GameChanging Procedures in Stroke Management Maximizing the Effectiveness














- Slides: 47

Game-Changing Procedures in Stroke Management: Maximizing the Effectiveness of Endovascular Treatment

Content Development Chairs Andrew W. Asimos, MD Medical Director, Neurological Emergencies Neurosciences Institute Carolinas Health. Care System Professor, Department of Emergency Medicine Carolinas Medical Center Charlotte, NC Dr. Asimos has nothing to disclose. Opeolu Adeoye, MD, MS, FACEP, FAHA Associate Professor, Department of Emergency Medicine Co-Director, UC Stroke Team University of Cincinnati, OH Dr. Adeoye owns stock in Sense Diagnostics, LLC. Nurse Planner Darby L. Copeland, Ed. D, RN, NRP, NCEE Executive Director West Virginia College of Emergency Physicians Wheeling, WV Dr. Copeland has nothing to disclose.

Presenter Hilary R. Rockwell, MD Emergency Medical Physician Avera Queen of Peach Hospital Mitchell, SD Dr. Rockwell has nothing to disclose.

What is one reason why the newer clinical trials (MR CLEAN, EXTEND-IA, ESCAPE, and SWIFT PRIME) showed benefit of endovascular stroke treatment compared with intravenous tissue plasminogen activator (IV t-PA) treatment? A. Treatment Selection B. Use of older-generation devices with only modest reperfusion efficacy C. Long interval between study entry and performance of endovascular reperfusion procedure D. Patients not within the time window to receive IV t-PA

According to the 2015 American Heart Association/American Stroke Association (AHA/ASA) update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment, patients should receive endovascular therapy with a stent retriever if they meet several criteria. What is one of the requirements? A. Acute ischemic stroke patient receiving IV t-PA within 3 hours of onset, according to guidelines from professional medical societies B. Age ≥ 18 years old C. Treatment can be initiated (groin puncture) within 4 hours of symptom onset D. Alberta Stroke Program Early CT Score (ASPECTS) ≥ 10

A 76 -year-old male presents to the emergency department (ED) at approximately 6: 15 PM. He is brought in by emergency medical services (EMS) and is accompanied by his wife. Upon physical exam you notice left arm weakness, facial droop, and slurred speech. His wife states that he started slurring his speech at approximately 4: 30 PM and fell a few minutes later, at which point she called 9 -1 -1. He has a history of hypertension and type 2 diabetes. If endovascular therapy is indicated, what is recommended according to AHA/ASA guidelines? A. Administer IV t-PA before any emergency imaging is conducted. B. Perform a noninvasive intracranial vascular study despite any delay in IV t-PA. C. Start IV t-PA if patient is eligible, even if endovascular treatment is being considered D. Initiate reperfusion with endovascular therapy as early as possible and within 10 hours of stroke onset.

RC is a 39 -year-old male being brought into the ED by ambulance after his wife called 9 -1 -1 saying he was having a stroke. RC’s wife reports that symptoms presented around 9 am, when RC was having trouble grabbing his utensils while eating breakfast. She did not call 9 -1 -1 until 10: 45 am, when he face began to droop and his speech was significantly slurred. RC arrives at the hospital at 11: 30 am. Upon his arrival, the nurse checks his vital signs, obtains IV access, and prepares him for a computed tomography (CT) scan. A causative occlusion involving the distal internal carotid artery is noticed. RC has an ASPECTS of 7. His imaging is negative for hemorrhage. Is RC a candidate for endovascular therapy based on the imaging studies performed? A. Yes, because he is within the window where endovascular therapy would be beneficial and his ASPECTS is >6. B. Yes, because his ASPECTS is >3, making him a good candidate for endovascular therapy. C. No, because he is outside the 2 -hour window when endovascular therapy would be beneficial. D. No, because his ASPECTS is too low. To be a candidate, a patient must have an ASPECTS of ≥ 10.

Which of the following is not a characteristic of a large-vessel occlusion (LVO)? A. LVOs are common and constitute 33% to 40% of all ischemic strokes. B. In LVOs, IV t-PA is very effective, especially if the embolus is in the carotid terminus. C. IV t-PA is more effective when opening occlusions at the distal M 1, M 2, M 3, and M 4. D. LVOs are severe emergencies that can increase mortality by 5 -fold.

Outline § Introductions § Overview of acute stroke § Evidence for endovascular therapy for ischemic stroke § Impact of emergency management of acute ischemic stroke – Imaging—what do we absolutely need? – Prehospital triage • Nursing implications – Interhospital triage § Conclusions and Future Directions

What You Need to Know About Stroke 1 # cause of disability among adults in the US 795, 000 33 -40% are large-vessel Americans each year occlusion (LVOs)1, 2 suffer a stroke KILLS 128, 000 people a year—that’s about 1 out of every 19 deaths EVERY 40 SECONDS someone has a stroke Statistics from: AHA/ASA, https: //www. heart. org/HEARTORG/General/Heart-and-Stroke-Association. Statistics_UCM_319064_Sub. Home. Page. jsp; CDC, http: //www. cdc. gov/stroke/facts. htm (03/15); WHO, http: //www. who. int/topics/cerebrovascular_accident/en/. 1. Skagen K, et al. J Stroke Cerebrovasc Dis. 2015; 24(7): 1532 -1539. 2. Turc G, et al. Stroke. 2016; 47: 1466 -1472. # 5 cause of death among adults in the US

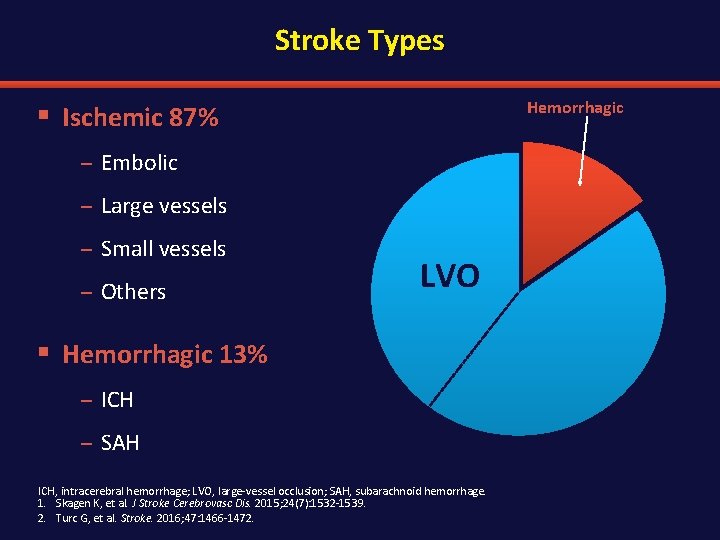
Stroke Types Hemorrhagic § Ischemic 87% – Embolic – Large vessels – Small vessels – Others LVO § Hemorrhagic 13% – ICH – SAH ICH, intracerebral hemorrhage; LVO, large-vessel occlusion; SAH, subarachnoid hemorrhage. 1. Skagen K, et al. J Stroke Cerebrovasc Dis. 2015; 24(7): 1532 -1539. 2. Turc G, et al. Stroke. 2016; 47: 1466 -1472.

Large-Vessel Occlusions (LVOs): Scope of the Problem § Common: 33%to 40% of all ischemic stroke 1, 2 § Severe: 5 x higher mortality; 3 -fold reduction in good outcome § Respond poorly to intravenous thrombolytic (IV t-PA) § Successful opening of occlusion by IV t-PA 3 – Distal M 1, M 2, M 3, and M 4: 78% to 86% – Carotid terminus: ~28% t-PA, tissue plasminogen activator. 1. Skagen K, et al. J Stroke Cerebrovasc Dis. 2015; 24(7): 1532 -1539. 2. Turc G, et al. Stroke. 2016; 47: 1466 -1472. 3. Demchuck AM, et al. Neuroradiol. 2014; 273(1): 202 -210.

Acute Ischemic Stroke Treatment Options Medical Management § IV t-PA is the thrombolytic drug used in stroke patients Mechanical Thrombectomy § This procedure uses a stent retriever that is placed in the occluded vessel through a catheter placed in the groin § Patients must be within the time window of 0 to 3 hours from symptom onset § The time window for mechanical thrombectomy is up to 6 hours § There are other from symptom onset contraindications associated with use of the drug as well § If the patient fails IV t-PA or is ineligible for IV t-PA, he/she may be eligible for mechanical thrombectomy Jauch EC, et al. Stroke. 2013; 44: 870 -947. Powers WJ, et al. Stroke. 2015; 46(10): 3020 -3035.

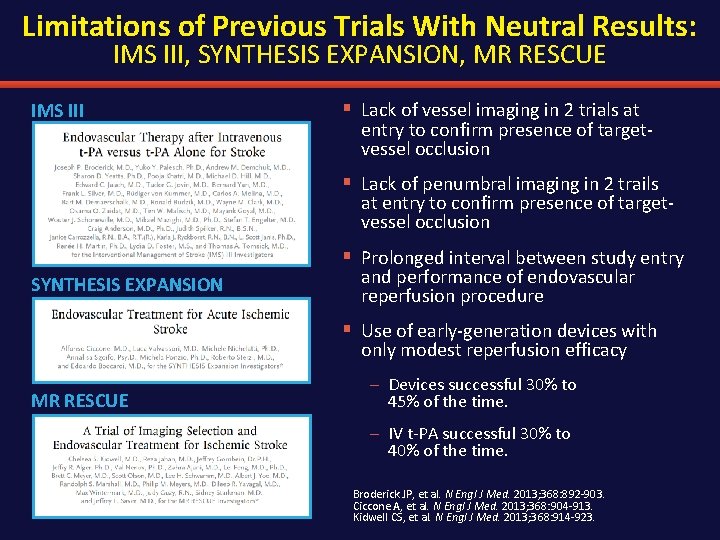
Limitations of Previous Trials With Neutral Results: IMS III, SYNTHESIS EXPANSION, MR RESCUE IMS III § Lack of vessel imaging in 2 trials at entry to confirm presence of targetvessel occlusion § Lack of penumbral imaging in 2 trails at entry to confirm presence of targetvessel occlusion § Prolonged interval between study entry SYNTHESIS EXPANSION and performance of endovascular reperfusion procedure § Use of early-generation devices with only modest reperfusion efficacy MR RESCUE – Devices successful 30% to 45% of the time. – IV t-PA successful 30% to 40% of the time. Broderick JP, et al. N Engl J Med. 2013; 368: 892 -903. Ciccone A, et al. N Engl J Med. 2013; 368: 904 -913. Kidwell CS, et al. N Engl J Med. 2013; 368: 914 -923.

Rationale for MR CLEAN, EXTEND-IA, ESCAPE, and SWIFT PRIME § Targeted patients with best chance of response to reperfusion – Proven major-vessel occlusion and – Salvageable tissue § Treat as fast as possible – No waiting to assess “t-PA failure” – Use the most effective device (stent retriever)

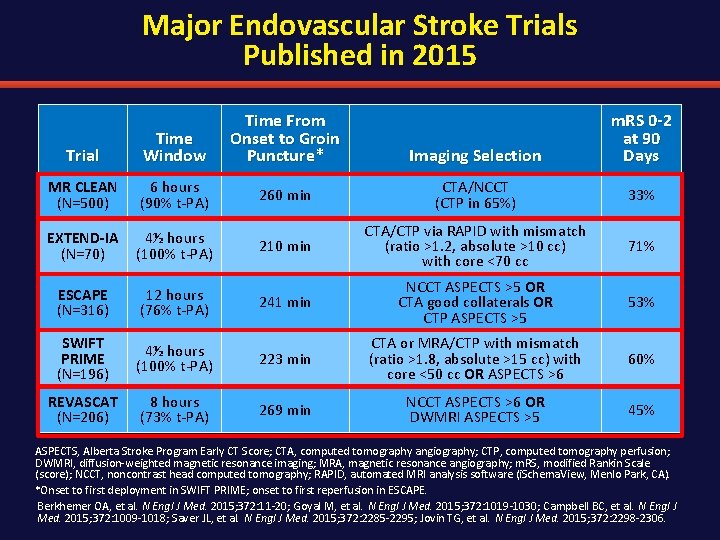
Major Endovascular Stroke Trials Published in 2015 Trial Time Window Time From Onset to Groin Puncture* Imaging Selection m. RS 0 -2 at 90 Days MR CLEAN (N=500) 6 hours (90% t-PA) 260 min CTA/NCCT (CTP in 65%) 33% EXTEND-IA (N=70) 4½ hours (100% t-PA) 210 min CTA/CTP via RAPID with mismatch (ratio >1. 2, absolute >10 cc) with core <70 cc 71% ESCAPE (N=316) 12 hours (76% t-PA) 241 min NCCT ASPECTS >5 OR CTA good collaterals OR CTP ASPECTS >5 53% SWIFT PRIME (N=196) 4½ hours (100% t-PA) 223 min CTA or MRA/CTP with mismatch (ratio >1. 8, absolute >15 cc) with core <50 cc OR ASPECTS >6 60% REVASCAT (N=206) 8 hours (73% t-PA) 269 min NCCT ASPECTS >6 OR DWMRI ASPECTS >5 45% ASPECTS, Alberta Stroke Program Early CT Score; CTA, computed tomography angiography; CTP, computed tomography perfusion; DWMRI, diffusion-weighted magnetic resonance imaging; MRA, magnetic resonance angiography; m. RS, modified Rankin Scale (score); NCCT, noncontrast head computed tomography; RAPID, automated MRI analysis software (i. Schema. View, Menlo Park, CA). *Onset to first deployment in SWIFT PRIME; onset to first reperfusion in ESCAPE. Berkhemer OA, et al. N Engl J Med. 2015; 372: 11 -20; Goyal M, et al. N Engl J Med. 2015; 372: 1019 -1030; Campbell BC, et al. N Engl J Med. 2015; 372: 1009 -1018; Saver JL, et al. N Engl J Med. 2015; 372: 2285 -2295; Jovin TG, et al. N Engl J Med. 2015; 372: 2298 -2306.

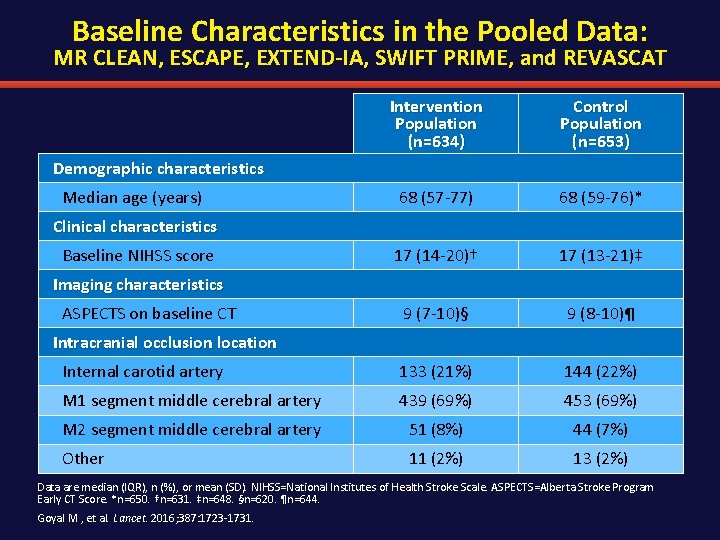
Baseline Characteristics in the Pooled Data: MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, and REVASCAT Intervention Population (n=634) Control Population (n=653) 68 (57 -77) 68 (59 -76)* 17 (14 -20)† 17 (13 -21)‡ 9 (7 -10)§ 9 (8 -10)¶ Internal carotid artery 133 (21%) 144 (22%) M 1 segment middle cerebral artery 439 (69%) 453 (69%) M 2 segment middle cerebral artery 51 (8%) 44 (7%) Other 11 (2%) 13 (2%) Demographic characteristics Median age (years) Clinical characteristics Baseline NIHSS score Imaging characteristics ASPECTS on baseline CT Intracranial occlusion location Data are median (IQR), n (%), or mean (SD). NIHSS=National Institutes of Health Stroke Scale. ASPECTS=Alberta Stroke Program Early CT Score. *n=650. †n=631. ‡n=648. §n=620. ¶n=644. Goyal M , et al. Lancet. 2016; 387: 1723 -1731.

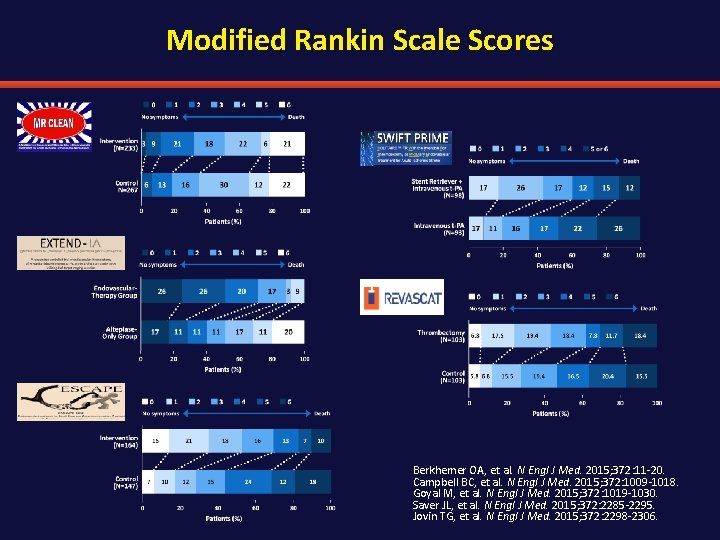
Modified Rankin Scale Scores Berkhemer OA, et al. N Engl J Med. 2015; 372: 11 -20. Campbell BC, et al. N Engl J Med. 2015; 372: 1009 -1018. Goyal M, et al. N Engl J Med. 2015; 372: 1019 -1030. Saver JL, et al. N Engl J Med. 2015; 372: 2285 -2295. Jovin TG, et al. N Engl J Med. 2015; 372: 2298 -2306.

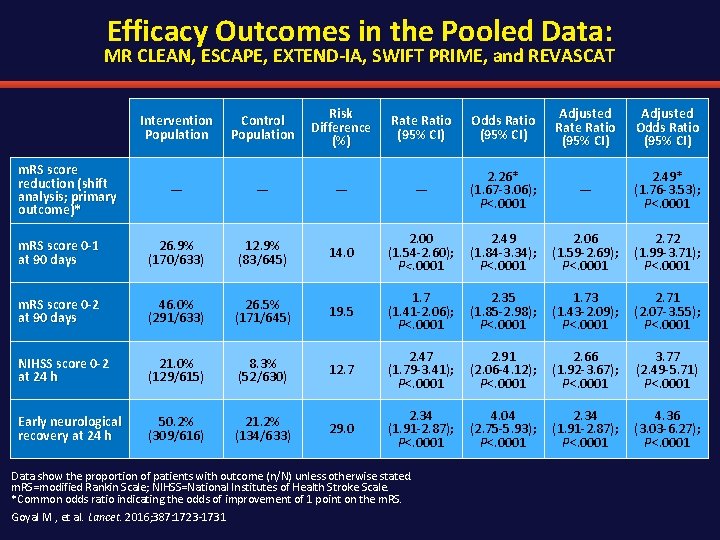
Efficacy Outcomes in the Pooled Data: MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME, and REVASCAT Intervention Population Control Population Risk Difference (%) Rate Ratio (95% CI) Odds Ratio (95% CI) Adjusted Rate Ratio (95% CI) Adjusted Odds Ratio (95% CI) — — 2. 26* (1. 67 -3. 06); P<. 0001 — 2. 49* (1. 76 -3. 53); P<. 0001 m. RS score 0 -1 at 90 days 26. 9% (170/633) 12. 9% (83/645) 14. 0 2. 00 (1. 54 -2. 60); P<. 0001 2. 49 (1. 84 -3. 34); P<. 0001 2. 06 (1. 59 -2. 69); P<. 0001 2. 72 (1. 99 -3. 71); P<. 0001 m. RS score 0 -2 at 90 days 46. 0% (291/633) 26. 5% (171/645) 19. 5 1. 7 (1. 41 -2. 06); P<. 0001 2. 35 (1. 85 -2. 98); P<. 0001 1. 73 (1. 43 -2. 09); P<. 0001 2. 71 (2. 07 -3. 55); P<. 0001 NIHSS score 0 -2 at 24 h 21. 0% (129/615) 8. 3% (52/630) 12. 7 2. 47 (1. 79 -3. 41); P<. 0001 2. 91 (2. 06 -4. 12); P<. 0001 2. 66 (1. 92 -3. 67); P<. 0001 3. 77 (2. 49 -5. 71) P<. 0001 Early neurological recovery at 24 h 50. 2% (309/616) 21. 2% (134/633) 29. 0 2. 34 (1. 91 -2. 87); P<. 0001 4. 04 (2. 75 -5. 93); P<. 0001 2. 34 (1. 91 -2. 87); P<. 0001 4. 36 (3. 03 -6. 27); P<. 0001 m. RS score reduction (shift analysis; primary outcome)* Data show the proportion of patients with outcome (n/N) unless otherwise stated. m. RS=modified Rankin Scale; NIHSS=National Institutes of Health Stroke Scale. *Common odds ratio indicating the odds of improvement of 1 point on the m. RS. Goyal M , et al. Lancet. 2016; 387: 1723 -1731

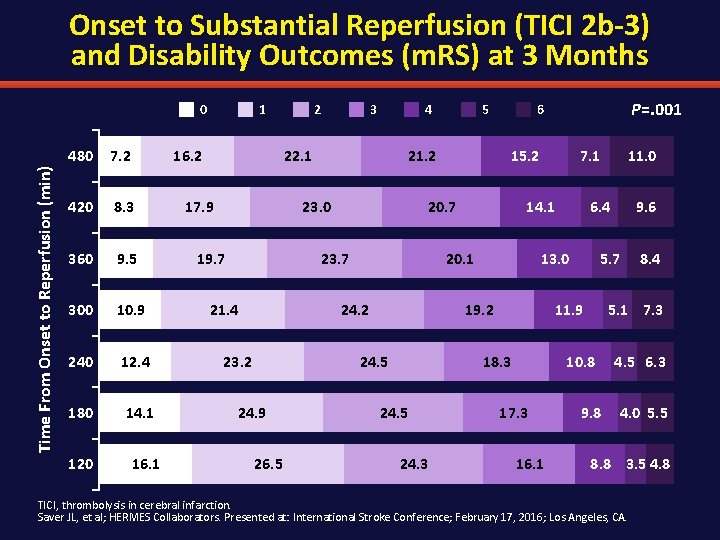
Onset to Substantial Reperfusion (TICI 2 b-3) and Disability Outcomes (m. RS) at 3 Months Time From Onset to Reperfusion (min) 0 480 7. 2 420 8. 3 360 9. 5 300 10. 9 240 12. 4 180 14. 1 120 1 16. 2 16. 1 2 3 4 22. 1 17. 9 21. 2 23. 0 19. 7 23. 2 26. 5 7. 1 14. 1 24. 5 24. 3 16. 1 8. 4 5. 1 7. 3 10. 8 17. 3 9. 6 5. 7 11. 9 18. 3 11. 0 6. 4 13. 0 19. 2 24. 5 24. 9 15. 2 20. 1 24. 2 P=. 001 6 20. 7 23. 7 21. 4 5 9. 8 4. 5 6. 3 4. 0 5. 5 8. 8 3. 5 4. 8 TICI, thrombolysis in cerebral infarction. Saver JL, et al; HERMES Collaborators. Presented at: International Stroke Conference; February 17, 2016; Los Angeles, CA.

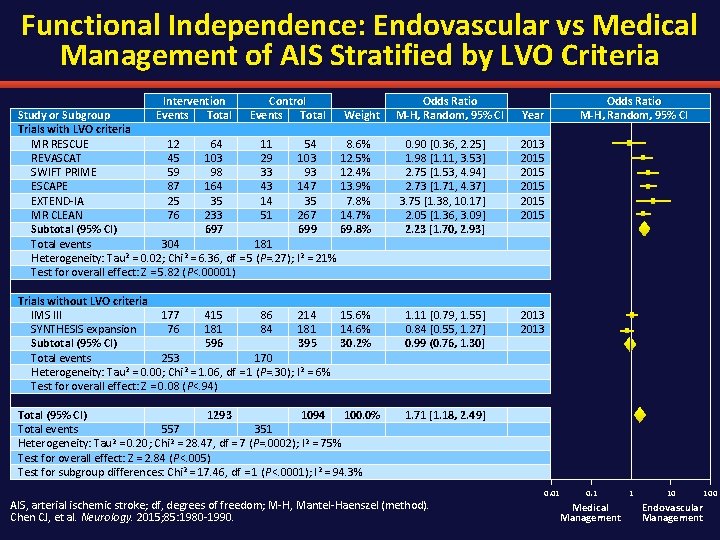
Functional Independence: Endovascular vs Medical Management of AIS Stratified by LVO Criteria Intervention Control Study or Subgroup Events Total Trials with LVO criteria MR RESCUE 12 64 11 54 REVASCAT 45 103 29 103 SWIFT PRIME 59 98 33 93 ESCAPE 87 164 43 147 EXTEND-IA 25 35 14 35 MR CLEAN 76 233 51 267 Subtotal (95% CI) 697 699 Total events 304 181 Heterogeneity: Tau 2 = 0. 02; Chi 2 = 6. 36, df = 5 (P=. 27); I 2 = 21% Test for overall effect: Z = 5. 82 (P<. 00001) Weight 8. 6% 12. 5% 12. 4% 13. 9% 7. 8% 14. 7% 69. 8% Trials without LVO criteria IMS III 177 415 86 214 15. 6% SYNTHESIS expansion 76 181 84 181 14. 6% Subtotal (95% CI) 596 395 30. 2% Total events 253 170 Heterogeneity: Tau 2 = 0. 00; Chi 2 = 1. 06, df = 1 (P=. 30); I 2 = 6% Test for overall effect: Z = 0. 08 (P<. 94) Total (95% CI) 1293 1094 100. 0% Total events 557 351 Heterogeneity: Tau 2 = 0. 20; Chi 2 = 28. 47, df = 7 (P=. 0002); I 2 = 75% Test for overall effect: Z = 2. 84 (P<. 005) Test for subgroup differences: Chi 2 = 17. 46, df = 1 (P<. 0001); I 2 = 94. 3% Odds Ratio M-H, Random, 95% CI Year 0. 90 [0. 36, 2. 25] 1. 98 [1. 11, 3. 53] 2. 75 [1. 53, 4. 94] 2. 73 [1. 71, 4. 37] 3. 75 [1. 38, 10. 17] 2. 05 [1. 36, 3. 09] 2. 23 [1. 70, 2. 93] 2013 2015 2015 1. 11 [0. 79, 1. 55] 0. 84 [0. 55, 1. 27] 0. 99 (0. 76, 1. 30] 2013 1. 71 [1. 18, 2. 49] AIS, arterial ischemic stroke; df, degrees of freedom; M-H, Mantel-Haenszel (method). Chen CJ, et al. Neurology. 2015; 85: 1980 -1990. 0. 01 0. 1 Medical Management 1 10 Endovascular Management 100

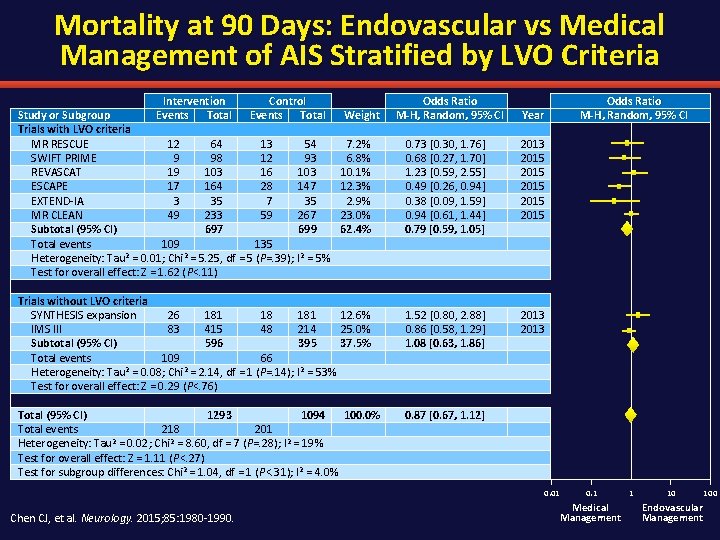
Mortality at 90 Days: Endovascular vs Medical Management of AIS Stratified by LVO Criteria Intervention Control Study or Subgroup Events Total Trials with LVO criteria MR RESCUE 12 64 13 54 SWIFT PRIME 9 98 12 93 REVASCAT 19 103 16 103 ESCAPE 17 164 28 147 EXTEND-IA 3 35 7 35 MR CLEAN 49 233 59 267 Subtotal (95% CI) 697 699 Total events 109 135 Heterogeneity: Tau 2 = 0. 01; Chi 2 = 5. 25, df = 5 (P=. 39); I 2 = 5% Test for overall effect: Z = 1. 62 (P<. 11) Weight 7. 2% 6. 8% 10. 1% 12. 3% 2. 9% 23. 0% 62. 4% Trials without LVO criteria SYNTHESIS expansion 26 181 18 181 12. 6% IMS III 83 415 48 214 25. 0% Subtotal (95% CI) 596 395 37. 5% Total events 109 66 Heterogeneity: Tau 2 = 0. 08; Chi 2 = 2. 14, df = 1 (P=. 14); I 2 = 53% Test for overall effect: Z = 0. 29 (P<. 76) Total (95% CI) 1293 1094 100. 0% Total events 218 201 Heterogeneity: Tau 2 = 0. 02; Chi 2 = 8. 60, df = 7 (P=. 28); I 2 = 19% Test for overall effect: Z = 1. 11 (P<. 27) Test for subgroup differences: Chi 2 = 1. 04, df = 1 (P<. 31); I 2 = 4. 0% Odds Ratio M-H, Random, 95% CI Year 0. 73 [0. 30, 1. 76] 0. 68 [0. 27, 1. 70] 1. 23 [0. 59, 2. 55] 0. 49 [0. 26, 0. 94] 0. 38 [0. 09, 1. 59] 0. 94 [0. 61, 1. 44] 0. 79 [0. 59, 1. 05] 2013 2015 2015 1. 52 [0. 80, 2. 88] 0. 86 [0. 58, 1. 29] 1. 08 [0. 63, 1. 86] 2013 0. 87 [0. 67, 1. 12] 0. 01 Chen CJ, et al. Neurology. 2015; 85: 1980 -1990. 0. 1 Medical Management 1 10 Endovascular Management 100

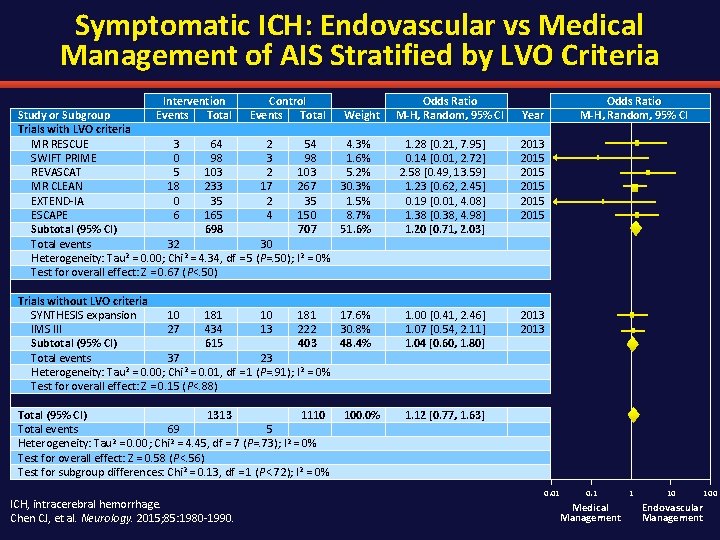
Symptomatic ICH: Endovascular vs Medical Management of AIS Stratified by LVO Criteria Intervention Control Study or Subgroup Events Total Weight Trials with LVO criteria MR RESCUE 3 64 2 54 4. 3% SWIFT PRIME 0 98 3 98 1. 6% REVASCAT 5 103 2 103 5. 2% MR CLEAN 18 233 17 267 30. 3% EXTEND-IA 0 35 2 35 1. 5% ESCAPE 6 165 4 150 8. 7% Subtotal (95% CI) 698 707 51. 6% Total events 32 30 Heterogeneity: Tau 2 = 0. 00; Chi 2 = 4. 34, df = 5 (P=. 50); I 2 = 0% Test for overall effect: Z = 0. 67 (P<. 50) Trials without LVO criteria SYNTHESIS expansion 10 181 17. 6% IMS III 27 434 13 222 30. 8% Subtotal (95% CI) 615 403 48. 4% Total events 37 23 Heterogeneity: Tau 2 = 0. 00; Chi 2 = 0. 01, df = 1 (P=. 91); I 2 = 0% Test for overall effect: Z = 0. 15 (P<. 88) Total (95% CI) 1313 1110 Total events 69 5 Heterogeneity: Tau 2 = 0. 00; Chi 2 = 4. 45, df = 7 (P=. 73); I 2 = 0% Test for overall effect: Z = 0. 58 (P<. 56) Test for subgroup differences: Chi 2 = 0. 13, df = 1 (P<. 72); I 2 = 0% ICH, intracerebral hemorrhage. Chen CJ, et al. Neurology. 2015; 85: 1980 -1990. 100. 0% Odds Ratio M-H, Random, 95% CI Year 1. 28 [0. 21, 7. 95] 0. 14 [0. 01, 2. 72] 2. 58 [0. 49, 13. 59] 1. 23 [0. 62, 2. 45] 0. 19 [0. 01, 4. 08] 1. 38 [0. 38, 4. 98] 1. 20 [0. 71, 2. 03] 2013 2015 2015 1. 00 [0. 41, 2. 46] 1. 07 [0. 54, 2. 11] 1. 04 [0. 60, 1. 80] 2013 1. 12 [0. 77, 1. 63] 0. 01 0. 1 Medical Management 1 10 Endovascular Management 100

Outline § Introductions § Overview of acute stroke § Evidence for endovascular therapy for ischemic stroke § Impact of emergency management of acute ischemic stroke – Imaging—what do we absolutely need? – Prehospital triage • Nursing implications – Interhospital triage § Conclusions and Future Directions

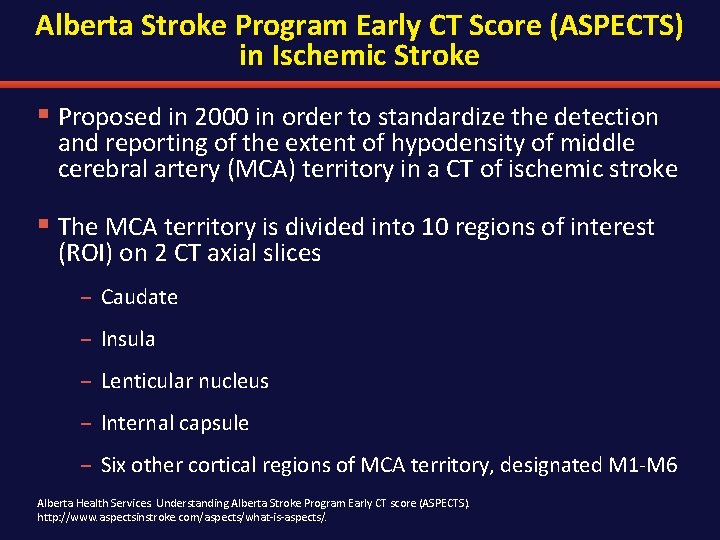
Alberta Stroke Program Early CT Score (ASPECTS) in Ischemic Stroke § Proposed in 2000 in order to standardize the detection and reporting of the extent of hypodensity of middle cerebral artery (MCA) territory in a CT of ischemic stroke § The MCA territory is divided into 10 regions of interest (ROI) on 2 CT axial slices – Caudate – Insula – Lenticular nucleus – Internal capsule – Six other cortical regions of MCA territory, designated M 1 -M 6 Alberta Health Services. Understanding Alberta Stroke Program Early CT score (ASPECTS). http: //www. aspectsinstroke. com/aspects/what-is-aspects/.

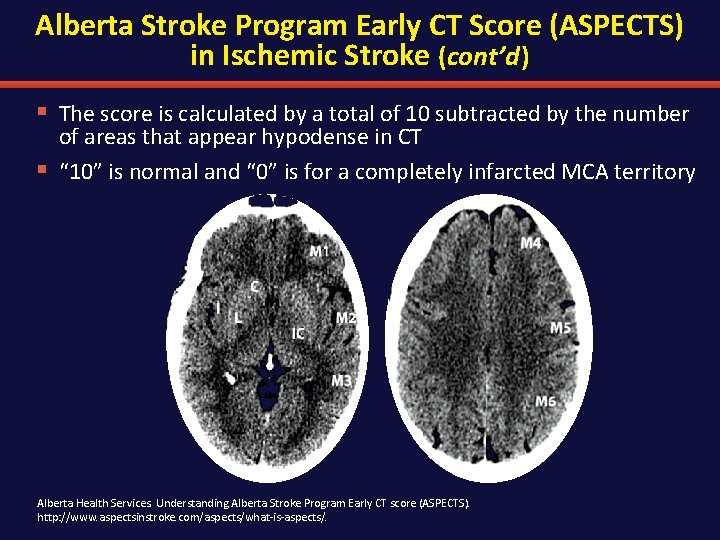
Alberta Stroke Program Early CT Score (ASPECTS) in Ischemic Stroke (cont’d ) § The score is calculated by a total of 10 subtracted by the number of areas that appear hypodense in CT § “ 10” is normal and “ 0” is for a completely infarcted MCA territory Alberta Health Services. Understanding Alberta Stroke Program Early CT score (ASPECTS). http: //www. aspectsinstroke. com/aspects/what-is-aspects/.

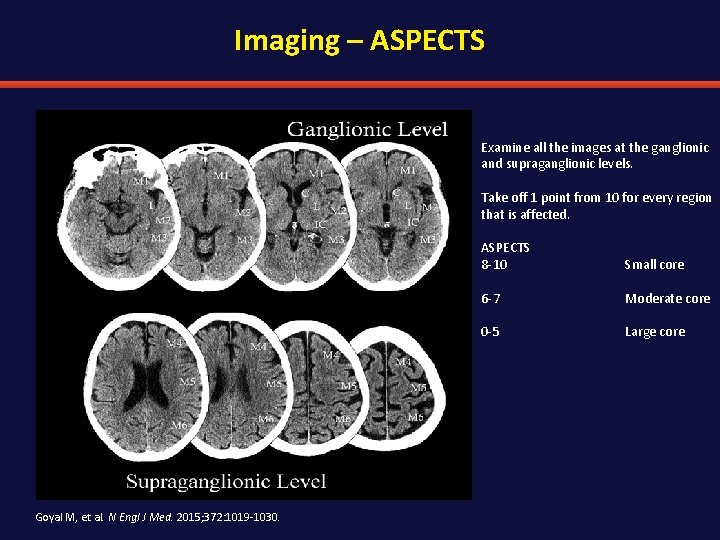
Imaging – ASPECTS Examine all the images at the ganglionic and supraganglionic levels. Take off 1 point from 10 for every region that is affected. ASPECTS 8 -10 Small core 6 -7 Moderate core 0 -5 Large core Goyal M, et al. N Engl J Med. 2015; 372: 1019 -1030.

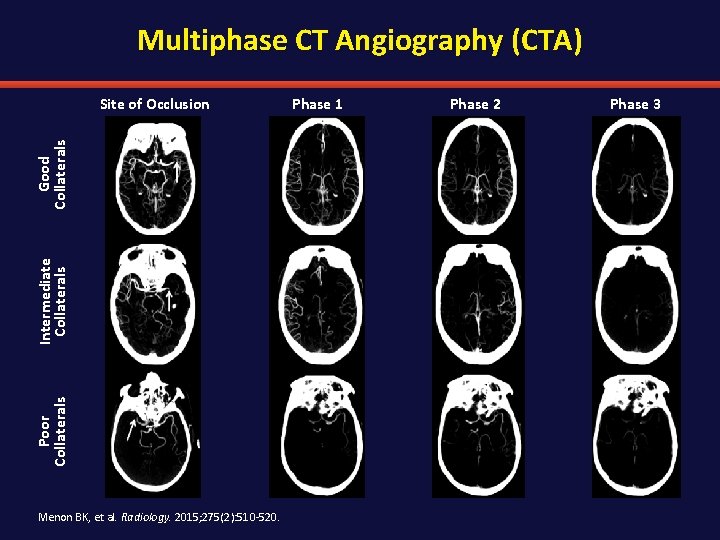
Multiphase CT Angiography (CTA) Poor Collaterals Intermediate Collaterals Good Collaterals Site of Occlusion Menon BK, et al. Radiology. 2015; 275(2): 510 -520. Phase 1 Phase 2 Phase 3

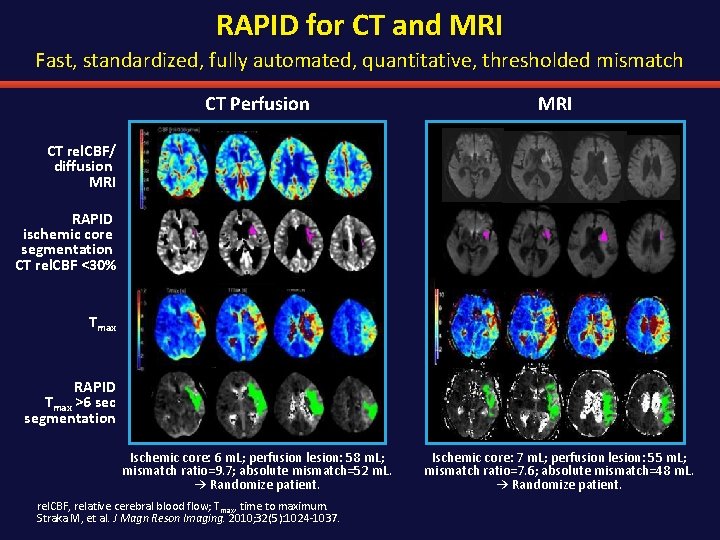
RAPID for CT and MRI Fast, standardized, fully automated, quantitative, thresholded mismatch CT Perfusion MRI Ischemic core: 6 m. L; perfusion lesion: 58 m. L; mismatch ratio=9. 7; absolute mismatch=52 m. L. → Randomize patient. Ischemic core: 7 m. L; perfusion lesion: 55 m. L; mismatch ratio=7. 6; absolute mismatch=48 m. L. → Randomize patient. CT rel. CBF/ diffusion MRI RAPID ischemic core segmentation CT rel. CBF <30% Tmax RAPID Tmax >6 sec segmentation rel. CBF, relative cerebral blood flow; Tmax, time to maximum. Straka M, et al. J Magn Reson Imaging. 2010; 32(5): 1024 -1037.

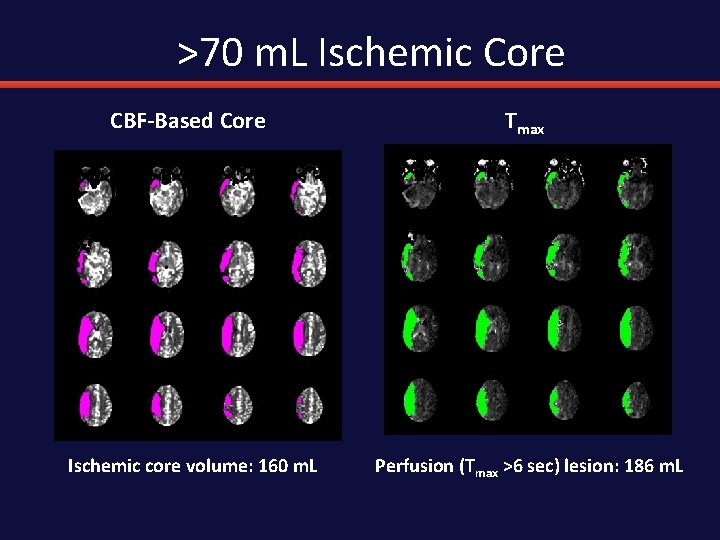
>70 m. L Ischemic Core CBF-Based Core Tmax Ischemic core volume: 160 m. L Perfusion (Tmax >6 sec) lesion: 186 m. L

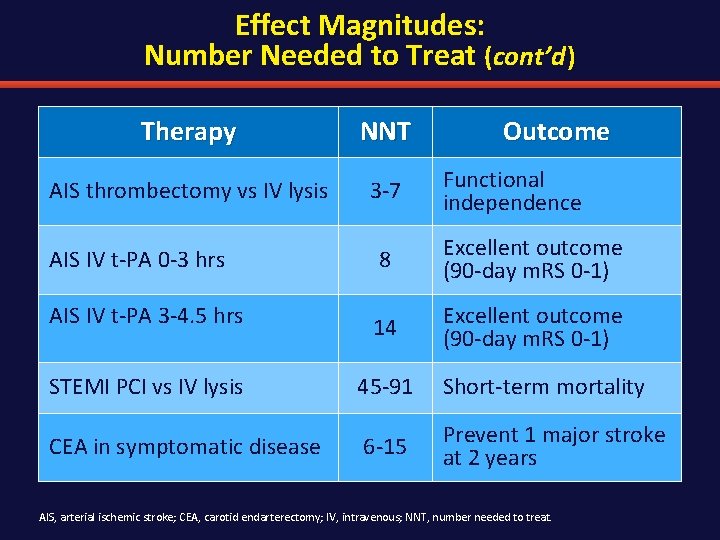
Effect Magnitudes: Number Needed to Treat (NNT) In order to have 1 additional stroke patient be independent: MR CLEAN ESCAPE EXTEND-IA SWIFT PRIME Primary PCI vs systemic thrombolysis for STEMI— prevention of MI/stroke/death: MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST segment elevation myocardial infarction.

Effect Magnitudes: Number Needed to Treat (cont’d ) Therapy NNT AIS thrombectomy vs IV lysis 3 -7 Outcome Functional independence AIS IV t-PA 0 -3 hrs 8 Excellent outcome (90 -day m. RS 0 -1) AIS IV t-PA 3 -4. 5 hrs 14 Excellent outcome (90 -day m. RS 0 -1) STEMI PCI vs IV lysis 45 -91 Short-term mortality CEA in symptomatic disease 6 -15 Prevent 1 major stroke at 2 years AIS, arterial ischemic stroke; CEA, carotid endarterectomy; IV, intravenous; NNT, number needed to treat.

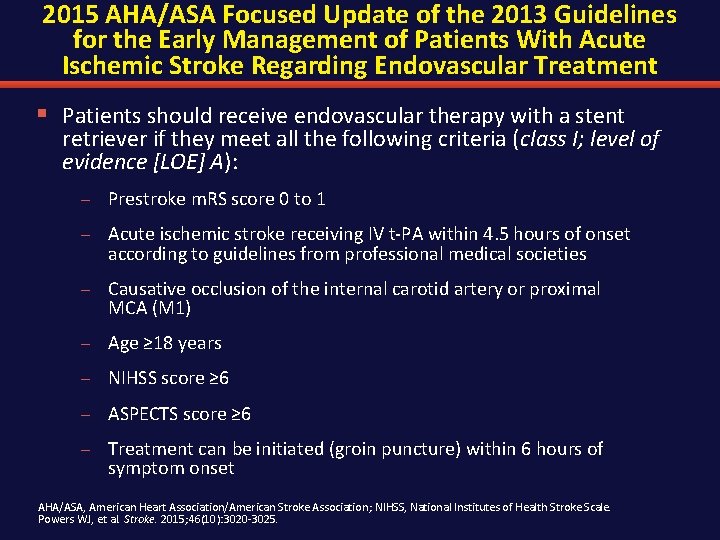
2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment § Patients should receive endovascular therapy with a stent retriever if they meet all the following criteria (class I; level of evidence [LOE] A): – Prestroke m. RS score 0 to 1 – Acute ischemic stroke receiving IV t-PA within 4. 5 hours of onset according to guidelines from professional medical societies – Causative occlusion of the internal carotid artery or proximal MCA (M 1) – Age ≥ 18 years – NIHSS score ≥ 6 – ASPECTS score ≥ 6 – Treatment can be initiated (groin puncture) within 6 hours of symptom onset AHA/ASA, American Heart Association/American Stroke Association; NIHSS, National Institutes of Health Stroke Scale. Powers WJ, et al. Stroke. 2015; 46(10): 3020 -3025.

2015 AHA/ASA Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment (cont’d ) § If endovascular therapy is contemplated, a noninvasive intracranial vascular (ICV) study is strongly recommended during the initial imaging evaluation of the acute stroke patient, but it should not delay IV t-PA if indicated § For patients who qualify for IV t-PA according to guidelines from professional medical societies, initiating IV t-PA before noninvasive vascular imaging is recommended in patients who have not had noninvasive vascular imaging as part of their initial imaging assessment for stroke − Noninvasive ICV imaging should then be obtained as quickly as possible Class I; LOE A. Powers WJ, et al. Stroke. 2015; 46(10): 3020 -3025.

Outline § Introductions § Overview of acute stroke § Evidence for endovascular therapy for ischemic stroke § Impact of emergency management of acute ischemic stroke – Imaging—what do we absolutely need? – Prehospital triage • Nursing implications – Interhospital triage § Conclusions and Future Directions

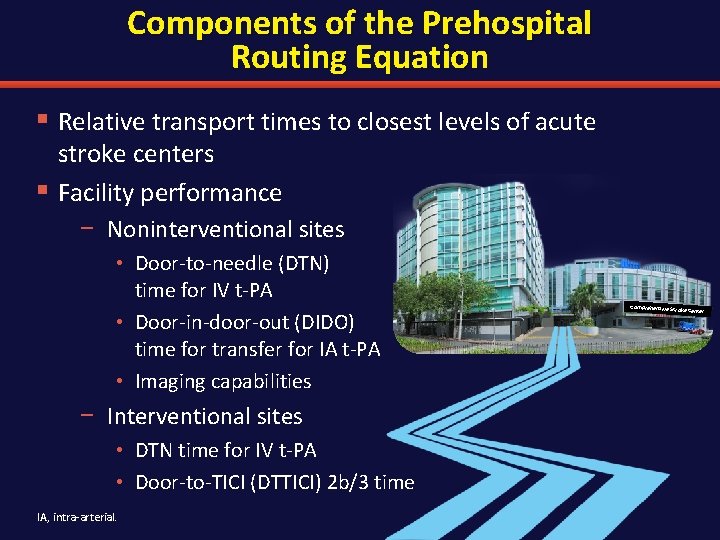
Components of the Prehospital Routing Equation § Relative transport times to closest levels of acute stroke centers § Facility performance − Noninterventional sites • Door-to-needle (DTN) time for IV t-PA • Door-in-door-out (DIDO) time for transfer for IA t-PA • Imaging capabilities − Interventional sites • DTN time for IV t-PA • Door-to-TICI (DTTICI) 2 b/3 time IA, intra-arterial. Comprehensive Str oke Center

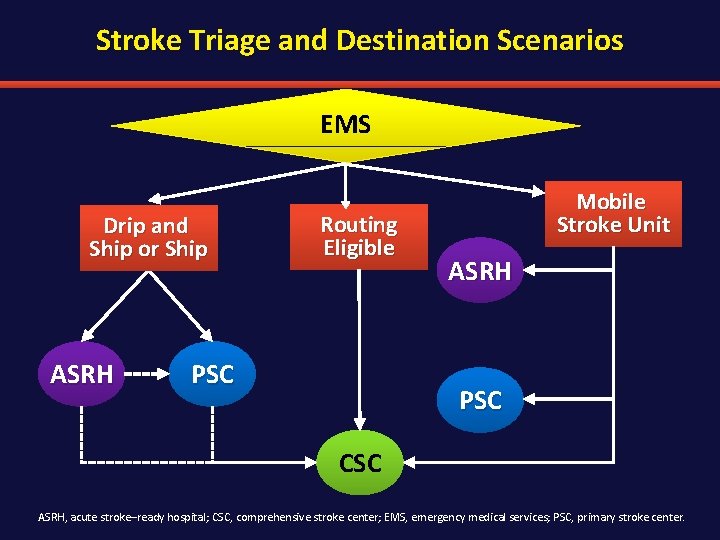
Stroke Triage and Destination Scenarios EMS Drip and Ship or Ship ASRH Routing Eligible PSC Mobile Stroke Unit ASRH PSC CSC ASRH, acute stroke–ready hospital; CSC, comprehensive stroke center; EMS, emergency medical services; PSC, primary stroke center.

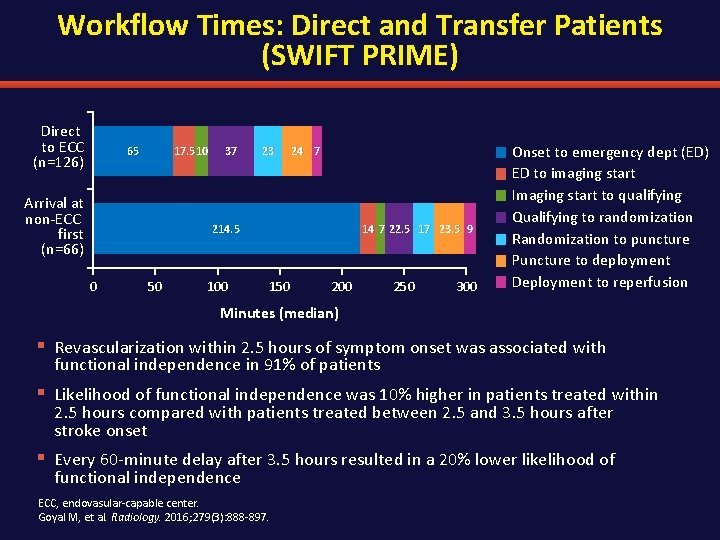
Workflow Times: Direct and Transfer Patients (SWIFT PRIME) Direct to ECC (n=126) 65 17. 5 10 Arrival at non-ECC first (n=66) 37 23 24 7 214. 5 0 50 100 14 7 22. 5 17 23. 5 9 150 200 250 300 Onset to emergency dept (ED) ED to imaging start Imaging start to qualifying Qualifying to randomization Randomization to puncture Puncture to deployment Deployment to reperfusion Minutes (median) § Revascularization within 2. 5 hours of symptom onset was associated with functional independence in 91% of patients § Likelihood of functional independence was 10% higher in patients treated within 2. 5 hours compared with patients treated between 2. 5 and 3. 5 hours after stroke onset § Every 60 -minute delay after 3. 5 hours resulted in a 20% lower likelihood of functional independence ECC, endovasular-capable center. Goyal M, et al. Radiology. 2016; 279(3): 888 -897.

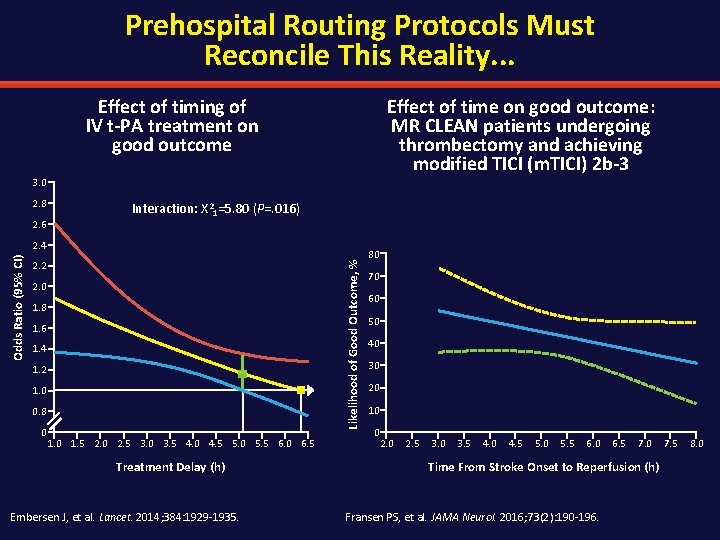
Prehospital Routing Protocols Must Reconcile This Reality. . . Effect of timing of IV t-PA treatment on good outcome Effect of time on good outcome: MR CLEAN patients undergoing thrombectomy and achieving modified TICI (m. TICI) 2 b-3 3. 0 2. 8 2. 6 Interaction: Х 21=5. 80 (P=. 016) 2. 2 Likelihood of Good Outcome, % Odds Ratio (95% CI) 2. 4 2. 0 1. 8 1. 6 1. 4 1. 2 1. 0 0. 8 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 4. 5 5. 0 5. 5 6. 0 6. 5 Treatment Delay (h) Embersen J, et al. Lancet. 2014; 384: 1929 -1935. 80 70 60 50 40 30 20 10 0 2. 5 3. 0 3. 5 4. 0 4. 5 5. 0 5. 5 6. 0 6. 5 7. 0 Time From Stroke Onset to Reperfusion (h) Fransen PS, et al. JAMA Neurol. 2016; 73(2): 190 -196. 7. 5 8. 0

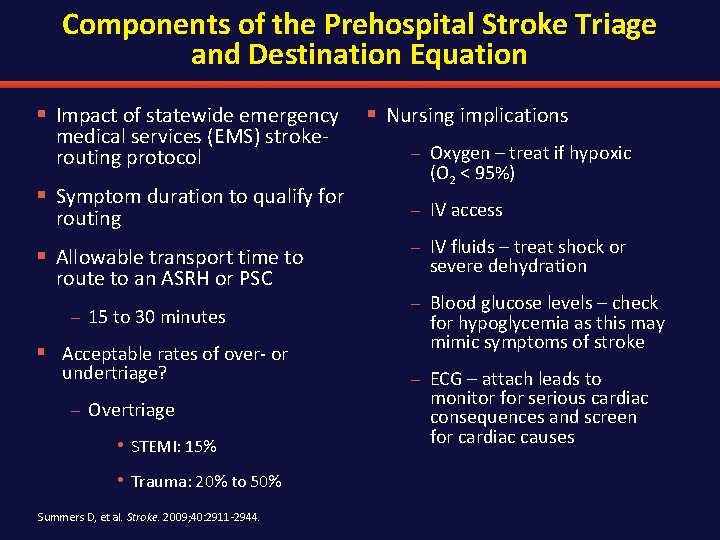
Components of the Prehospital Stroke Triage and Destination Equation § Impact of statewide emergency § Nursing implications medical services (EMS) strokerouting protocol § Symptom duration to qualify for routing § Allowable transport time to route to an ASRH or PSC – 15 to 30 minutes § Acceptable rates of over- or undertriage? – Overtriage • STEMI: 15% • Trauma: 20% to 50% Summers D, et al. Stroke. 2009; 40: 2911 -2944. – Oxygen – treat if hypoxic (O 2 < 95%) – IV access – IV fluids – treat shock or severe dehydration – Blood glucose levels – check for hypoglycemia as this may mimic symptoms of stroke – ECG – attach leads to monitor for serious cardiac consequences and screen for cardiac causes

Number Needed to Route (NNR) § Retrospective review – Polk County, Florida § Stroke severity–adjusted EMS triage § For every 8 presumed acute severe strokes bypassed to a CSC, 1 received a neurosurgical or endovascular intervention not available at the PSC Allen E, et al. Stroke. 2014; 45: A 213.

Components of the Prehospital Stroke Triage and Destination Equation (cont’d) § Dispatch accuracy § Last known well/symptom duration (agreement with hospital? ) – 0 to 3. 5 h; 3. 6 to 7 h; >7 h; wake-up stroke § Qualitative stroke-detection screen and/or quantitative stroke-severity measurement/scale – Validity, reliability, and predictive values § Pre-event level of function/disability? § Anticoagulation status? § Recent surgery? § Patient/family preference § If MSU, identification of ICH or LVO MSU, mobile stroke unit.

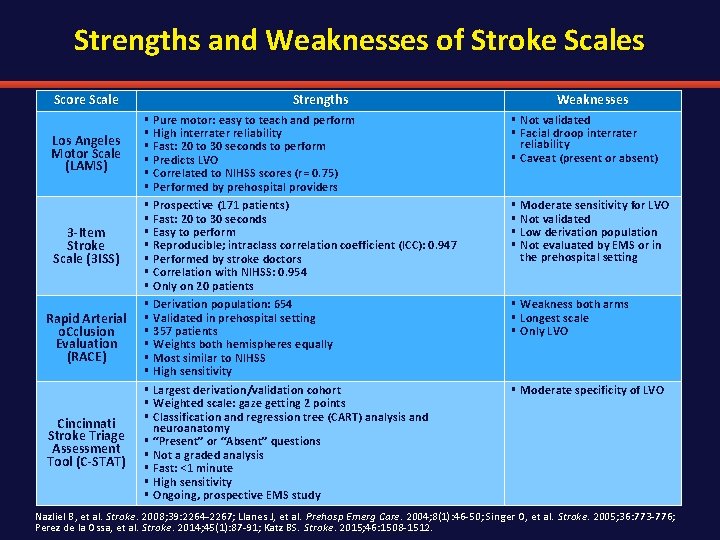
Strengths and Weaknesses of Stroke Scales Score Scale Strengths Los Angeles Motor Scale (LAMS) § § § 3 -Item Stroke Scale (3 ISS) § § § § Rapid Arterial o. Cclusion Evaluation (RACE) § § § Cincinnati Stroke Triage Assessment Tool (C-STAT) § § § § Pure motor: easy to teach and perform High interrater reliability Fast: 20 to 30 seconds to perform Predicts LVO Correlated to NIHSS scores (r = 0. 75) Performed by prehospital providers Prospective (171 patients) Fast: 20 to 30 seconds Easy to perform Reproducible; intraclass correlation coefficient (ICC): 0. 947 Performed by stroke doctors Correlation with NIHSS: 0. 954 Only on 20 patients Derivation population: 654 Validated in prehospital setting 357 patients Weights both hemispheres equally Most similar to NIHSS High sensitivity Largest derivation/validation cohort Weighted scale: gaze getting 2 points Classification and regression tree (CART) analysis and neuroanatomy “Present” or “Absent” questions Not a graded analysis Fast: <1 minute High sensitivity Ongoing, prospective EMS study Weaknesses § Not validated § Facial droop interrater reliability § Caveat (present or absent) § § Moderate sensitivity for LVO Not validated Low derivation population Not evaluated by EMS or in the prehospital setting § Weakness both arms § Longest scale § Only LVO § Moderate specificity of LVO Nazliel B, et al. Stroke. 2008; 39: 2264 -2267; Llanes J, et al. Prehosp Emerg Care. 2004; 8(1): 46 -50; Singer O, et al. Stroke. 2005; 36: 773 -776; Perez de la Ossa, et al. Stroke. 2014; 45(1): 87 -91; Katz BS. Stroke. 2015; 46: 1508 -1512.

Components of the Between-Hospital Stroke Triage Equation § Imaging at the noninterventional center – Who / What / Where • Centralized vs noncentralized imaging interpretation and/or viewing capability • Standardization § Telestroke – Independent challenges • Nonagreement among interventionalists regarding required imaging and overall candidacy Ø Posterior circulation Ø Wake-up strokes – Well coordinated with the destination interventional center is optimal

What Imaging? Guidance from the Guidelines § Canadian guidelines – All suspected acute strokes within intervention window • Noncontrast head computed tomography (NCCT) scan • Extracranial and intracranial CTA – Eligible for treatment • Computed tomography perfusion (CTP) or dynamic CTA (initial imaging if no substantial delay) mechanical thrombectomy § AHA/ASA guidelines – If endovascular therapy is contemplated, a noninvasive intracranial vascular (ICV) study is strongly recommended during the initial imaging evaluation of the acute stroke patient, but it should not delay IV t-PA if indicated – Benefits of CTP for making endovascular therapy decisions are “unknown” § European Stroke Organisation (ESO) guidelines – Intracranial vessel occlusion must be diagnosed with noninvasive imaging whenever possible before considering treatment with mechanical thrombectomy Powers WJ, et al. Stroke. 2015; 46: 3020 -3035. Casaubon LK, et al. Int J Stroke. 2015; 10: 924 -940.

What it all means. . § Call fast – Increase public awareness to better recognize signs/symptoms § EMS is part of the team – Educate EMS on field stroke severity assessments – Review levels of AIS treatment available at community, primary, comprehensive stroke centers § Nursing – Timely neurological assessments – Fluid and nutrition management – IV access § Faster treatment times – Decreased door-to-CT times – Decreased door-to-needle times

Questions?