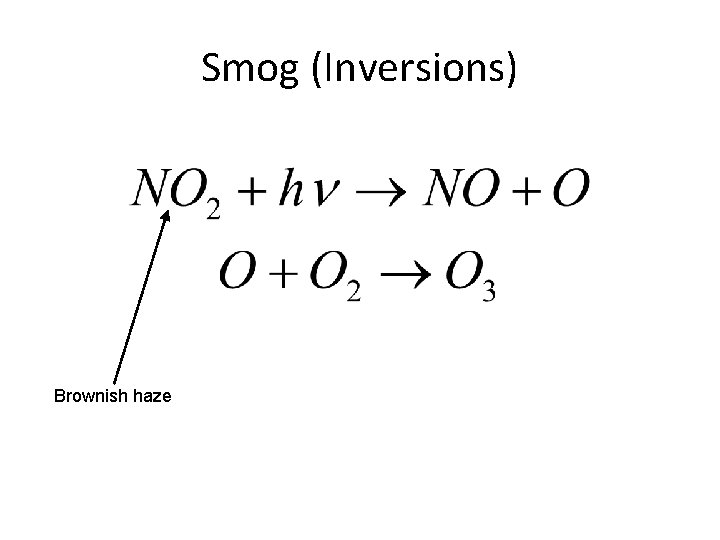
Smog (Inversions) Brownish haze

Acid Rain

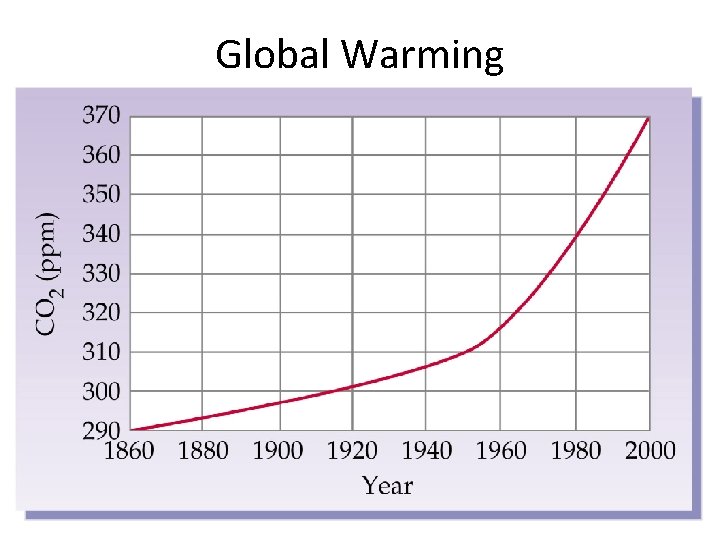
Global Warming
 Gas pressure units
Gas pressure units Air higroskopis adalah
Air higroskopis adalah Air pressure units
Air pressure units Air pressure units
Air pressure units Barometer
Barometer Air mass vocabulary
Air mass vocabulary Derive ideal gas equation
Derive ideal gas equation Imaginary gas
Imaginary gas Gas law
Gas law Sutherland's law
Sutherland's law Conclusion bhopal gas tragedy
Conclusion bhopal gas tragedy Gas leaked in bhopal gas tragedy
Gas leaked in bhopal gas tragedy Volume molare
Volume molare Flue gas desulfurisation gas filter
Flue gas desulfurisation gas filter Poisonous gas leaked in bhopal gas tragedy
Poisonous gas leaked in bhopal gas tragedy Difference between ideal gas and real gas
Difference between ideal gas and real gas Kinetika kimia
Kinetika kimia Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Gas constant r value
Gas constant r value Botox brow lift technique
Botox brow lift technique When units manufactured exceed units sold:
When units manufactured exceed units sold: 1910 confined space
1910 confined space Avogadro's law
Avogadro's law Bacaan psi gas r32
Bacaan psi gas r32 Gas laws hot air balloon
Gas laws hot air balloon Air mixed gas surface supplied diving new orleans
Air mixed gas surface supplied diving new orleans Gas law hot air balloon
Gas law hot air balloon Air hujan sanggup menjadi air tanah lantaran proses …
Air hujan sanggup menjadi air tanah lantaran proses … Urutan kelarutan perak halida dalam pelarut air
Urutan kelarutan perak halida dalam pelarut air Single acting cylinder example
Single acting cylinder example Section 1 what causes air pollution
Section 1 what causes air pollution Can you feel it in the air in the air
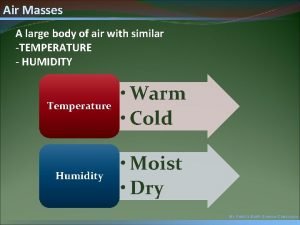
Can you feel it in the air in the air Large body of air
Large body of air Two cold air masses converge on a warm air mass
Two cold air masses converge on a warm air mass Put your right hand in the air
Put your right hand in the air Awan cumulus nimbus
Awan cumulus nimbus Is methane lighter than air
Is methane lighter than air Right hand in the air left hand in the air
Right hand in the air left hand in the air Air payau adalah campuran dari
Air payau adalah campuran dari A counterflow concentric tube heat exchanger
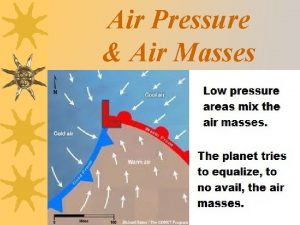
A counterflow concentric tube heat exchanger Cold front air movement
Cold front air movement Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Cop in refrigeration
Cop in refrigeration Pneumatic dcv
Pneumatic dcv Pneumatic
Pneumatic Maritime tropical air mass symbol
Maritime tropical air mass symbol Pekerjaan sanitasi
Pekerjaan sanitasi Chapter 12 air section 1 what causes air pollution
Chapter 12 air section 1 what causes air pollution Cold air mass overtakes warm air mass
Cold air mass overtakes warm air mass Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Air masses & frontswhat is an air mass?
Air masses & frontswhat is an air mass? Relation between pressure and kinetic energy of gas
Relation between pressure and kinetic energy of gas Sebuah tangki berisi alkohol yang massa jenisnya 800
Sebuah tangki berisi alkohol yang massa jenisnya 800 Which gas law relates pressure and temperature
Which gas law relates pressure and temperature Partial pressure gas law
Partial pressure gas law