The Gas Laws Pressure Liquid pressure exerted equally
















- Slides: 38

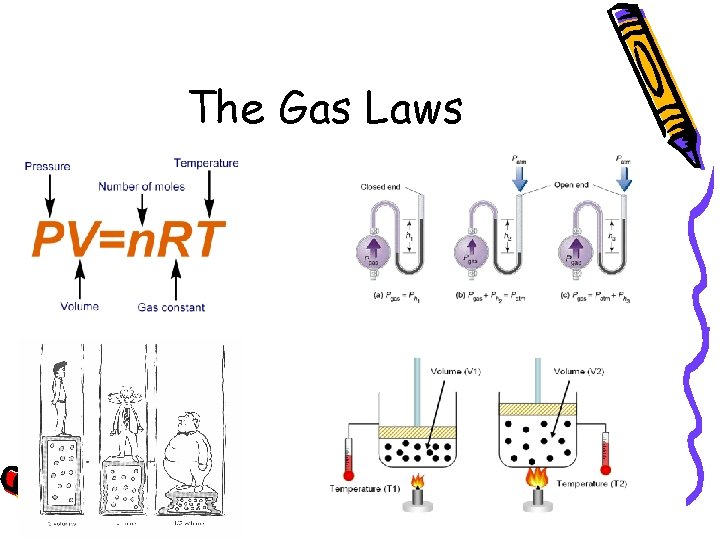
The Gas Laws

Pressure Liquid pressure – exerted equally in all directions - swimmers feel an increase in pressure as they go deeper down into the ocean

Pressure Atmospheric Pressure – (air pressure, barometric pressure) - at sea level, air pressure = weight of a kg mass on every square centimeter of surface exposed to it ** we are not conscious of air pressure because it is exerted in all directions

Measuring Pressure Hg Barometer – measures AIR PRESSURE - Pressure varies with altitude - Decrease in air pressure as you increase altitude - Drop in air pressure before a storm - Normal Atmospheric Pressure - 760 mm. Hg or 1 atm

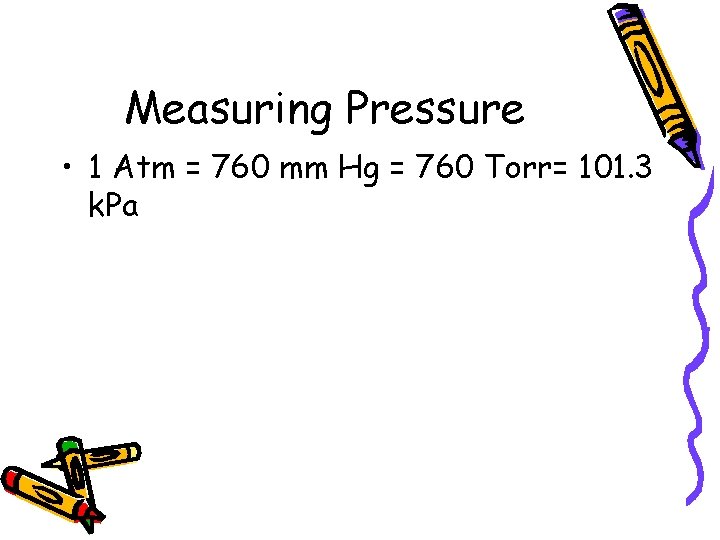
Measuring Pressure • 1 Atm = 760 mm Hg = 760 Torr= 101. 3 k. Pa

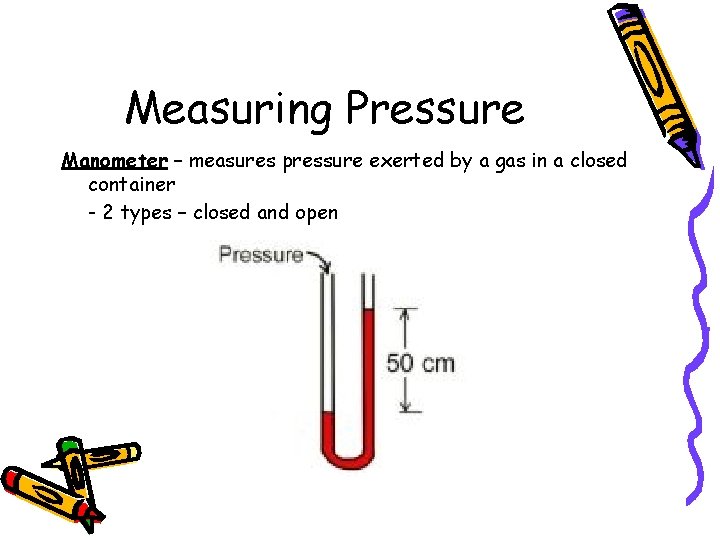
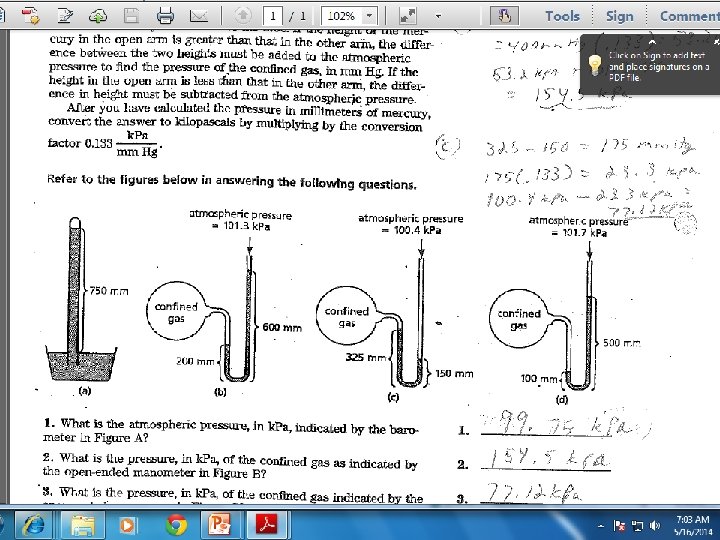
Measuring Pressure Manometer – measures pressure exerted by a gas in a closed container - 2 types – closed and open

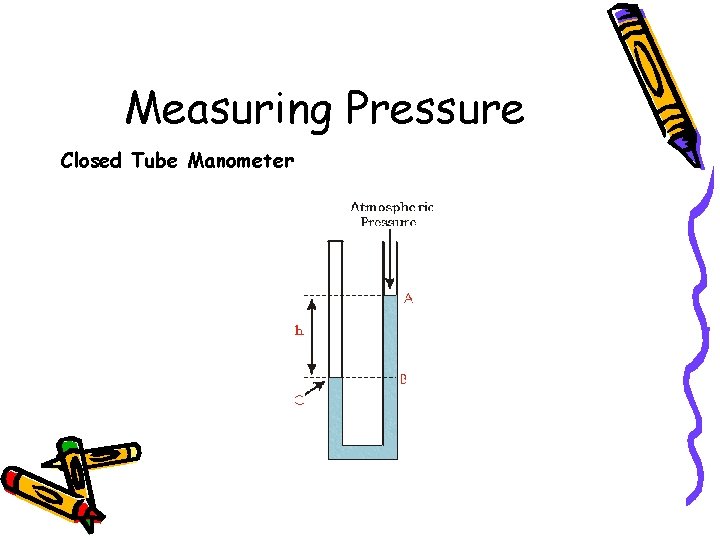
Measuring Pressure Closed Tube Manometer


Gas Laws We will be studying the behavior of gases under varying conditions including: Pressure, Volume and Temperature Watch Safari Montage video - Common Properties of Gases http: //econtent 2. bucksiu. org/SAFARI/montage/play. php? frompage=play&keyindex=3139&location=l ocal&chapterskeyindex=22429&sceneclipskeyindex=-1

Kinetic Theory of Gases • Gas is made up of very small particles that are in constant random motion • Gas particles are free to spread far apart from each other • The higher the temperature – the faster the particles move

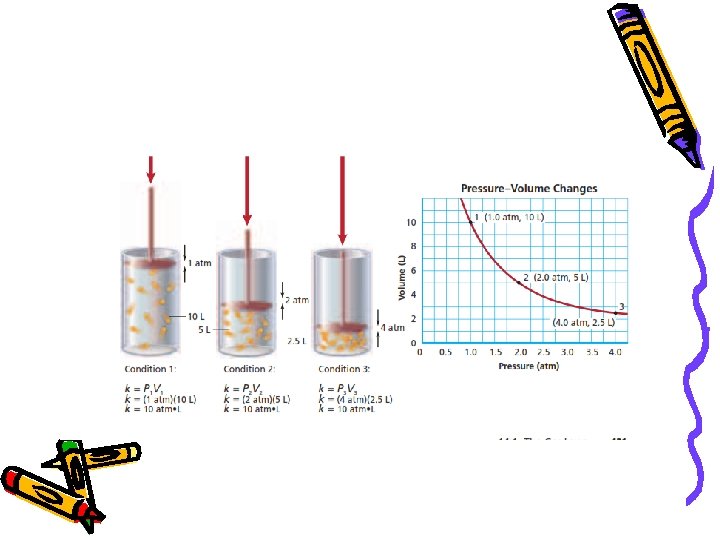
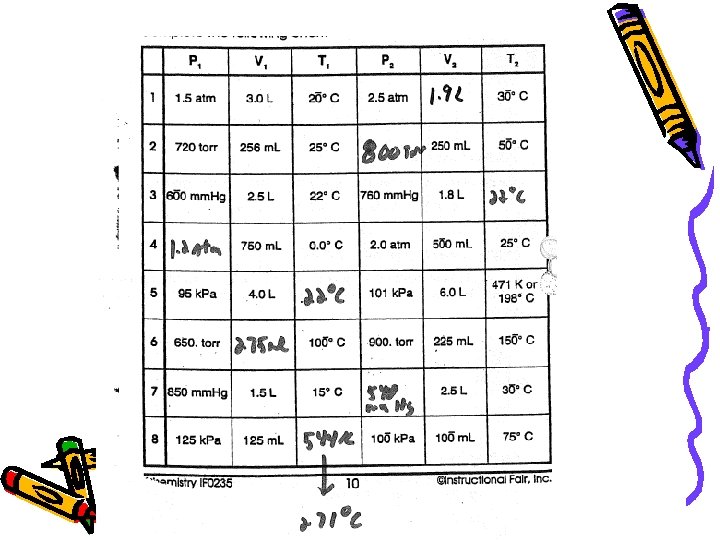
Boyle’s Law • Safari Montage Video – Boyles Law http: //econtent 2. bucksiu. org/SAFARI/montage/playlistedit. php? playlisttype=MY&Action=Make. Active&playlistkeyindex=978& location=local • At constant temperature, the volume of a gas varies inversely with pressure P 1 V 1 = P 2 V 2


Boyle’s Law • Safari Montage Video – Boyles Law http: //econtent 2. bucksiu. org/SAFARI/montage/playlistedit. php? playlisttype=MY&Action=Make. Active&playlistkeyindex=978& location=local • At constant temperature, the volume of a gas varies inversely with pressure P 1 V 1 = P 2 V 2

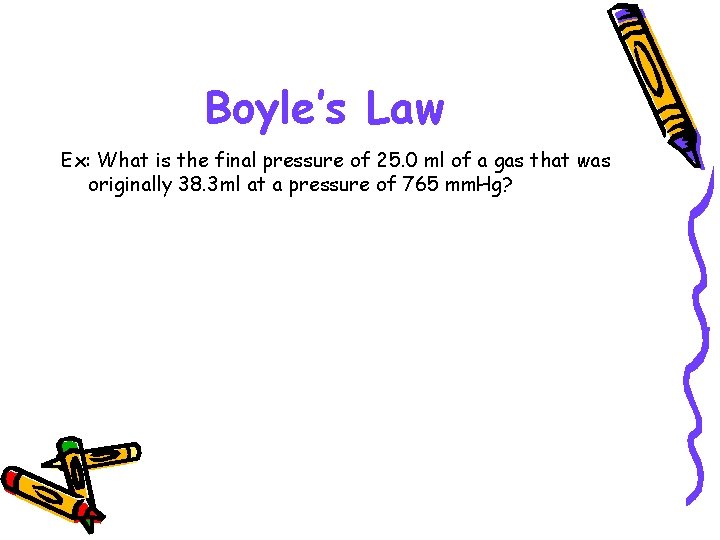
Boyle’s Law Ex: What is the final pressure of 25. 0 ml of a gas that was originally 38. 3 ml at a pressure of 765 mm. Hg?


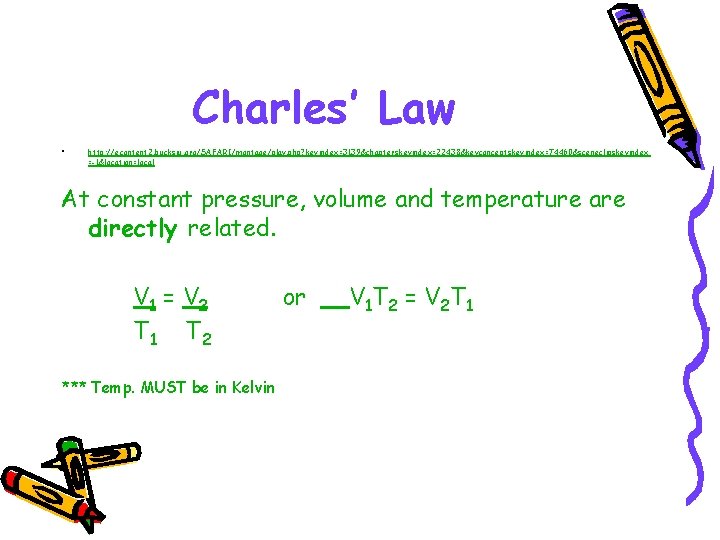
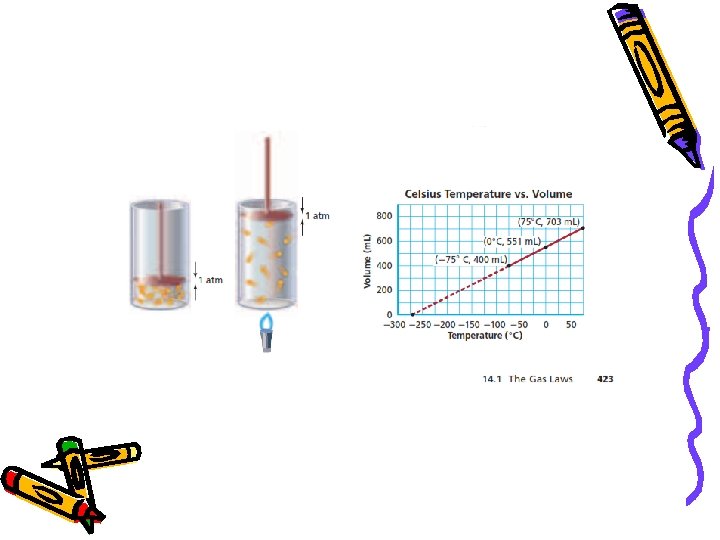
Charles’ Law • http: //econtent 2. bucksiu. org/SAFARI/montage/play. php? keyindex=3139&chapterskeyindex=22438&keyconceptskeyindex=74460&sceneclipskeyindex =-1&location=local At constant pressure, volume and temperature are directly related. V 1 = V 2 T 1 T 2 *** Temp. MUST be in Kelvin or V 1 T 2 = V 2 T 1

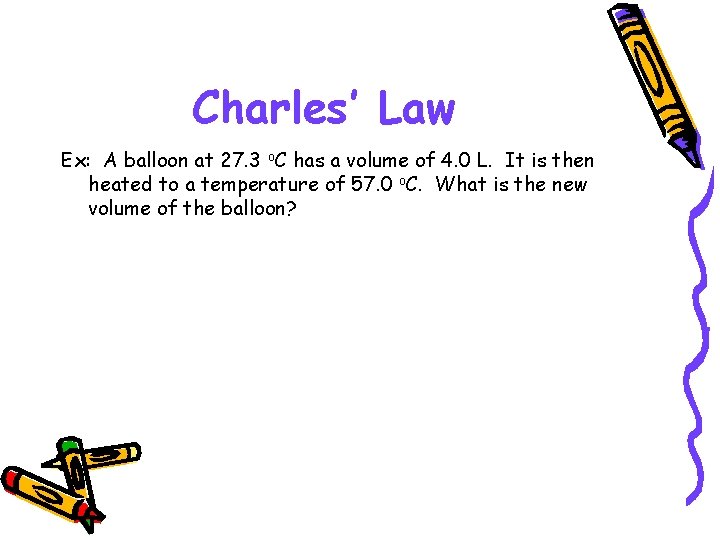
Charles’ Law Ex: A balloon at 27. 3 o. C has a volume of 4. 0 L. It is then heated to a temperature of 57. 0 o. C. What is the new volume of the balloon?



Gay-Lussac’s Law • http: //econtent 2. bucksiu. org/SAFARI/montage/play. php? keyindex=3139&chapterskeyindex=22438&keyconceptskeyindex=74460&sceneclipskeyindex =-1&location=local • At constant volume, pressure and temperature are DIRECTLY related P 1 = P 2 T 1 T 2 or P 1 T 2 = P 2 T 1 *** Temp. MUST be in Kelvin

Gay-Lussac’s Law Ex: A gas is left in a used aerosol can is at a pressure of 1. 0 atm at 27. 0 o. C. If this can is thrown onto a fire, what is the internal pressure of the gas when it reaches 927 o. C?


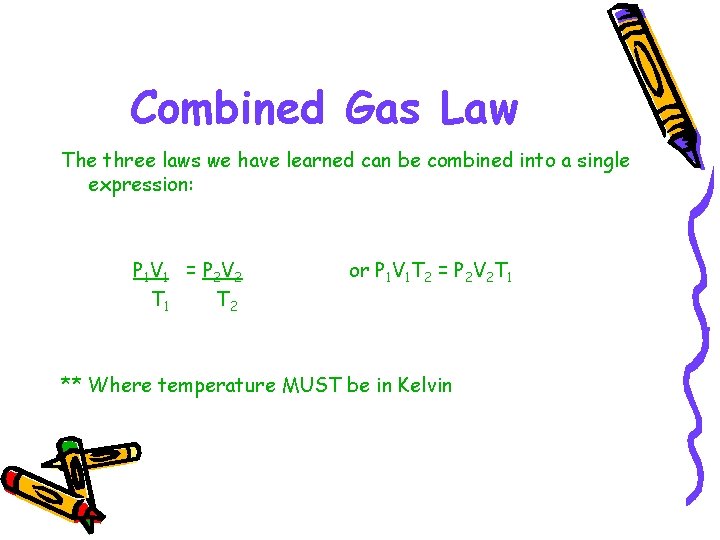
Combined Gas Law The three laws we have learned can be combined into a single expression: P 1 V 1 = P 2 V 2 T 1 T 2 or P 1 V 1 T 2 = P 2 V 2 T 1 ** Where temperature MUST be in Kelvin

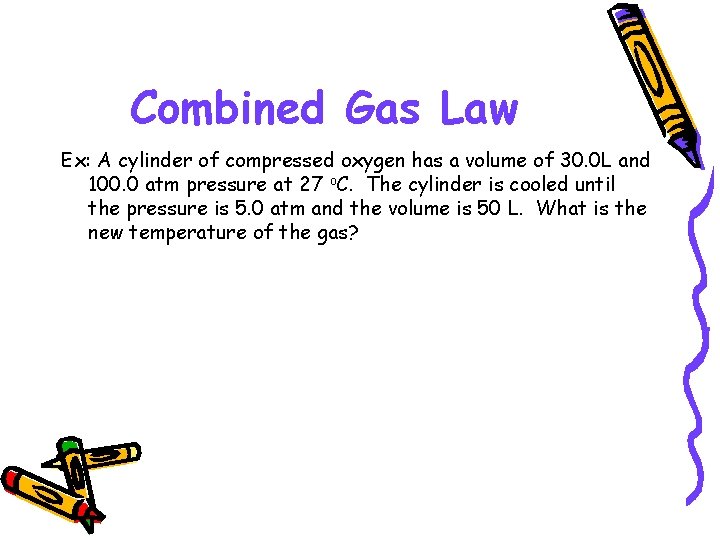
Combined Gas Law Ex: A cylinder of compressed oxygen has a volume of 30. 0 L and 100. 0 atm pressure at 27 o. C. The cylinder is cooled until the pressure is 5. 0 atm and the volume is 50 L. What is the new temperature of the gas?


Ideal Gas Law http: //econtent 2. bucksiu. org/SAFARI/montage/play. php? keyindex=3139&chapterskeyindex=22438&keyconceptskeyindex=74448&sceneclipskeyindex=1&location=local Ideal Gas – follows all the gas laws at ALL conditions of pressure and temperature. - Does not really exist. - Real gases usually start to liquefy at low temp. and high pressures. - We will use ideal gases for all problems in this class – therefore we will now learn the Ideal Gas Law

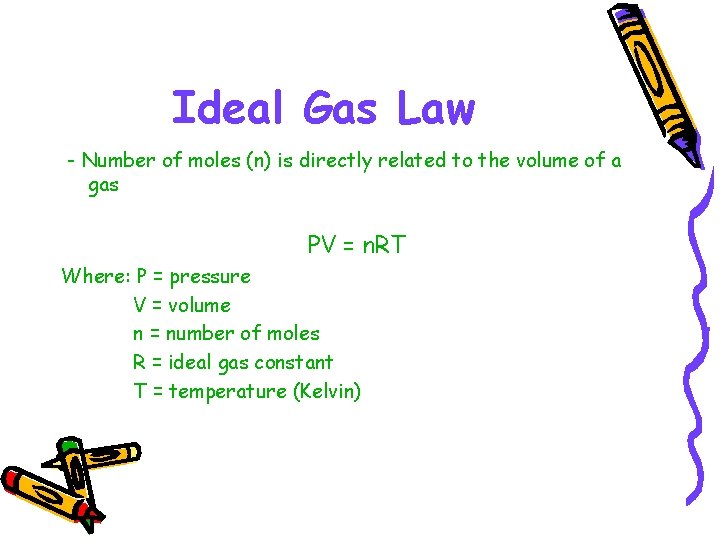
Ideal Gas Law - Number of moles (n) is directly related to the volume of a gas PV = n. RT Where: P = pressure V = volume n = number of moles R = ideal gas constant T = temperature (Kelvin)

Ideal Gas Law R = ideal gas constant - number depends on the units of P, V and T 0. 0821 L. Atm K. Mol 62. 4 L. mm. Hg K. mol 8. 31 L. k. Pa K. mol

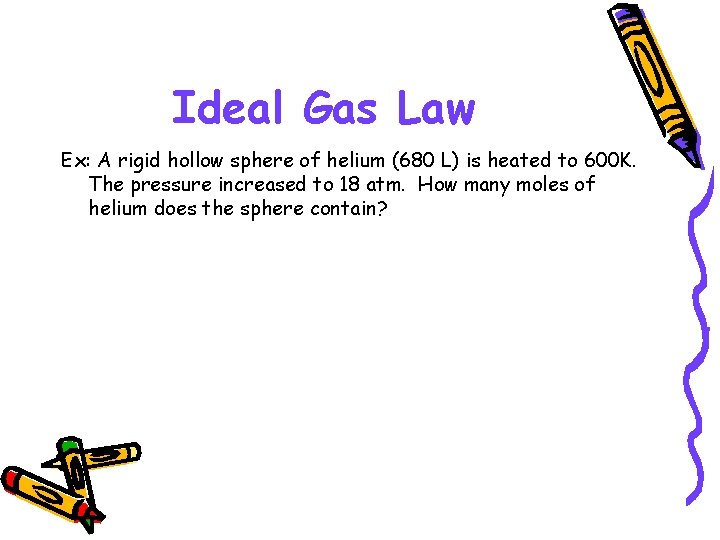
Ideal Gas Law Ex: A rigid hollow sphere of helium (680 L) is heated to 600 K. The pressure increased to 18 atm. How many moles of helium does the sphere contain?

Ideal Gas Law Ex: What is the pressure of 25. 0 g of carbon dioxide that occupies a volume of 350 ml at 25 o. C?

Dalton’s Law of Partial Pressure Ex: What would be the pressure inside a cylinder if we combined these three gases?

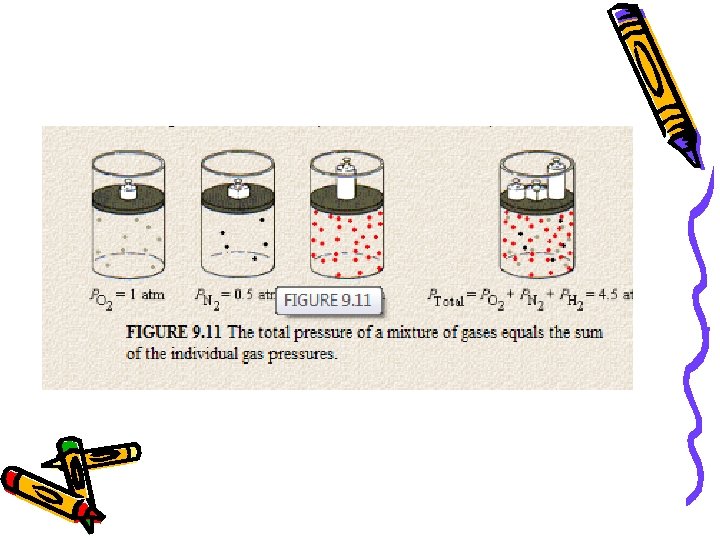
Dalton’s Law of Partial Pressure At constant volume and temperature, the pressure exerted by a mixture of gases is equal to the sum of the partial pressures. PT = P 1 + P 2 + P 3 + …

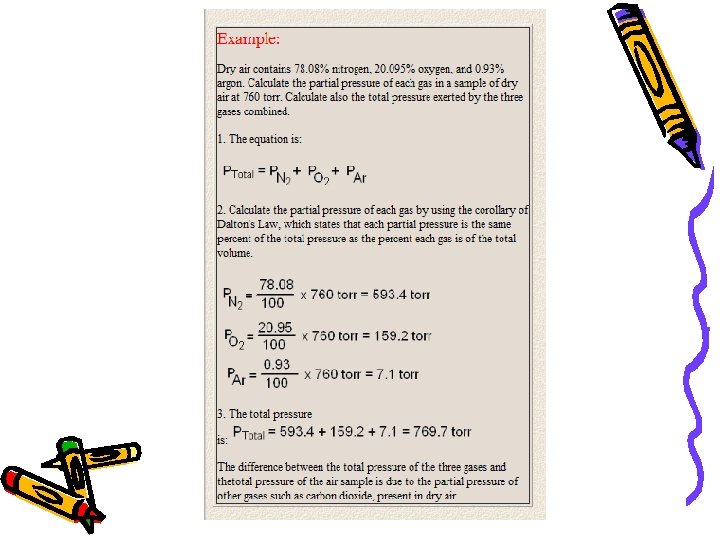
Dalton’s Law of Partial Pressure Ex: Air contains oxygen, nitrogen, carbon dioxide and trace amounts of other gases. What is the partial pressure of the oxygen gas given the following information? P of nitrogen = 593. 4 mm. Hg P of carbon dioxide = 0. 3 mm. Hg P of trace gases = 7. 1 mm. Hg Total air pressure = 760 mm. Hg



Graham’s Law of Diffusion – movement of particles Which do you think would travel faster? A light gas (such as H) or a heavy gas (such as CO 2)

Graham’s Law of Diffusion Law says – rate of diffusion of a gas is inversely proportional to the square root of its formula mass. In other words: Lighter gas travel faster!!!!! Mathematically written: Rate A = √MB Rate B √MA

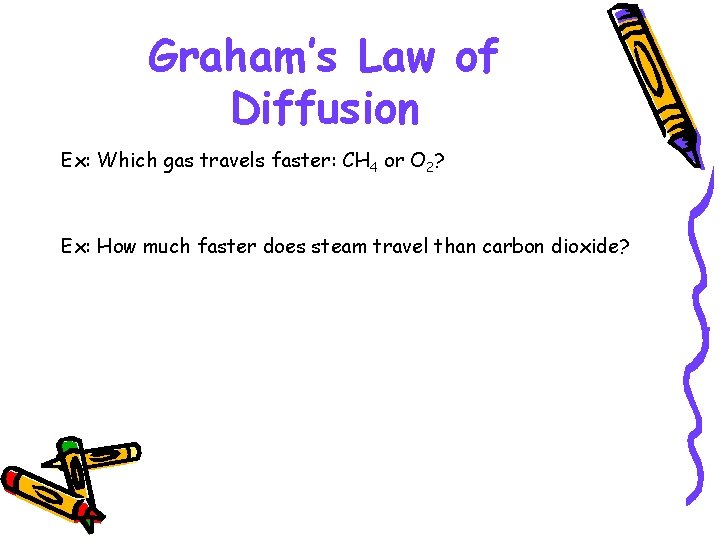
Graham’s Law of Diffusion Ex: Which gas travels faster: CH 4 or O 2? Ex: How much faster does steam travel than carbon dioxide?
 In which direction is air pressure exerted?
In which direction is air pressure exerted? Calculate pressure exerted by a screw on the wooden plank
Calculate pressure exerted by a screw on the wooden plank Definisi tekanan zat padat
Definisi tekanan zat padat Facts about montesquieu
Facts about montesquieu Conduction and breakdown in pure liquids
Conduction and breakdown in pure liquids Liquid liquid extraction unit
Liquid liquid extraction unit The force exerted on an object by a machine
The force exerted on an object by a machine Force exerted by jet on moving plate formula
Force exerted by jet on moving plate formula Force exerted by magnets
Force exerted by magnets Magnetic field lines of a bar magnet
Magnetic field lines of a bar magnet How to calculate applied force
How to calculate applied force Balanced force facts
Balanced force facts The force exerted by the air above is called
The force exerted by the air above is called What is the maximum torque exerted by a 55 kg person
What is the maximum torque exerted by a 55 kg person When we divide
When we divide Criterion of realism formula
Criterion of realism formula Equally likely decision criterion
Equally likely decision criterion Equally well symposium
Equally well symposium I wonder by my troth
I wonder by my troth Lonely metaphors
Lonely metaphors Equally shared
Equally shared Two equally charged pith balls are 3 cm apart
Two equally charged pith balls are 3 cm apart Kieron's cats answer
Kieron's cats answer Fundamental charge
Fundamental charge Electric charge
Electric charge Four brothers share 5 cookies equally
Four brothers share 5 cookies equally Equally well scotland
Equally well scotland Pr��ce ����ste��n�� ��vazek
Pr��ce ����ste��n�� ��vazek Gas laws crash course
Gas laws crash course Indirect vs direct correlation
Indirect vs direct correlation Empirical gas laws
Empirical gas laws The combined gas law
The combined gas law Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Charles law balloon
Charles law balloon Gas laws conceptual questions
Gas laws conceptual questions Charles law example
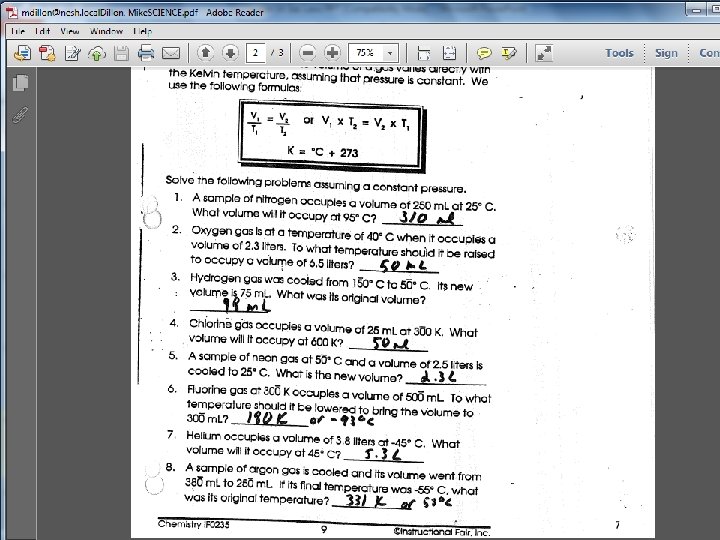
Charles law example Charles' law worksheet with answers
Charles' law worksheet with answers Boyle's gas law formula
Boyle's gas law formula