Gas Laws Pressure Pressure The amount of force







- Slides: 11

Gas Laws Pressure

Pressure The amount of force an object puts on a surface. Pressure is measured by a barometer. Atmospheric pressure comes from air being pulled down by gravity. Atmospheric pressure changes with altitude – higher elevations have lower atmospheric pressure.

Units of pressure There a few units of pressure that we use in chemistry. k. Pa = kilopascal atm = atmosphere Mm Hg = millimeters of mercury Torr Psi = pounds per square inch

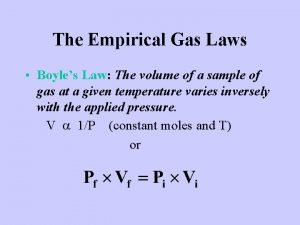
Pressure conversions All these units are different ways of measuring the same thing. We can convert from one unit to another using the following relationship: 1 atm = 760 mm Hg = 760 Torr = 101. 3 k. Pa = 14. 7 psi This relationship allows us to make conversion factors between units of pressure.

Examples 1. Convert the pressure of a gas from 2 atm to mm Hg. 2. Convert the pressure of a gas from 29. 4 psi to atm.

3. Convert 1520 mm Hg to k. Pa.

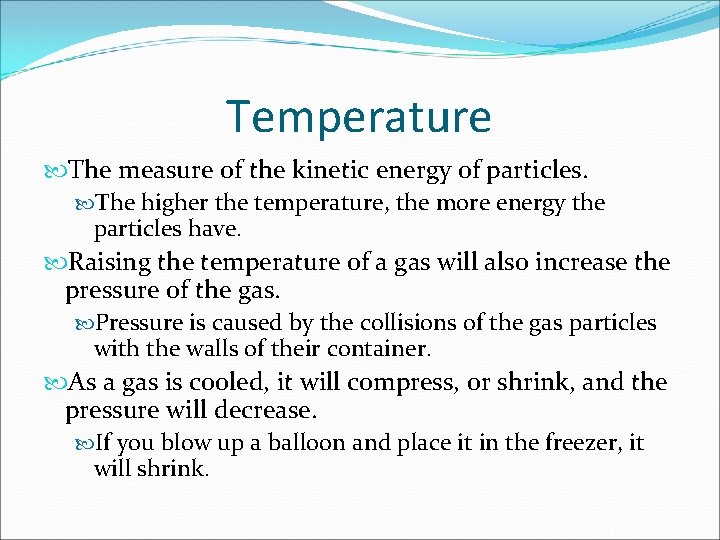
Temperature The measure of the kinetic energy of particles. The higher the temperature, the more energy the particles have. Raising the temperature of a gas will also increase the pressure of the gas. Pressure is caused by the collisions of the gas particles with the walls of their container. As a gas is cooled, it will compress, or shrink, and the pressure will decrease. If you blow up a balloon and place it in the freezer, it will shrink.

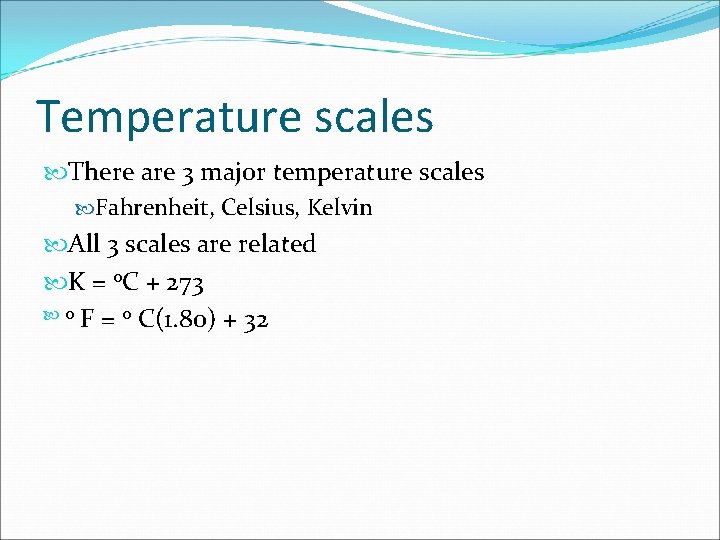
Temperature scales There are 3 major temperature scales Fahrenheit, Celsius, Kelvin All 3 scales are related K = o. C + 273 o F = o C(1. 80) + 32

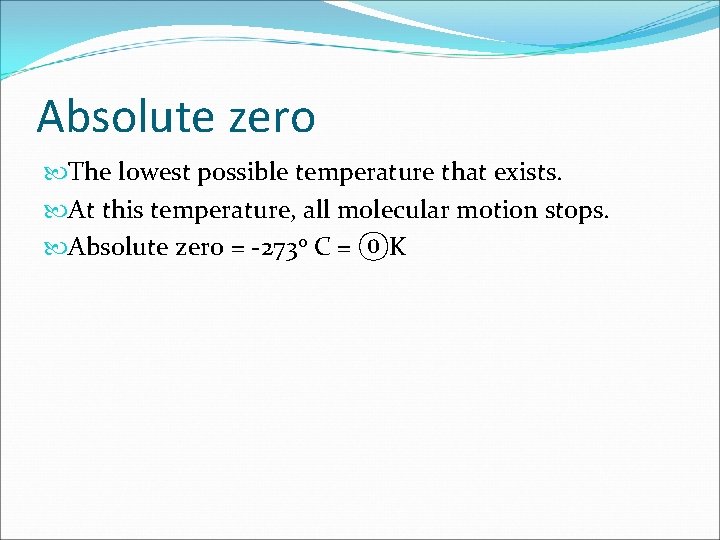
Absolute zero The lowest possible temperature that exists. At this temperature, all molecular motion stops. Absolute zero = -273 o C = ⓪K

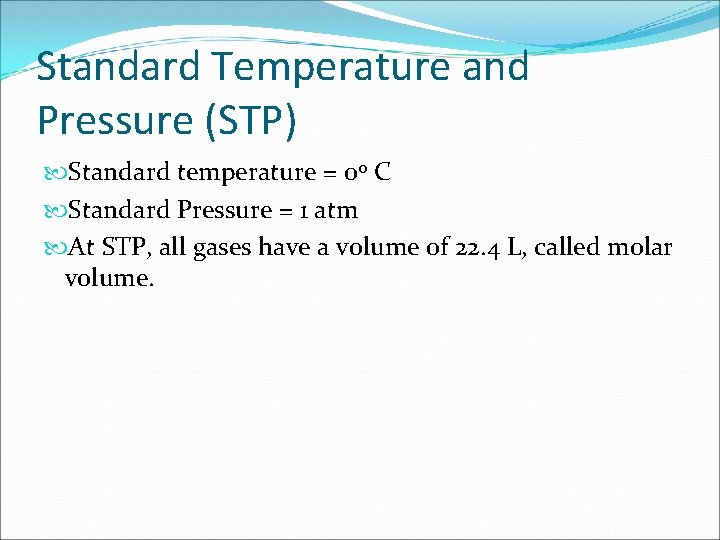
Standard Temperature and Pressure (STP) Standard temperature = 0 o C Standard Pressure = 1 atm At STP, all gases have a volume of 22. 4 L, called molar volume.

Temperature Conversions Convert 50 K to degrees Celsius. Convert 50 o C to Kelvin.
 Useless laws weaken the necessary laws
Useless laws weaken the necessary laws The amount of force applied per unit of area
The amount of force applied per unit of area Gas laws crash course
Gas laws crash course What is direct and indirect relationship
What is direct and indirect relationship Empirical gas laws
Empirical gas laws Gas laws calculations
Gas laws calculations Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Avogrados law
Avogrados law Gas laws conceptual questions
Gas laws conceptual questions Charles law formula for t2
Charles law formula for t2 Combined gas law practice worksheet answers
Combined gas law practice worksheet answers