Gas Laws Gas Pressure Pressure is defined as
















- Slides: 21

Gas Laws

Gas Pressure • Pressure is defined as force per unit area. • Gas particles exert pressure when they collide with the walls of their container. • The SI unit of pressure is the pascal (Pa). • However, there are several units of pressure – – – Pascal (Pa) Kilopascal (KPa) Atmosphere (atm) mm. Hg Torr

Boyle’s Law: Pressure and Volume • Boyle was an Irish chemist who studied the relationship between volume and pressure • Boyle’s law states that the pressure and volume of a gas at constant temperature are inversely proportional.

Boyle’s Law: Pressure and Volume • At a constant temperature, the pressure exerted by a gas depends on the frequency of collisions between gas particles and the container. • If the same number of particles is squeezed into a smaller space, the frequency of collisions increases, thereby increasing the pressure.

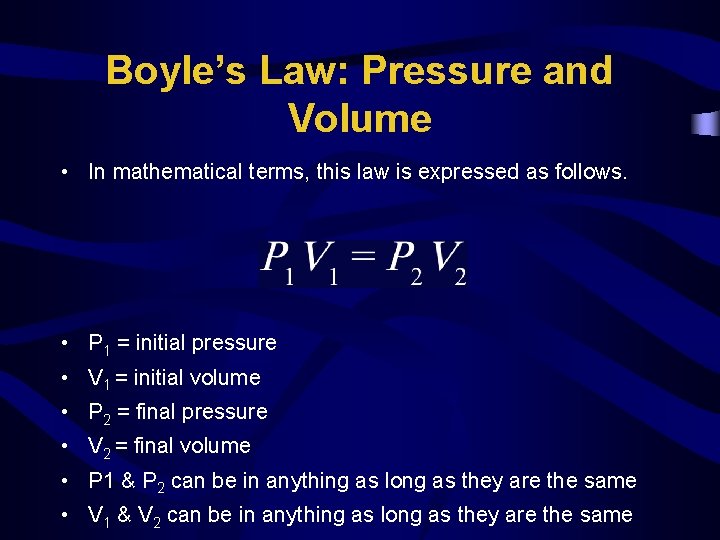
Boyle’s Law: Pressure and Volume • In mathematical terms, this law is expressed as follows. • P 1 = initial pressure • V 1 = initial volume • P 2 = final pressure • V 2 = final volume • P 1 & P 2 can be in anything as long as they are the same • V 1 & V 2 can be in anything as long as they are the same

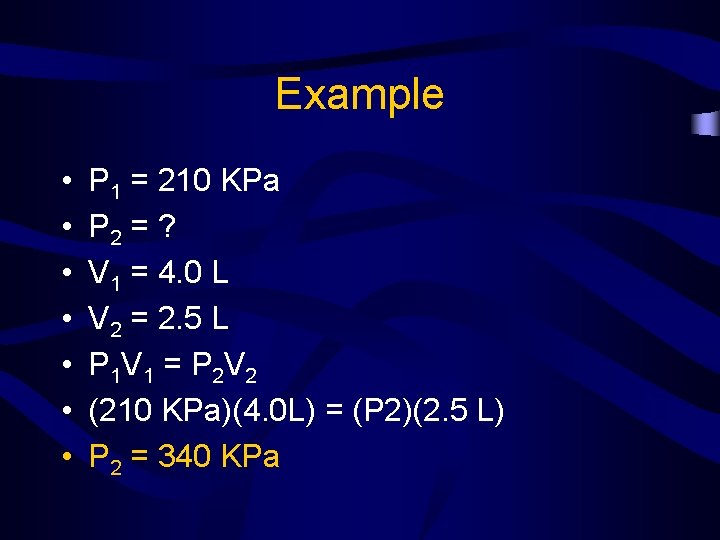
Example • A sample of Helium gas is compressed from 4. 0 L to 2. 5 L at a constant temperature. If the pressure of the gas in the 4. 0 L volume is 210 KPa, what will the pressure be at 2. 5 L?

Example • • P 1 = 210 KPa P 2 = ? V 1 = 4. 0 L V 2 = 2. 5 L P 1 V 1 = P 2 V 2 (210 KPa)(4. 0 L) = (P 2)(2. 5 L) P 2 = 340 KPa

Charles’ Law: Volume & Temperature • Charles was a French physicist who looked at the relationship between temperature and volume • He noted that as temperature went up, so did volume when pressure was held constant

Charles’ Law: Volume & Temperature • This observation is Charles’s law, which can be stated mathematically as follows.

Charles’ Law: Volume & Temperature • V 1 = V 2 T 1 T 2 • V 1 = initial volume • V 2 = final volume • T 1 = initial temperature • T 2 = final temperature • V 1 & V 2 can be in any unit as long as they are the same • T 1 & T 2 MUST be in Kelvin

Temperature conversions K = 273 + °C °C = 0. 56 (°F – 32) °F = 1. 8 °C + 32

Example • A sample of gas at 40. 0 °C occupies a volume of 2. 32 L. If the temperature is raised to 75. 0 °C what will the new volume be?

Example • • • V 1 = 2. 32 L V 2 = ? T 1 = 40. 0 °C = 313 K T 2 = 75. 0 °C = 348 K V 1 = V 2 T 1 T 2 • 2. 32 L = V 2 313 K 348 K • V 2 = 2. 58 L

Gay Lussac’s Law: Pressure & Temperature • Gay Lussac studied the relationship between pressure and temperature • He noticed that at a constant volume a direct relationship existed between the Kelvin temperature and volume • Giving the equation: • P 1 = P 2 T 1 T 2

Gay Lussac’s Law: Pressure & Temperature • P 1 = P 2 T 1 T 2 • P 1 = initial pressure • P 2 = final pressure • T 1 = initial temperature • T 2 = final temperature • P 1 & P 2 can be in any unit as long as they are the same • T 1 & T 2 MUST be in Kelvin

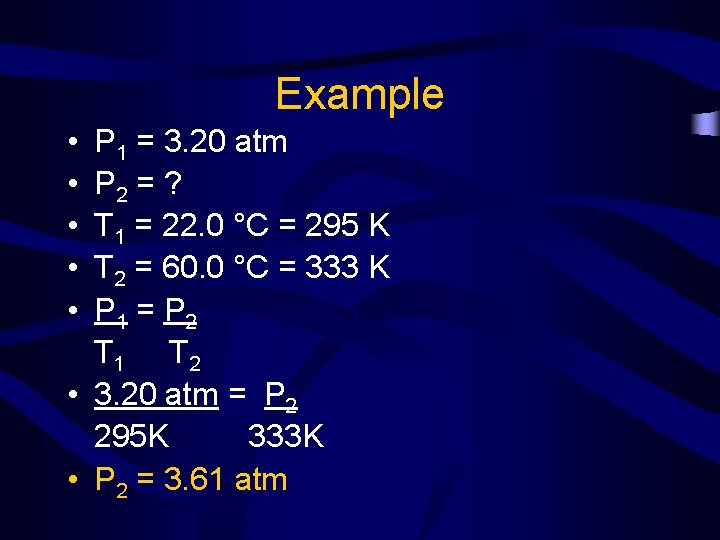
Example • The pressure of a gas in a tank is 3. 20 atm at 22. 0 °C. If the temperature rises to 60. 0 °C, what will the new pressure in the tank be?

Example • • • P 1 = 3. 20 atm P 2 = ? T 1 = 22. 0 °C = 295 K T 2 = 60. 0 °C = 333 K P 1 = P 2 T 1 T 2 • 3. 20 atm = P 2 295 K 333 K • P 2 = 3. 61 atm

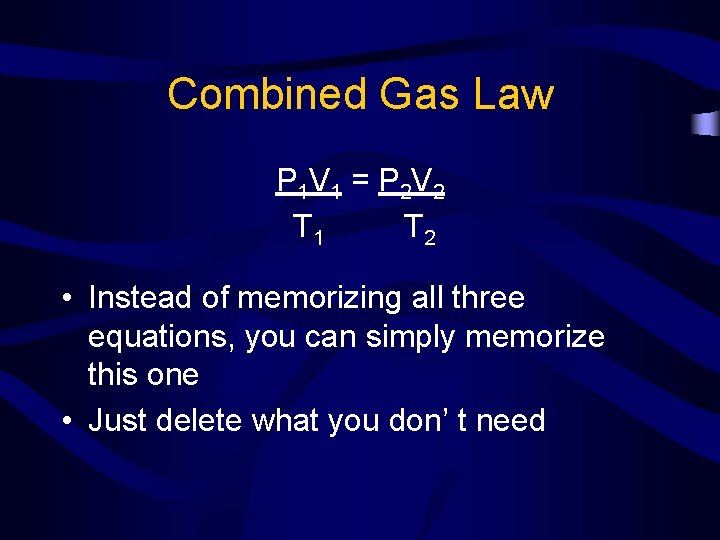
Combined Gas Law P 1 V 1 = P 2 V 2 T 1 T 2 • Instead of memorizing all three equations, you can simply memorize this one • Just delete what you don’ t need

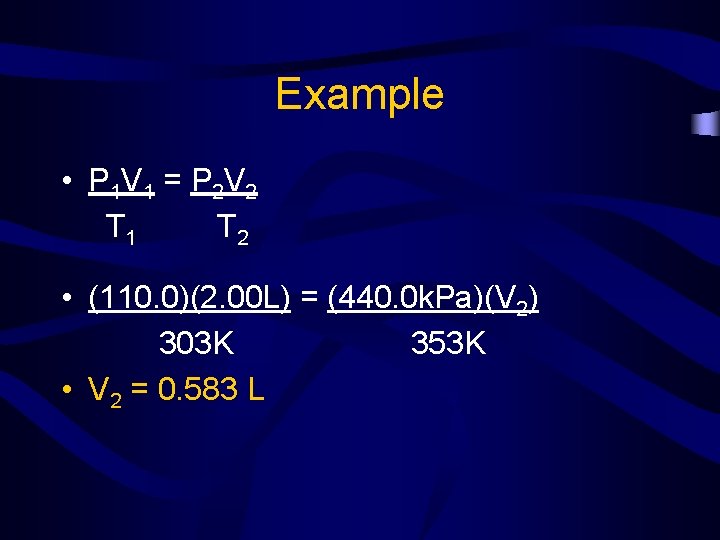
Example • A gas at 110. 0 k. Pa and 30. 0°C fills a flexible container to a volume of 2. 00 L. If the temperature was raised to 80. 0°C and the pressure was increased to 440. 0 k. Pa, what is the new volume?

Example • P 1 V 1 = P 2 V 2 T 1 T 2 • • • P 1 = 110. 0 k. Pa V 1 = 2. 00 L T 1 = 30. 0 °C = 303 K P 2 = 440. 0 k. Pa V 2 = ? T 2 = 80. 0 °C = 353 K

Example • P 1 V 1 = P 2 V 2 T 1 T 2 • (110. 0)(2. 00 L) = (440. 0 k. Pa)(V 2) 303 K 353 K • V 2 = 0. 583 L
 How is gas pressure defined
How is gas pressure defined Is a collection of well defined objects
Is a collection of well defined objects Charles de secondat
Charles de secondat Pressure is defined as force per unit area.
Pressure is defined as force per unit area. Pressure is defined as *
Pressure is defined as * Gas laws crash course
Gas laws crash course Direct and indirect gas laws
Direct and indirect gas laws Empirical gas law
Empirical gas law Combined gas laws
Combined gas laws All the gas laws
All the gas laws Different gas laws
Different gas laws Avogrados law
Avogrados law Gas law conceptual questions
Gas law conceptual questions Boyle's law practice problems
Boyle's law practice problems Boyle's law examples
Boyle's law examples Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas laws
Combined gas laws Chapter 13 gases
Chapter 13 gases Which gas laws are inversely proportional
Which gas laws are inversely proportional Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws