FREE RADICAL REACTIONS 9292020 Dr Mohammad Nahid Siddiqui



- Slides: 62

FREE RADICAL REACTIONS 9/29/2020 Dr. Mohammad Nahid Siddiqui 1

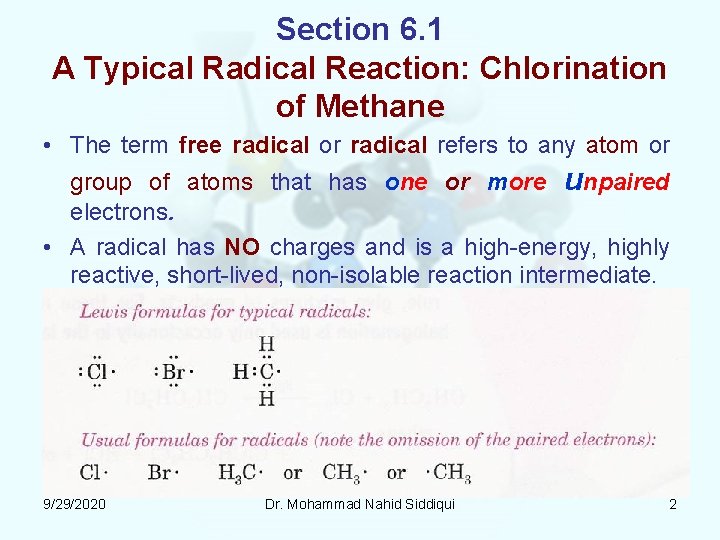
Section 6. 1 A Typical Radical Reaction: Chlorination of Methane • The term free radical or radical refers to any atom or group of atoms that has one or more unpaired electrons. • A radical has NO charges and is a high energy, highly reactive, short lived, non isolable reaction intermediate. 9/29/2020 Dr. Mohammad Nahid Siddiqui 2

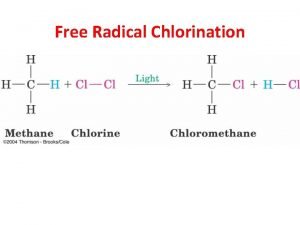
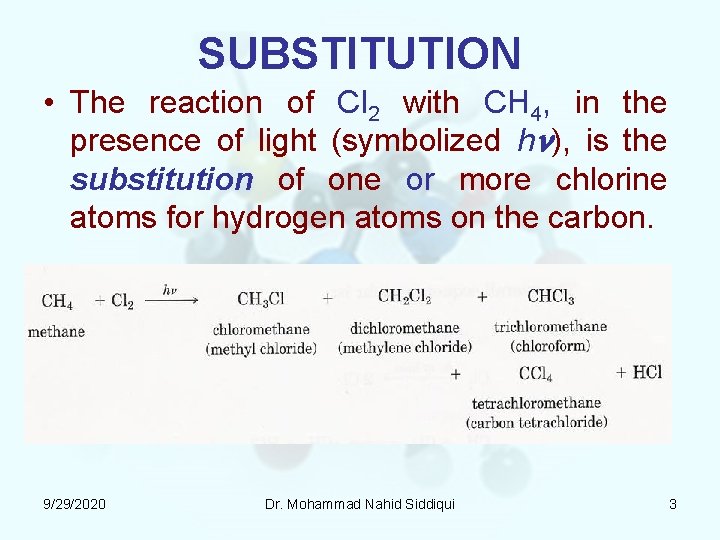
SUBSTITUTION • The reaction of Cl 2 with CH 4, in the presence of light (symbolized hn), is the substitution of one or more chlorine atoms for hydrogen atoms on the carbon. 9/29/2020 Dr. Mohammad Nahid Siddiqui 3

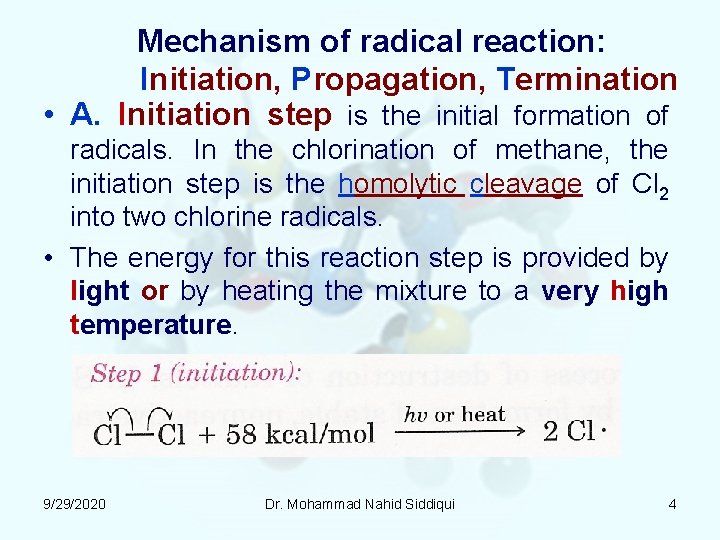
Mechanism of radical reaction: Initiation, Propagation, Termination • A. Initiation step is the initial formation of radicals. In the chlorination of methane, the initiation step is the homolytic cleavage of Cl 2 into two chlorine radicals. • The energy for this reaction step is provided by light or by heating the mixture to a very high temperature. 9/29/2020 Dr. Mohammad Nahid Siddiqui 4

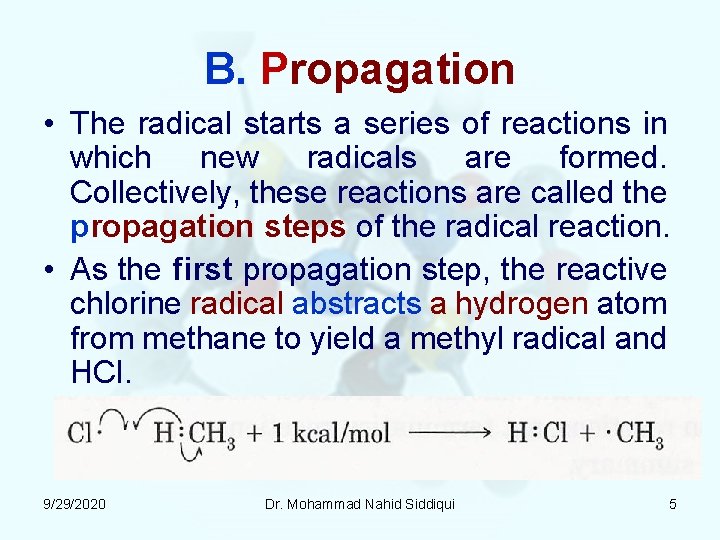
B. Propagation • The radical starts a series of reactions in which new radicals are formed. Collectively, these reactions are called the propagation steps of the radical reaction. • As the first propagation step, the reactive chlorine radical abstracts a hydrogen atom from methane to yield a methyl radical and HCl. 9/29/2020 Dr. Mohammad Nahid Siddiqui 5

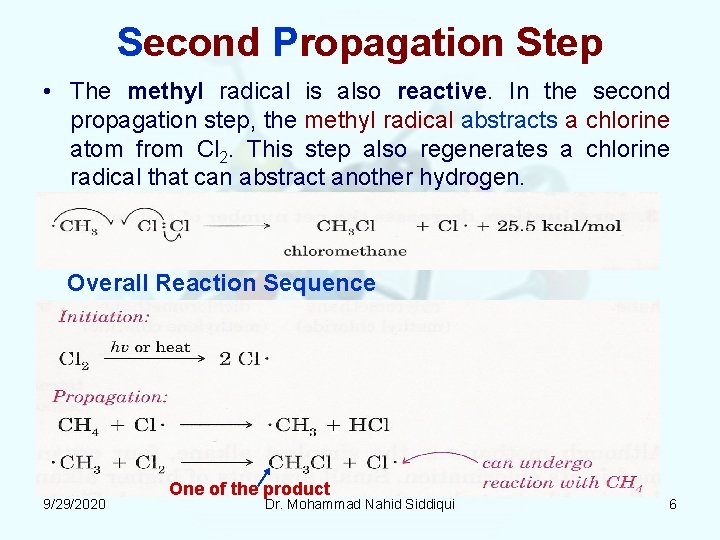
Second Propagation Step • The methyl radical is also reactive. In the second propagation step, the methyl radical abstracts a chlorine atom from Cl 2. This step also regenerates a chlorine radical that can abstract another hydrogen. Overall Reaction Sequence 9/29/2020 One of the product Dr. Mohammad Nahid Siddiqui 6

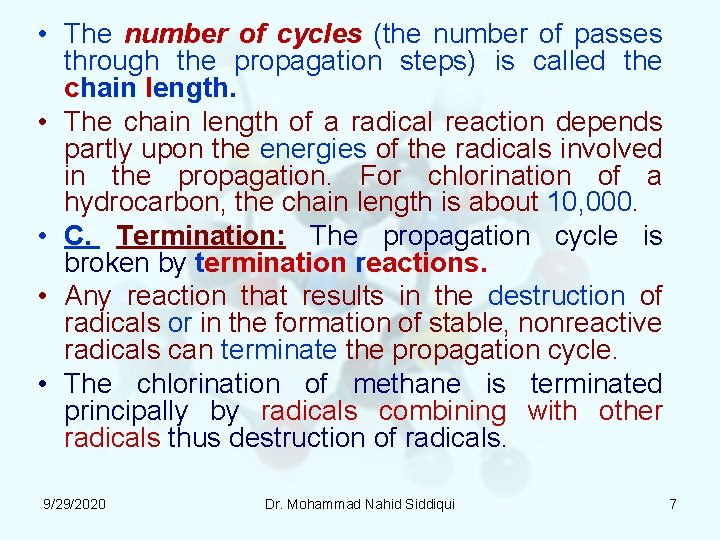
• The number of cycles (the number of passes through the propagation steps) is called the chain length. • The chain length of a radical reaction depends partly upon the energies of the radicals involved in the propagation. For chlorination of a hydrocarbon, the chain length is about 10, 000. • C. Termination: The propagation cycle is broken by termination reactions. • Any reaction that results in the destruction of radicals or in the formation of stable, nonreactive radicals can terminate the propagation cycle. • The chlorination of methane is terminated principally by radicals combining with other radicals thus destruction of radicals. 9/29/2020 Dr. Mohammad Nahid Siddiqui 7

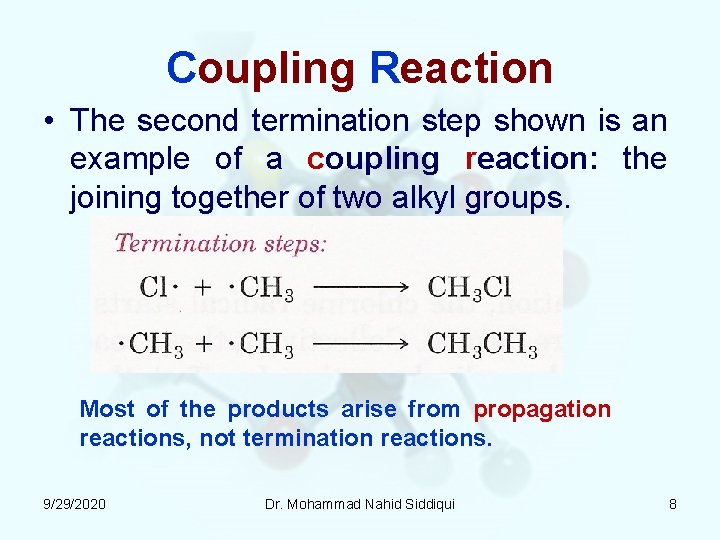
Coupling Reaction • The second termination step shown is an example of a coupling reaction: the joining together of two alkyl groups. Most of the products arise from propagation reactions, not termination reactions. 9/29/2020 Dr. Mohammad Nahid Siddiqui 8

Summary • • • initiation: increases the net number of radicals propagation: leaves the net number of radicals unchanged termination: decreases the net number of radicals. 9/29/2020 Dr. Mohammad Nahid Siddiqui 9

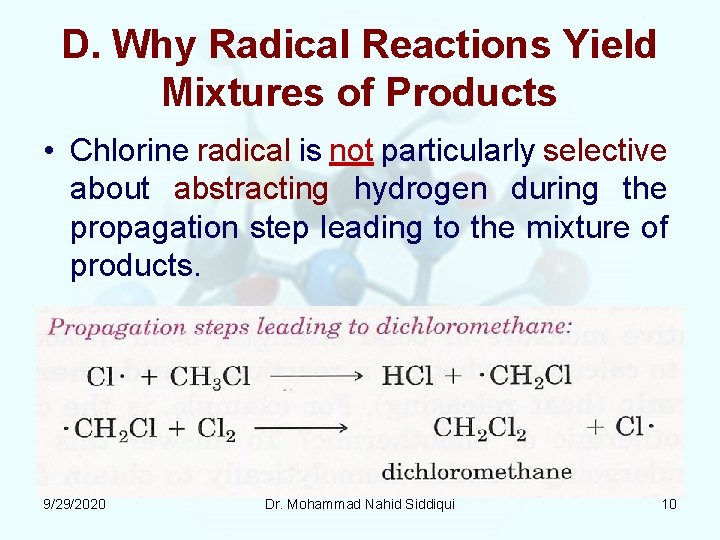
D. Why Radical Reactions Yield Mixtures of Products • Chlorine radical is not particularly selective about abstracting hydrogen during the propagation step leading to the mixture of products. 9/29/2020 Dr. Mohammad Nahid Siddiqui 10

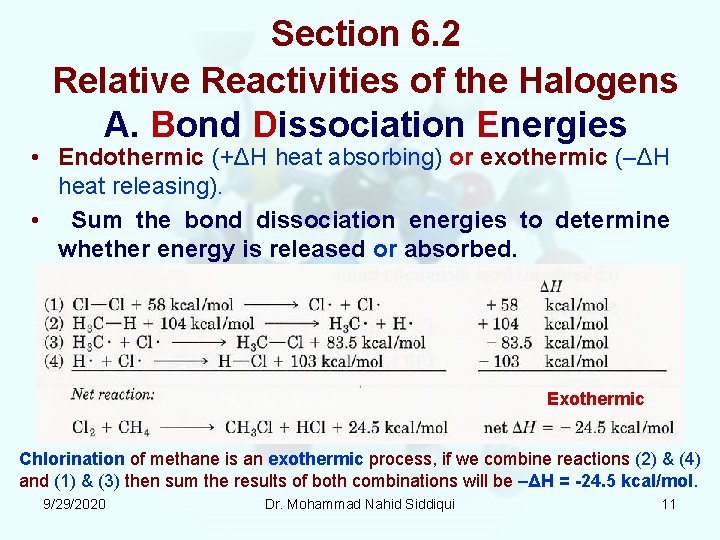
Section 6. 2 Relative Reactivities of the Halogens A. Bond Dissociation Energies • Endothermic (+ΔH heat absorbing) or exothermic (–ΔH heat releasing). • Sum the bond dissociation energies to determine whether energy is released or absorbed. Exothermic Chlorination of methane is an exothermic process, if we combine reactions (2) & (4) and (1) & (3) then sum the results of both combinations will be –ΔH = 24. 5 kcal/mol. 9/29/2020 Dr. Mohammad Nahid Siddiqui 11

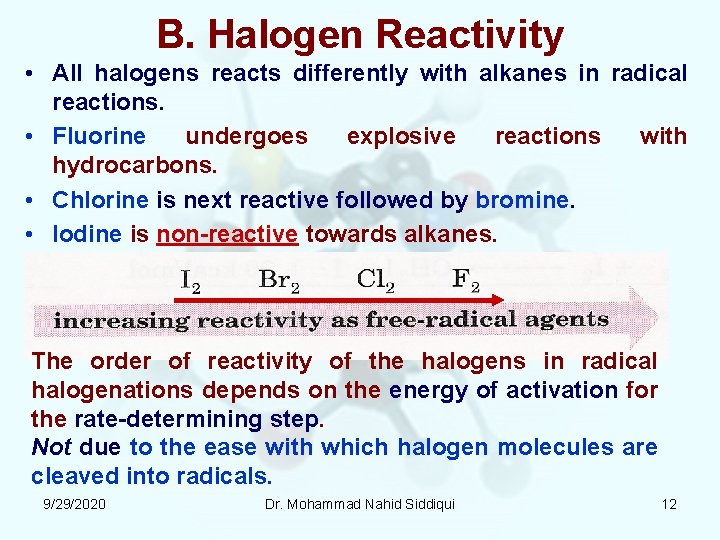
B. Halogen Reactivity • All halogens reacts differently with alkanes in radical reactions. • Fluorine undergoes explosive reactions with hydrocarbons. • Chlorine is next reactive followed by bromine. • Iodine is non reactive towards alkanes. The order of reactivity of the halogens in radical halogenations depends on the energy of activation for the rate determining step. Not due to the ease with which halogen molecules are cleaved into radicals. 9/29/2020 Dr. Mohammad Nahid Siddiqui 12

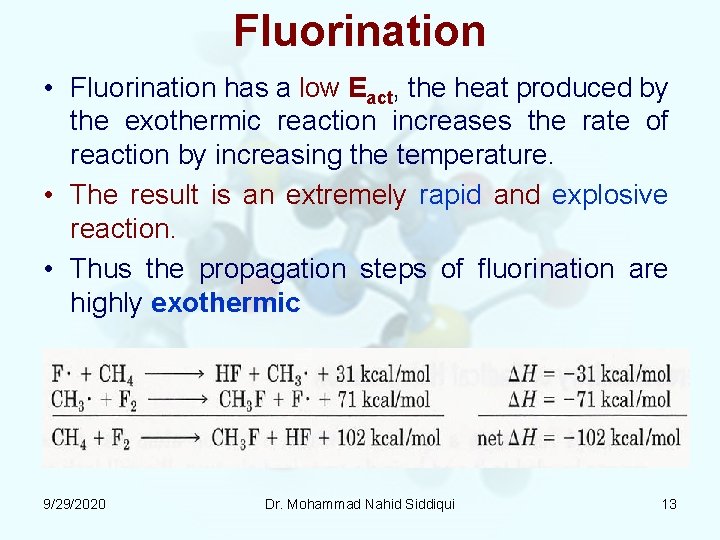
Fluorination • Fluorination has a low Eact, the heat produced by the exothermic reaction increases the rate of reaction by increasing the temperature. • The result is an extremely rapid and explosive reaction. • Thus the propagation steps of fluorination are highly exothermic 9/29/2020 Dr. Mohammad Nahid Siddiqui 13

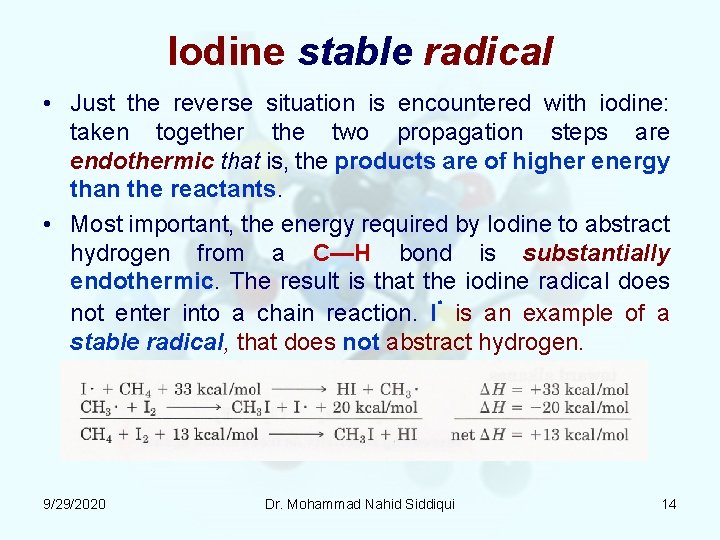
Iodine stable radical • Just the reverse situation is encountered with iodine: taken together the two propagation steps are endothermic that is, the products are of higher energy than the reactants. • Most important, the energy required by Iodine to abstract hydrogen from a C—H bond is substantially endothermic. The result is that the iodine radical does not enter into a chain reaction. I is an example of a stable radical, that does not abstract hydrogen. 9/29/2020 Dr. Mohammad Nahid Siddiqui 14

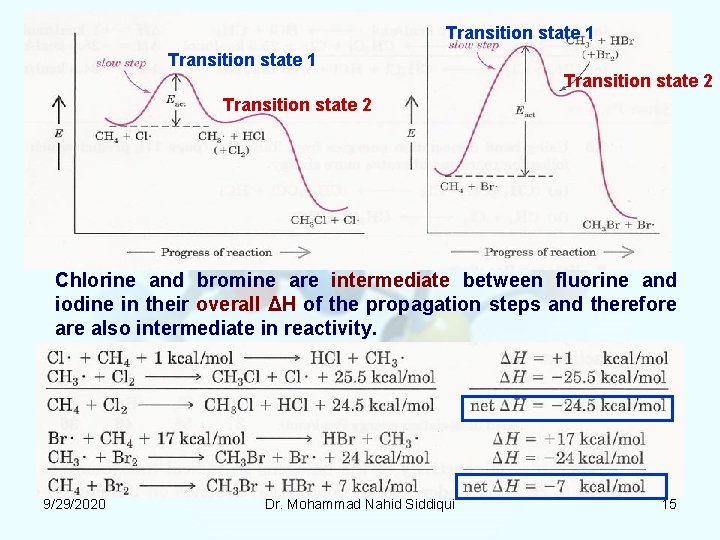
Transition state 1 Transition state 2 Chlorine and bromine are intermediate between fluorine and iodine in their overall ΔH of the propagation steps and therefore also intermediate in reactivity. 9/29/2020 Dr. Mohammad Nahid Siddiqui 15

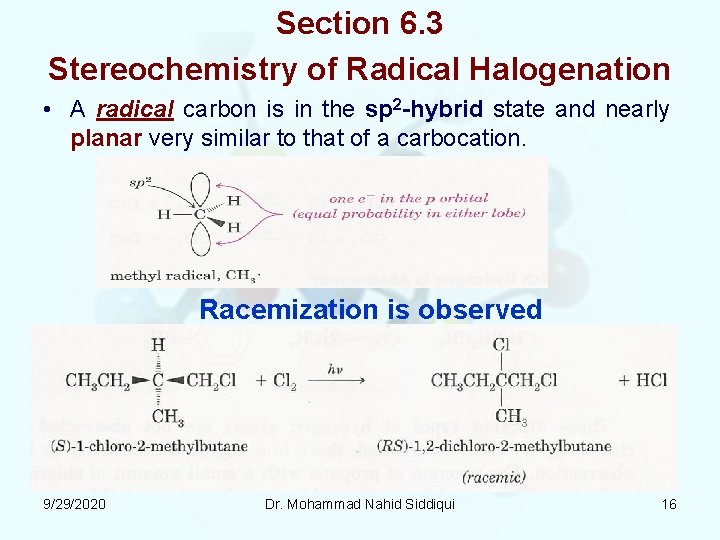
Section 6. 3 Stereochemistry of Radical Halogenation • A radical carbon is in the sp 2 hybrid state and nearly planar very similar to that of a carbocation. Racemization is observed 9/29/2020 Dr. Mohammad Nahid Siddiqui 16

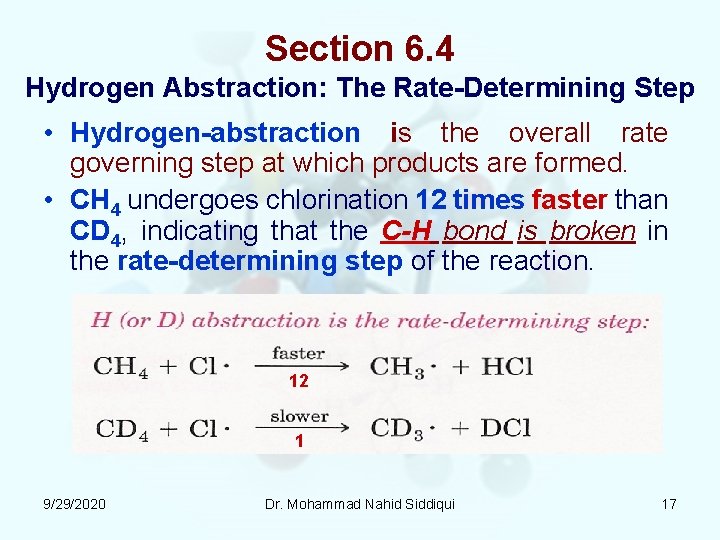
Section 6. 4 Hydrogen Abstraction: The Rate Determining Step • Hydrogen abstraction is the overall rate governing step at which products are formed. • CH 4 undergoes chlorination 12 times faster than CD 4, indicating that the C-H bond is broken in the rate determining step of the reaction. 12 1 9/29/2020 Dr. Mohammad Nahid Siddiqui 17

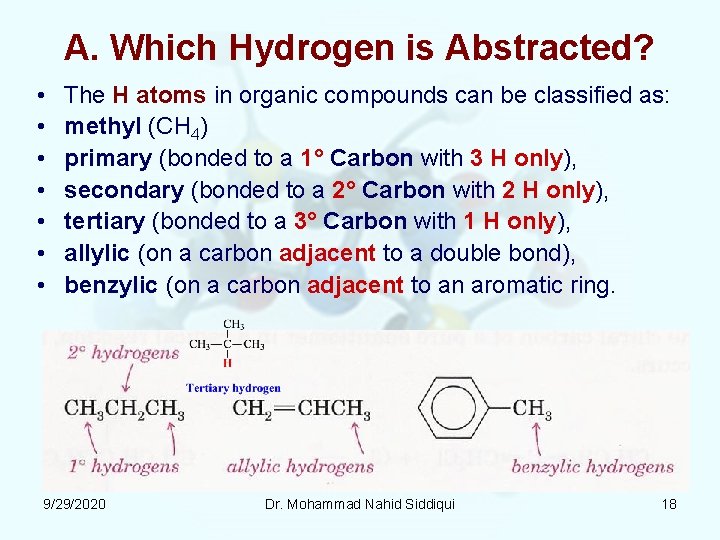
A. Which Hydrogen is Abstracted? • • The H atoms in organic compounds can be classified as: methyl (CH 4) primary (bonded to a 1° Carbon with 3 H only), secondary (bonded to a 2° Carbon with 2 H only), tertiary (bonded to a 3° Carbon with 1 H only), allylic (on a carbon adjacent to a double bond), benzylic (on a carbon adjacent to an aromatic ring. 9/29/2020 Dr. Mohammad Nahid Siddiqui 18

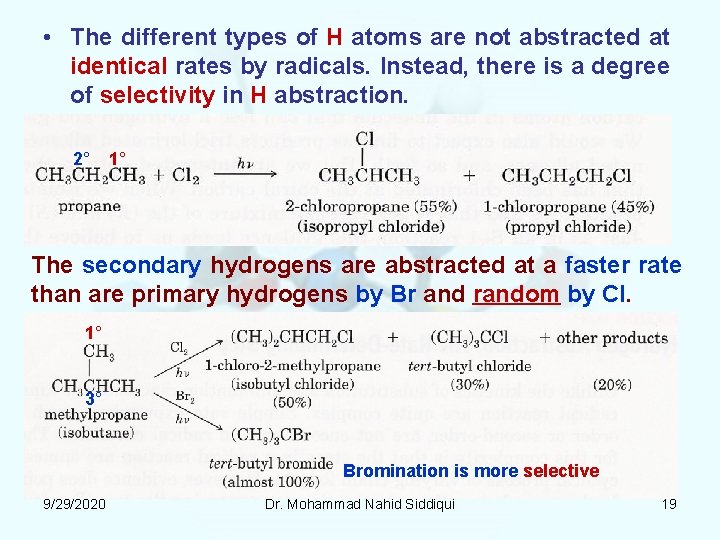
• The different types of H atoms are not abstracted at identical rates by radicals. Instead, there is a degree of selectivity in H abstraction. 2° 1° The secondary hydrogens are abstracted at a faster rate than are primary hydrogens by Br and random by Cl. 1° 3° Bromination is more selective 9/29/2020 Dr. Mohammad Nahid Siddiqui 19

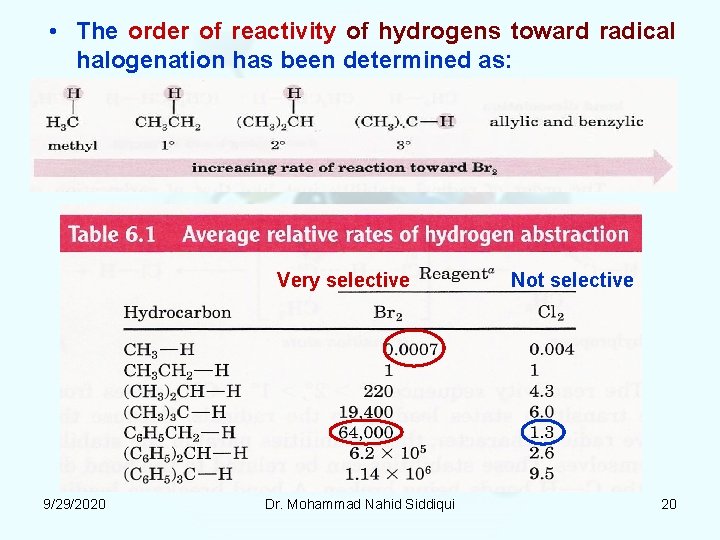
• The order of reactivity of hydrogens toward radical halogenation has been determined as: Very selective 9/29/2020 Dr. Mohammad Nahid Siddiqui Not selective 20

B. Relative Stabilities of Alkyl Radicals • Transition states in the chlorination of methane and methylpropane clarifies the H priority abstraction. • The symbol δ. is used to show that both the chlorine atoms and the carbon atoms have partial radical character in the transition states. 9/29/2020 Dr. Mohammad Nahid Siddiqui 21

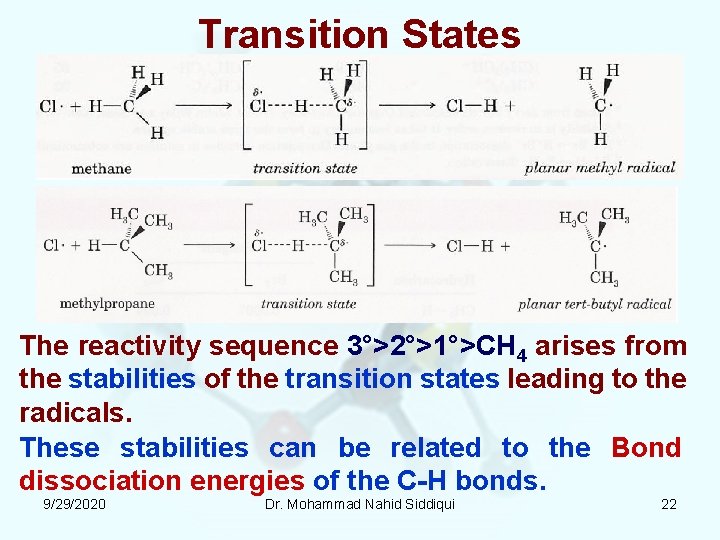
Transition States The reactivity sequence 3°>2°>1°>CH 4 arises from the stabilities of the transition states leading to the radicals. These stabilities can be related to the Bond dissociation energies of the C H bonds. 9/29/2020 Dr. Mohammad Nahid Siddiqui 22

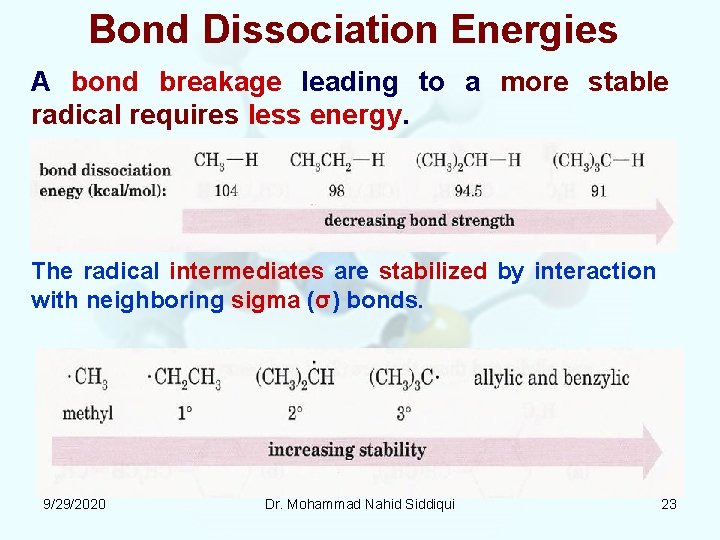
Bond Dissociation Energies A bond breakage leading to a more stable radical requires less energy. The radical intermediates are stabilized by interaction with neighboring sigma (σ) bonds. 9/29/2020 Dr. Mohammad Nahid Siddiqui 23

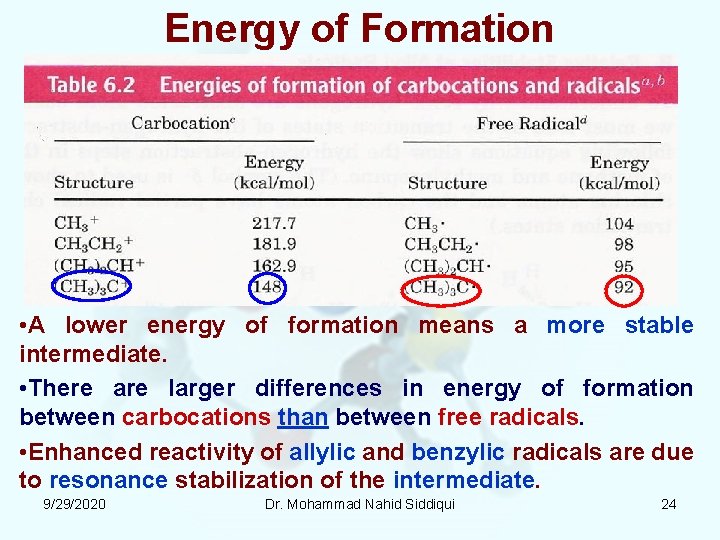
Energy of Formation • A lower energy of formation means a more stable intermediate. • There are larger differences in energy of formation between carbocations than between free radicals. • Enhanced reactivity of allylic and benzylic radicals are due to resonance stabilization of the intermediate. 9/29/2020 Dr. Mohammad Nahid Siddiqui 24

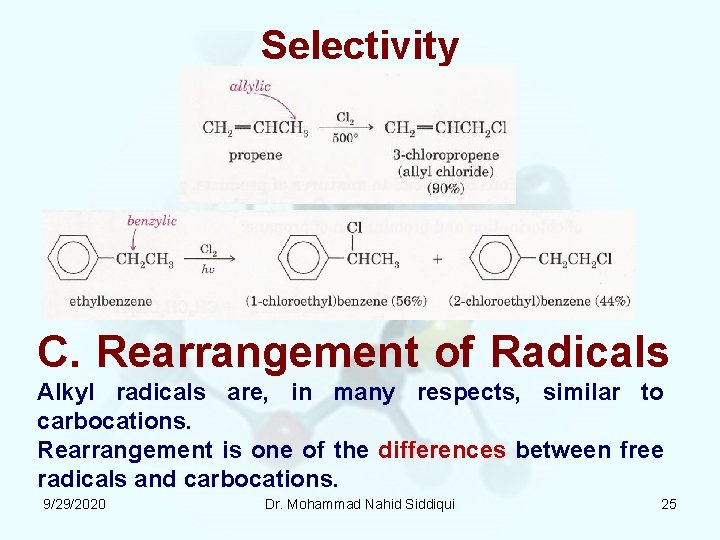
Selectivity C. Rearrangement of Radicals Alkyl radicals are, in many respects, similar to carbocations. Rearrangement is one of the differences between free radicals and carbocations. 9/29/2020 Dr. Mohammad Nahid Siddiqui 25

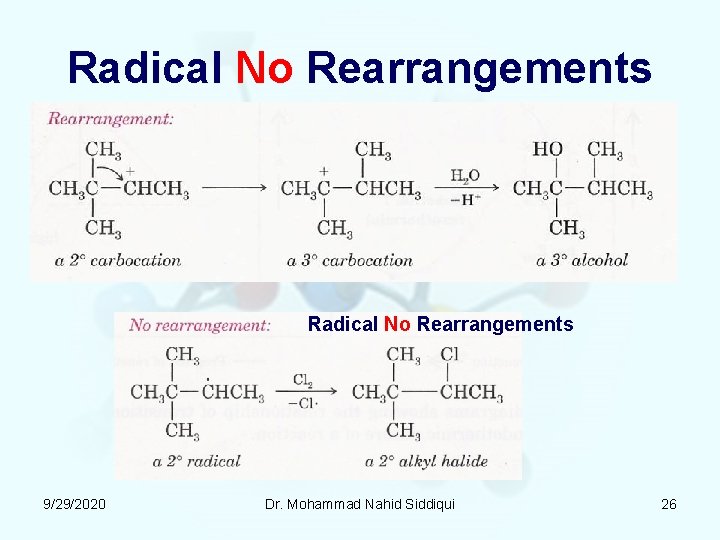
Radical No Rearrangements 9/29/2020 Dr. Mohammad Nahid Siddiqui 26

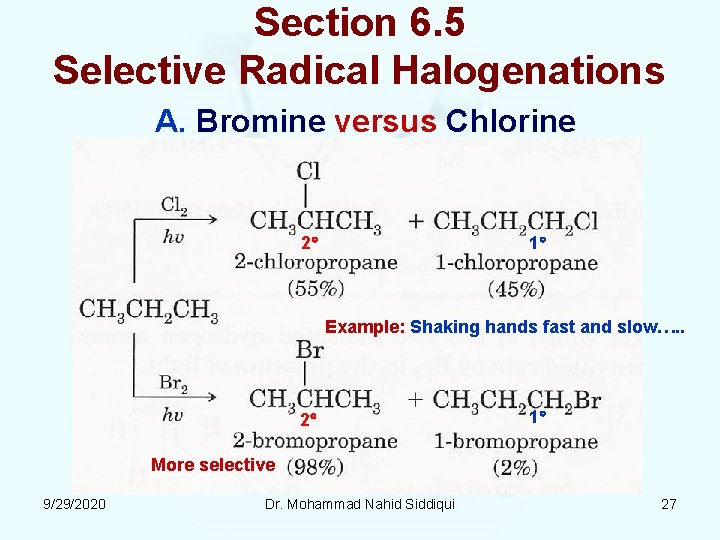
Section 6. 5 Selective Radical Halogenations A. Bromine versus Chlorine 2 1 Example: Shaking hands fast and slow…. . 2 1 More selective 9/29/2020 Dr. Mohammad Nahid Siddiqui 27

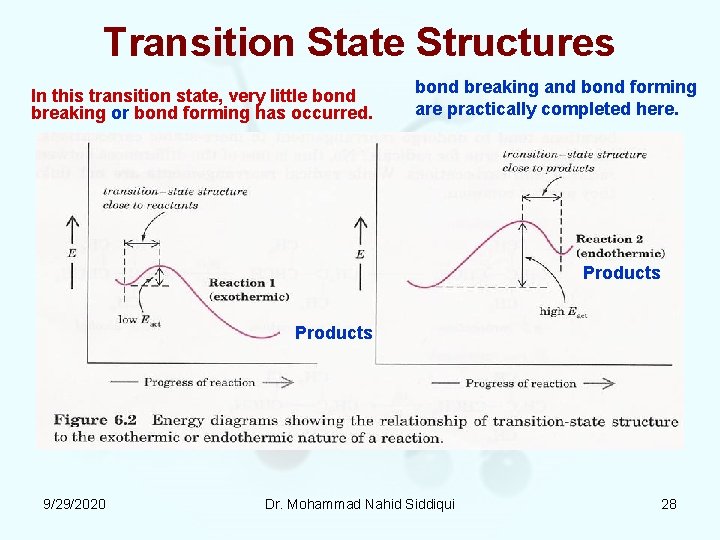
Transition State Structures In this transition state, very little bond breaking or bond forming has occurred. bond breaking and bond forming are practically completed here. Products 9/29/2020 Dr. Mohammad Nahid Siddiqui 28

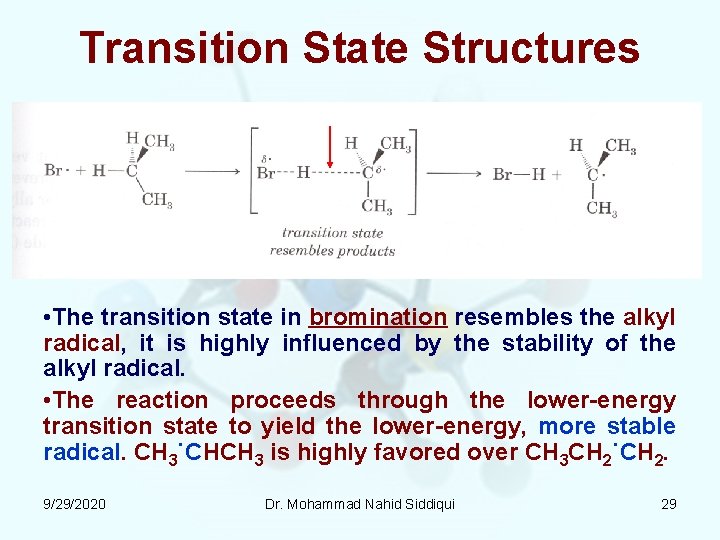
Transition State Structures • The transition state in bromination resembles the alkyl radical, it is highly influenced by the stability of the alkyl radical. • The reaction proceeds through the lower energy transition state to yield the lower energy, more stable radical. CH 3˙CHCH 3 is highly favored over CH 3 CH 2˙CH 2. 9/29/2020 Dr. Mohammad Nahid Siddiqui 29

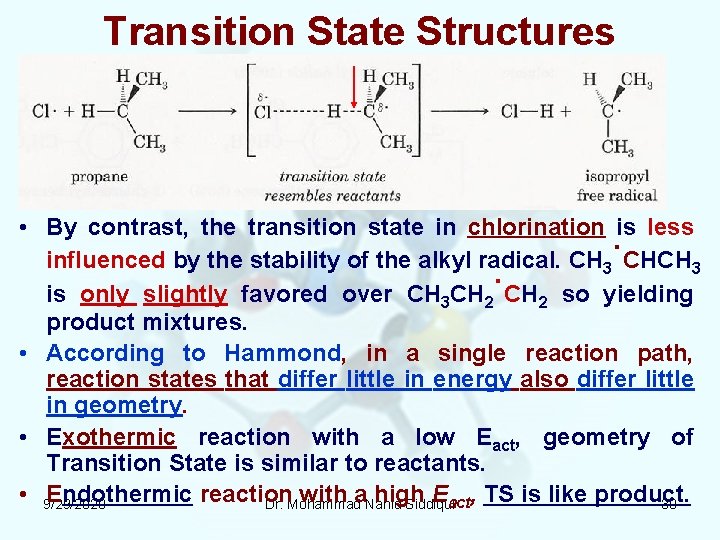
Transition State Structures • By contrast, the transition state in chlorination is less influenced by the stability of the alkyl radical. CH 3˙CHCH 3 is only slightly favored over CH 3 CH 2˙CH 2 so yielding product mixtures. • According to Hammond, in a single reaction path, reaction states that differ little in energy also differ little in geometry. • Exothermic reaction with a low Eact, geometry of Transition State is similar to reactants. • 9/29/2020 Endothermic reaction with a. Nahid high Eact, TS is like product. Dr. Mohammad Siddiqui 30

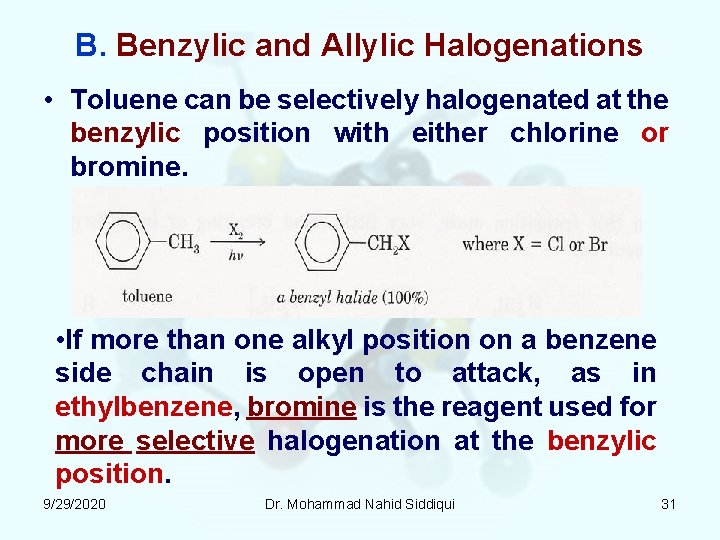
B. Benzylic and Allylic Halogenations • Toluene can be selectively halogenated at the benzylic position with either chlorine or bromine. • If more than one alkyl position on a benzene side chain is open to attack, as in ethylbenzene, bromine is the reagent used for more selective halogenation at the benzylic position. 9/29/2020 Dr. Mohammad Nahid Siddiqui 31

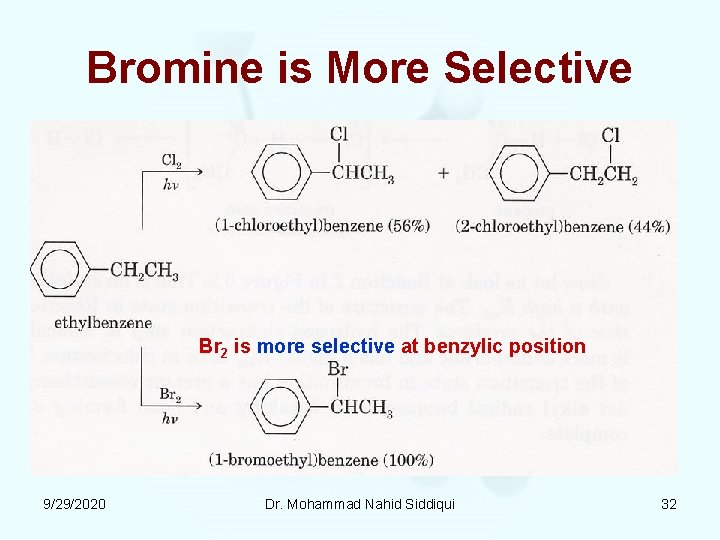
Bromine is More Selective Br 2 is more selective at benzylic position 9/29/2020 Dr. Mohammad Nahid Siddiqui 32

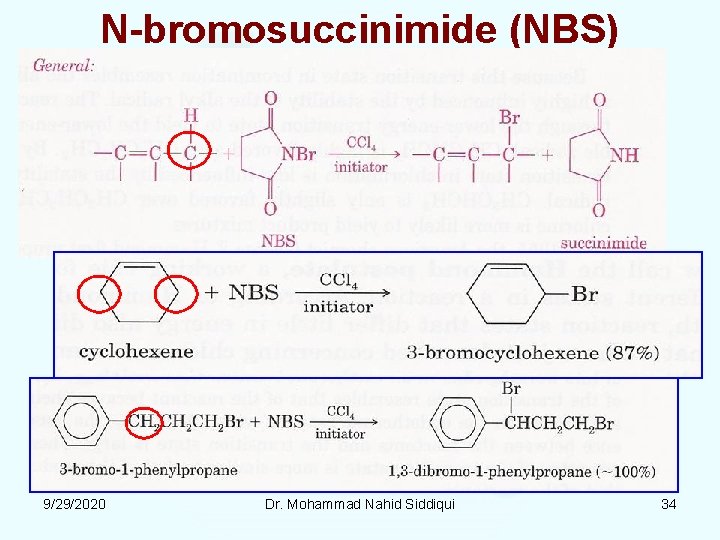
Allylic/Benzylic Halogenation • Alkenes can be directly halogenated at the allylic position, but very high temperatures and a low concentration of halogen must be used to prevent addition reaction at the double bond. • N bromosuccinimide (NBS) is a more specific reagent than Br 2 for allylic and benzylic halogenations. • An NBS reaction can be initiated by either light or free radicals from a peroxide (ROOR 2 RO. ). 9/29/2020 Dr. Mohammad Nahid Siddiqui 33

N bromosuccinimide (NBS) 9/29/2020 Dr. Mohammad Nahid Siddiqui 34

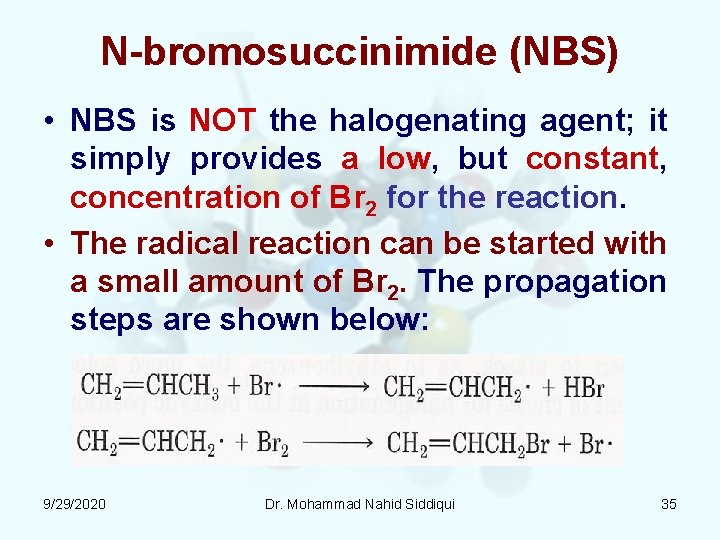
N bromosuccinimide (NBS) • NBS is NOT the halogenating agent; it simply provides a low, but constant, concentration of Br 2 for the reaction. • The radical reaction can be started with a small amount of Br 2. The propagation steps are shown below: 9/29/2020 Dr. Mohammad Nahid Siddiqui 35

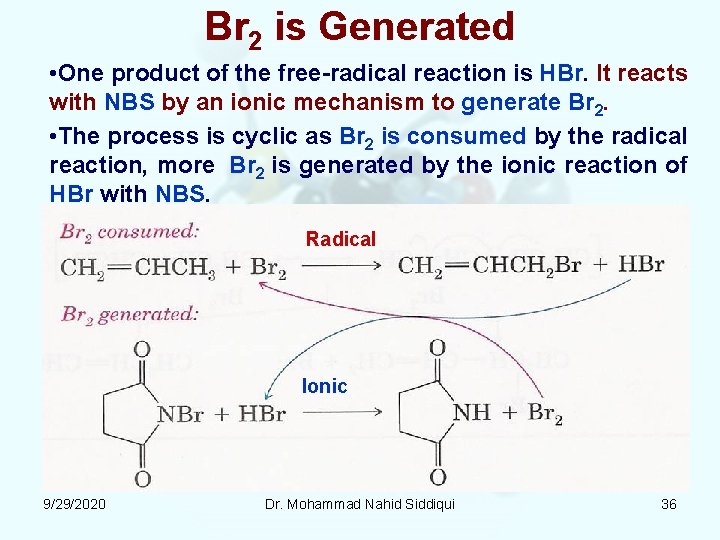
Br 2 is Generated • One product of the free radical reaction is HBr. It reacts with NBS by an ionic mechanism to generate Br 2. • The process is cyclic as Br 2 is consumed by the radical reaction, more Br 2 is generated by the ionic reaction of HBr with NBS. Radical Ionic 9/29/2020 Dr. Mohammad Nahid Siddiqui 36

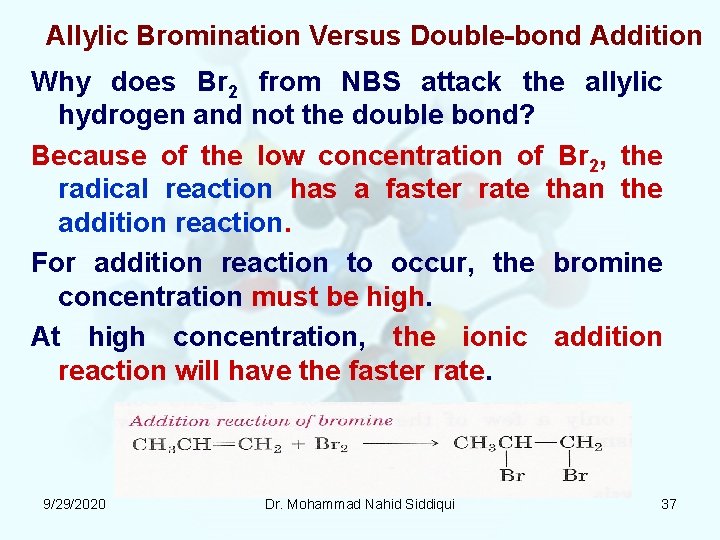
Allylic Bromination Versus Double bond Addition Why does Br 2 from NBS attack the allylic hydrogen and not the double bond? Because of the low concentration of Br 2, the radical reaction has a faster rate than the addition reaction. For addition reaction to occur, the bromine concentration must be high. At high concentration, the ionic addition reaction will have the faster rate. 9/29/2020 Dr. Mohammad Nahid Siddiqui 37

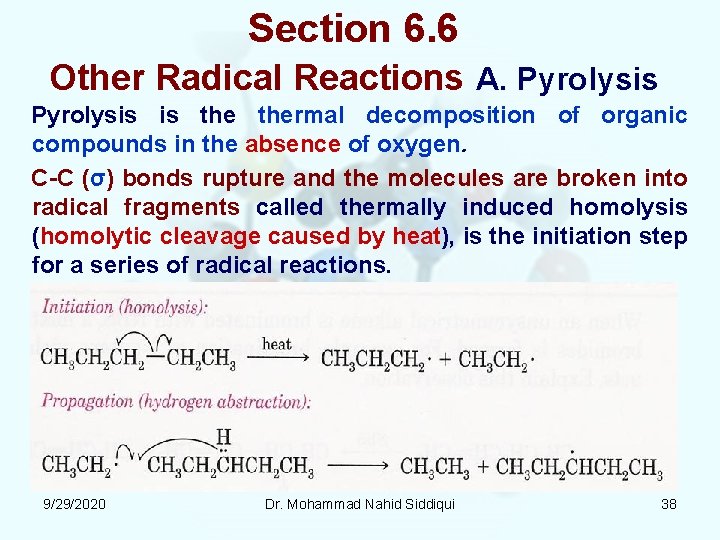
Section 6. 6 Other Radical Reactions A. Pyrolysis is thermal decomposition of organic compounds in the absence of oxygen. C C (σ) bonds rupture and the molecules are broken into radical fragments called thermally induced homolysis (homolytic cleavage caused by heat), is the initiation step for a series of radical reactions. 9/29/2020 Dr. Mohammad Nahid Siddiqui 38

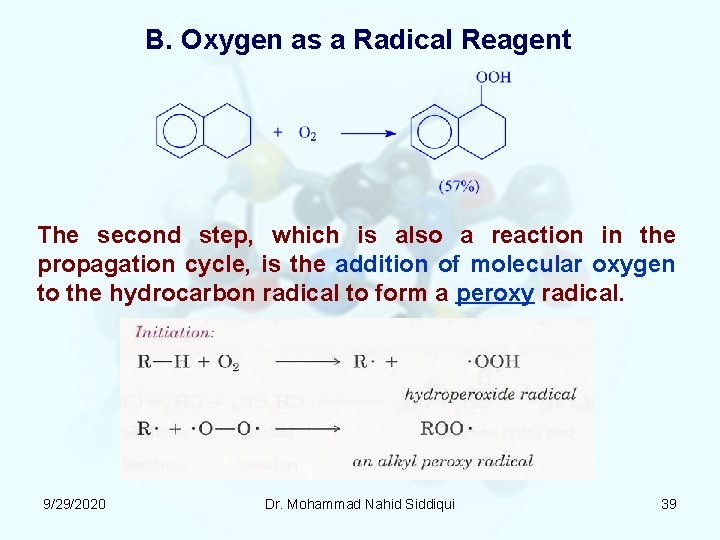
B. Oxygen as a Radical Reagent The second step, which is also a reaction in the propagation cycle, is the addition of molecular oxygen to the hydrocarbon radical to form a peroxy radical. 9/29/2020 Dr. Mohammad Nahid Siddiqui 39

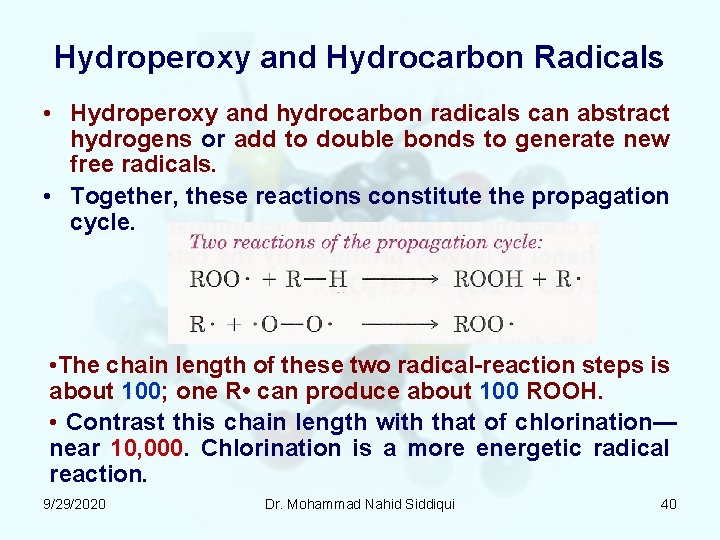
Hydroperoxy and Hydrocarbon Radicals • Hydroperoxy and hydrocarbon radicals can abstract hydrogens or add to double bonds to generate new free radicals. • Together, these reactions constitute the propagation cycle. • The chain length of these two radical reaction steps is about 100; one R • can produce about 100 ROOH. • Contrast this chain length with that of chlorination— near 10, 000. Chlorination is a more energetic radical reaction. 9/29/2020 Dr. Mohammad Nahid Siddiqui 40

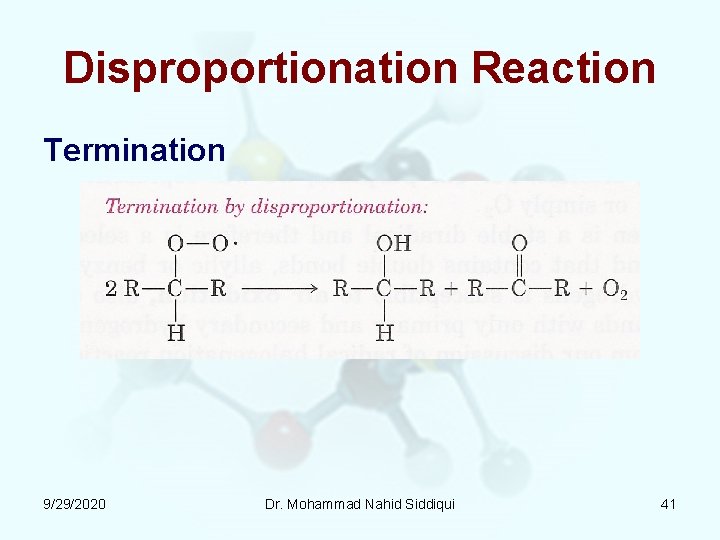
Disproportionation Reaction Termination 9/29/2020 Dr. Mohammad Nahid Siddiqui 41

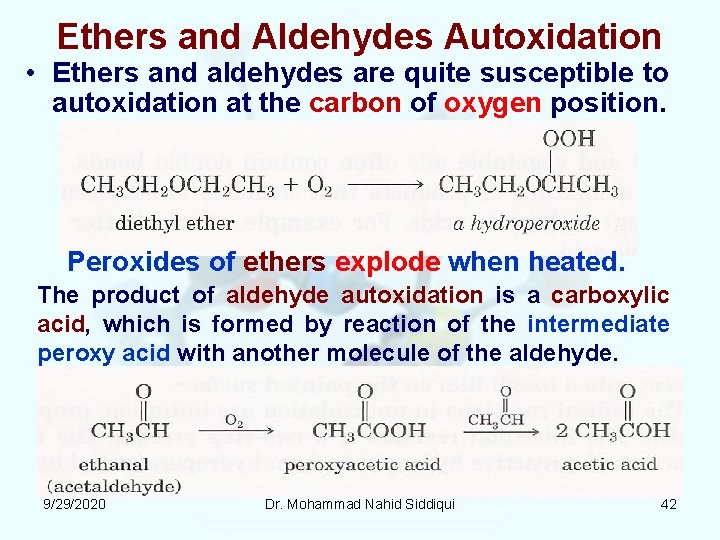
Ethers and Aldehydes Autoxidation • Ethers and aldehydes are quite susceptible to autoxidation at the carbon of oxygen position. Peroxides of ethers explode when heated. The product of aldehyde autoxidation is a carboxylic acid, which is formed by reaction of the intermediate peroxy acid with another molecule of the aldehyde. 9/29/2020 Dr. Mohammad Nahid Siddiqui 42

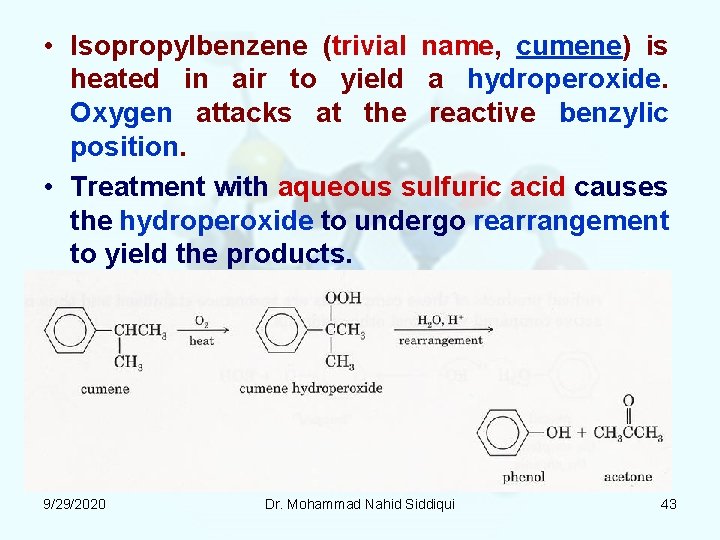
• Isopropylbenzene (trivial name, cumene) is heated in air to yield a hydroperoxide. Oxygen attacks at the reactive benzylic position. • Treatment with aqueous sulfuric acid causes the hydroperoxide to undergo rearrangement to yield the products. 9/29/2020 Dr. Mohammad Nahid Siddiqui 43

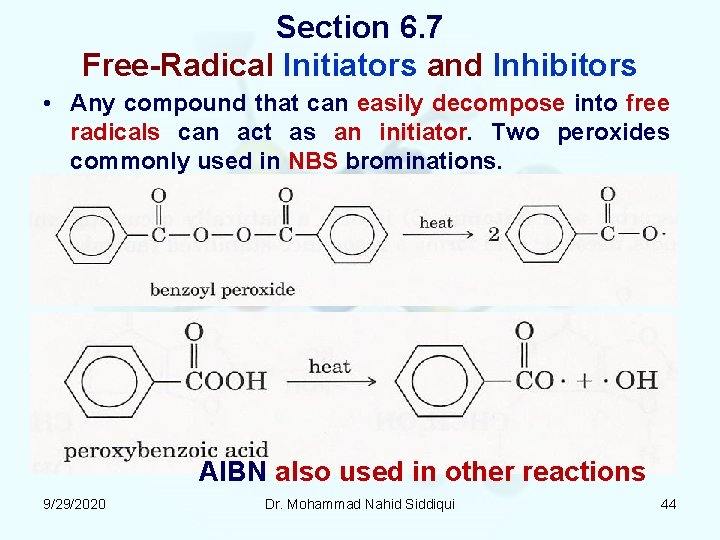
Section 6. 7 Free Radical Initiators and Inhibitors • Any compound that can easily decompose into free radicals can act as an initiator. Two peroxides commonly used in NBS brominations. AIBN also used in other reactions 9/29/2020 Dr. Mohammad Nahid Siddiqui 44

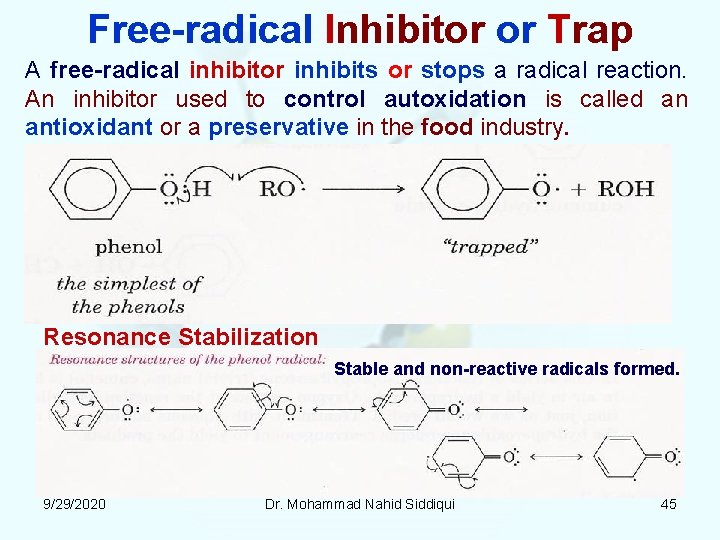
Free radical Inhibitor or Trap A free radical inhibitor inhibits or stops a radical reaction. An inhibitor used to control autoxidation is called an antioxidant or a preservative in the food industry. Resonance Stabilization Stable and non reactive radicals formed. 9/29/2020 Dr. Mohammad Nahid Siddiqui 45

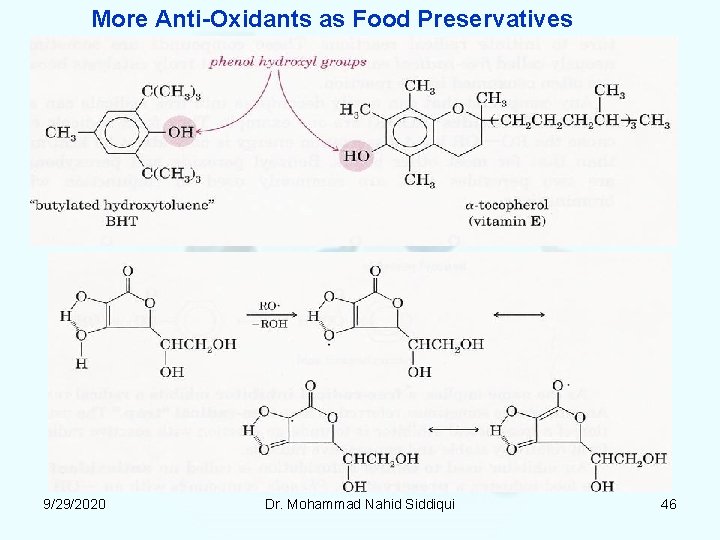
More Anti Oxidants as Food Preservatives 9/29/2020 Dr. Mohammad Nahid Siddiqui 46

Section 6. 8 Polymers Classification of Polymers • Elastomers are those polymers with elastic properties, like rubber. • Fibers are threadlike polymers, such as cotton, silk, or nylon. • Plastics can be thin sheets (kitchen wrap), hard and moldable solids (piping, children's toys), or coatings (car finishes, varnishes). • A polymer is made up of many repeating units of small parts, called monomers ("one part"). • In a polymerization reaction, the first products are dimers ("two parts"), then trimers, tetramers and so on. • The polymers called addition polymers because they are formed by the addition of monomers to each other without the loss of atoms or groups. 9/29/2020 Dr. Mohammad Nahid Siddiqui 47

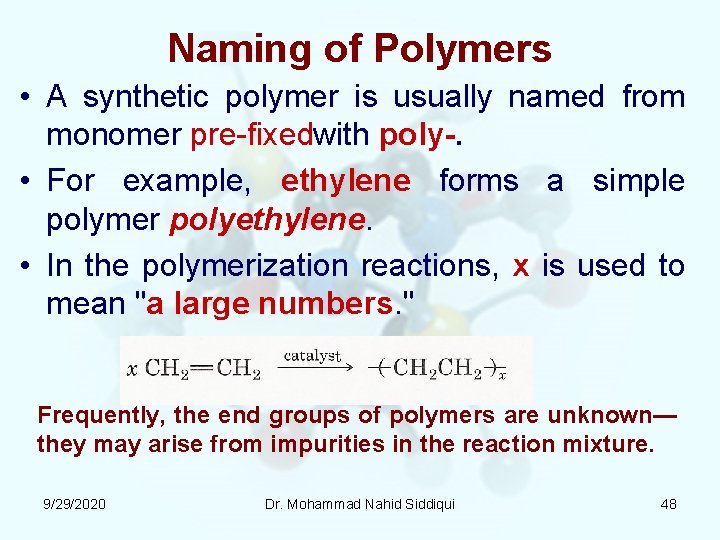
Naming of Polymers • A synthetic polymer is usually named from monomer pre fixedwith poly. • For example, ethylene forms a simple polymer polyethylene. • In the polymerization reactions, x is used to mean "a large numbers. " Frequently, the end groups of polymers are unknown— they may arise from impurities in the reaction mixture. 9/29/2020 Dr. Mohammad Nahid Siddiqui 48

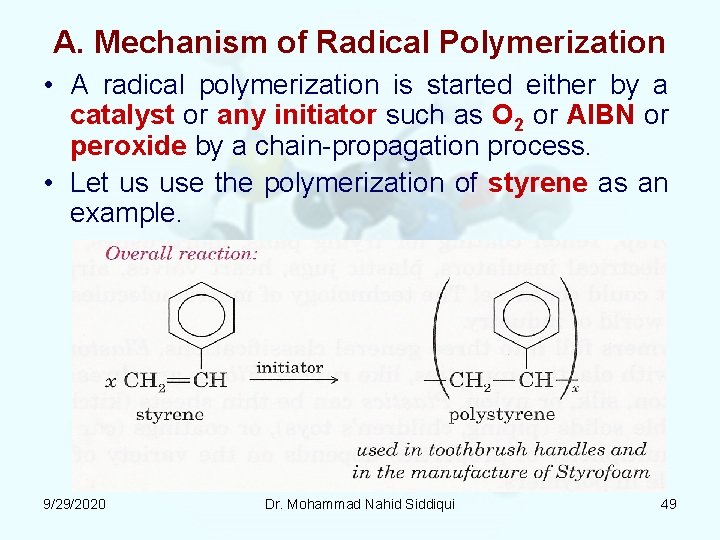
A. Mechanism of Radical Polymerization • A radical polymerization is started either by a catalyst or any initiator such as O 2 or AIBN or peroxide by a chain propagation process. • Let us use the polymerization of styrene as an example. 9/29/2020 Dr. Mohammad Nahid Siddiqui 49

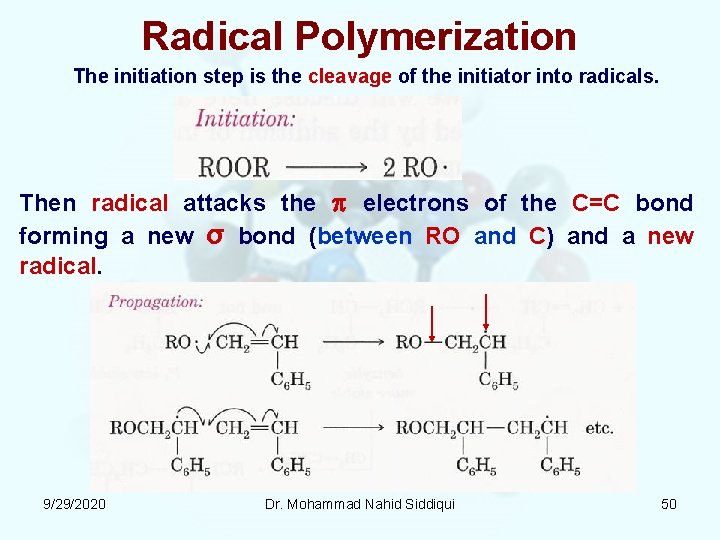
Radical Polymerization The initiation step is the cleavage of the initiator into radicals. Then radical attacks the electrons of the C=C bond forming a new σ bond (between RO and C) and a new radical. 9/29/2020 Dr. Mohammad Nahid Siddiqui 50

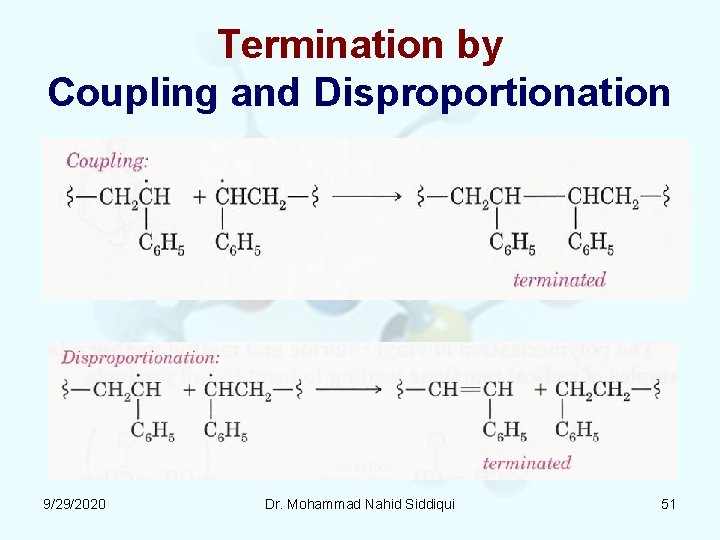
Termination by Coupling and Disproportionation 9/29/2020 Dr. Mohammad Nahid Siddiqui 51

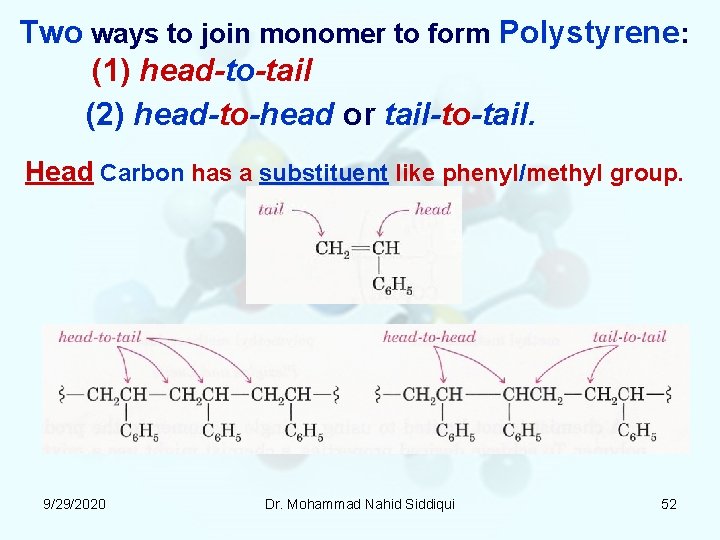
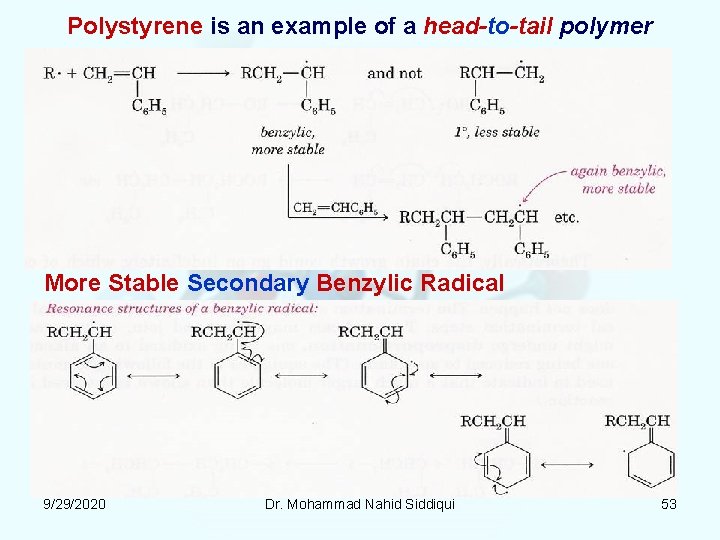
Two ways to join monomer to form Polystyrene: (1) head-to-tail (2) head-to-head or tail-to-tail. Head Carbon has a substituent like phenyl/methyl group. 9/29/2020 Dr. Mohammad Nahid Siddiqui 52

Polystyrene is an example of a head-to-tail polymer More Stable Secondary Benzylic Radical 9/29/2020 Dr. Mohammad Nahid Siddiqui 53

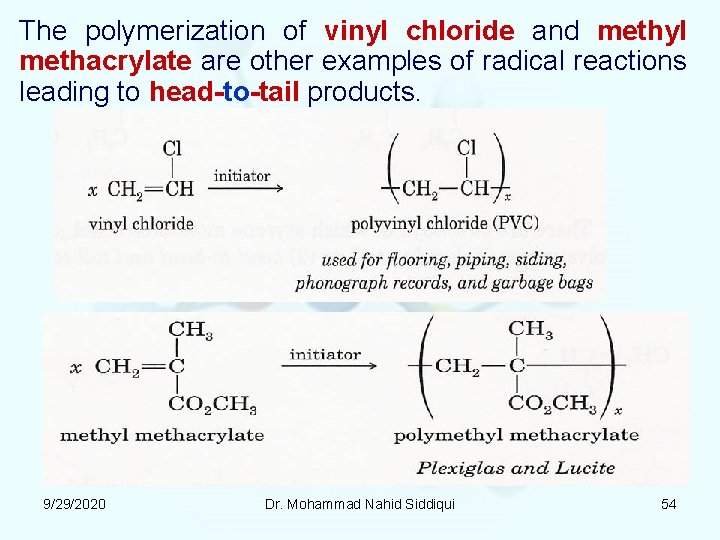
The polymerization of vinyl chloride and methyl methacrylate are other examples of radical reactions leading to head to tail products. 9/29/2020 Dr. Mohammad Nahid Siddiqui 54

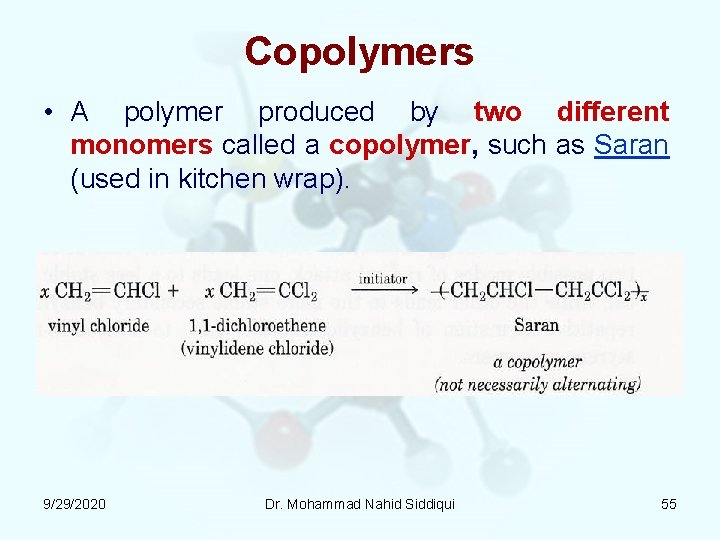
Copolymers • A polymer produced by two different monomers called a copolymer, such as Saran (used in kitchen wrap). 9/29/2020 Dr. Mohammad Nahid Siddiqui 55

B. Structure and Stereochemistry of Polymers • Polymers may have functional groups and chiral carbons and can also undergo hydrogen bonding and dipole–dipole interactions. • The chemical composition of a polymer chain is called its primary structure. • The arrangement of itself relative to other chains is called the secondary structure. 9/29/2020 Dr. Mohammad Nahid Siddiqui 56

Crystalline and Non crystalline Polymer • A polymer may be a tangled/twisted mass of continuous chains or branched chains. The result is a soft amorphous solid such as soft rubber. • A polymer composed of continuous chains held together by hydrogen bonds or by other dipole— dipole attractions is more ordered in nature. • A more ordered polymer is said to have a higher degree of crystallinity than the amorphous or non crystalline polymer. 9/29/2020 Dr. Mohammad Nahid Siddiqui 57

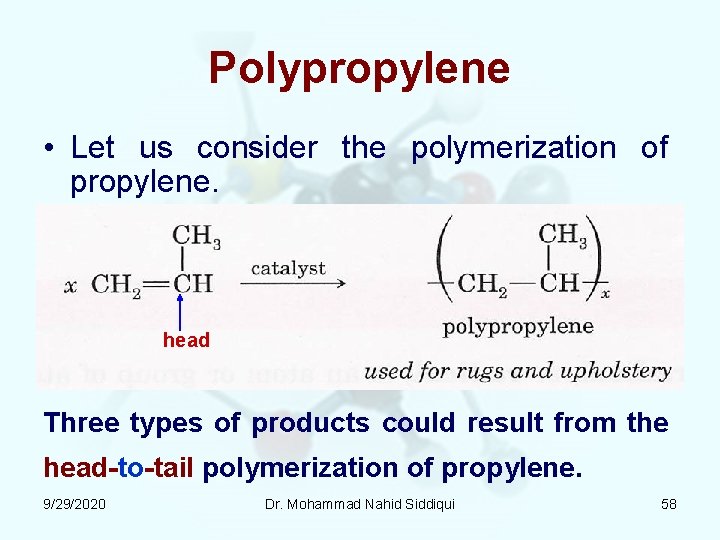
Polypropylene • Let us consider the polymerization of propylene. head Three types of products could result from the head to tail polymerization of propylene. 9/29/2020 Dr. Mohammad Nahid Siddiqui 58

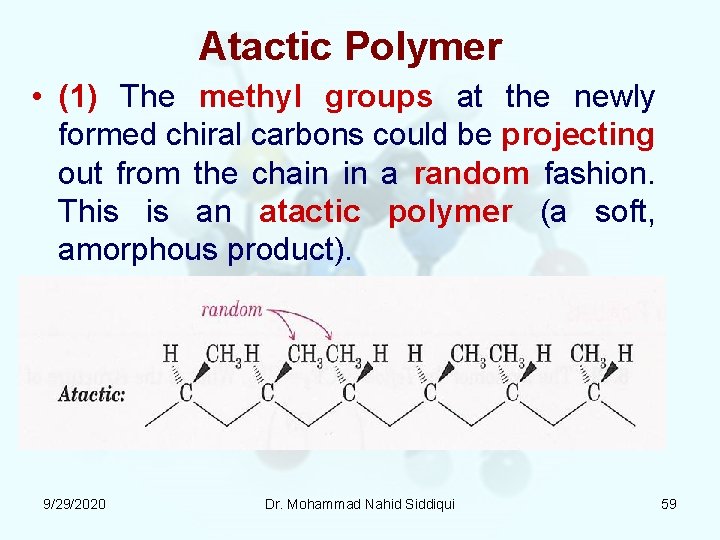
Atactic Polymer • (1) The methyl groups at the newly formed chiral carbons could be projecting out from the chain in a random fashion. This is an atactic polymer (a soft, amorphous product). 9/29/2020 Dr. Mohammad Nahid Siddiqui 59

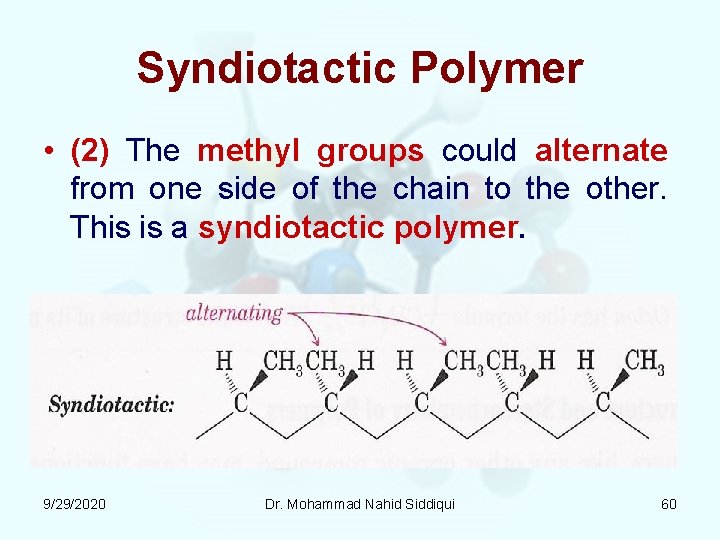
Syndiotactic Polymer • (2) The methyl groups could alternate from one side of the chain to the other. This is a syndiotactic polymer. 9/29/2020 Dr. Mohammad Nahid Siddiqui 60

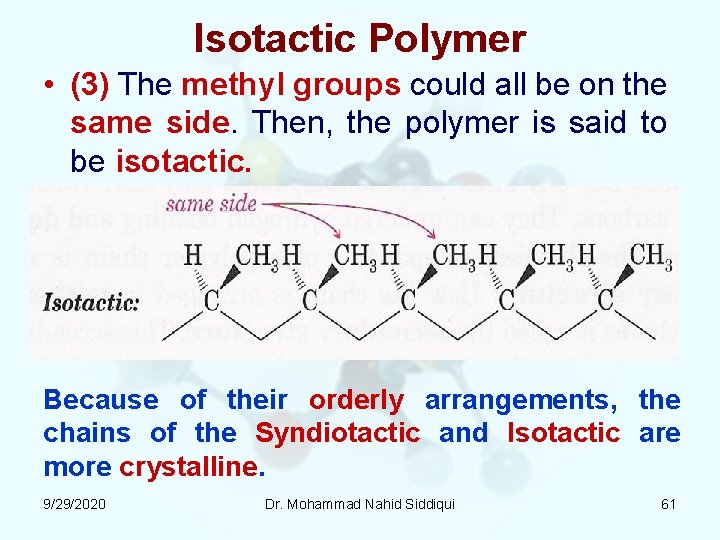
Isotactic Polymer • (3) The methyl groups could all be on the same side. Then, the polymer is said to be isotactic. Because of their orderly arrangements, the chains of the Syndiotactic and Isotactic are more crystalline. 9/29/2020 Dr. Mohammad Nahid Siddiqui 61

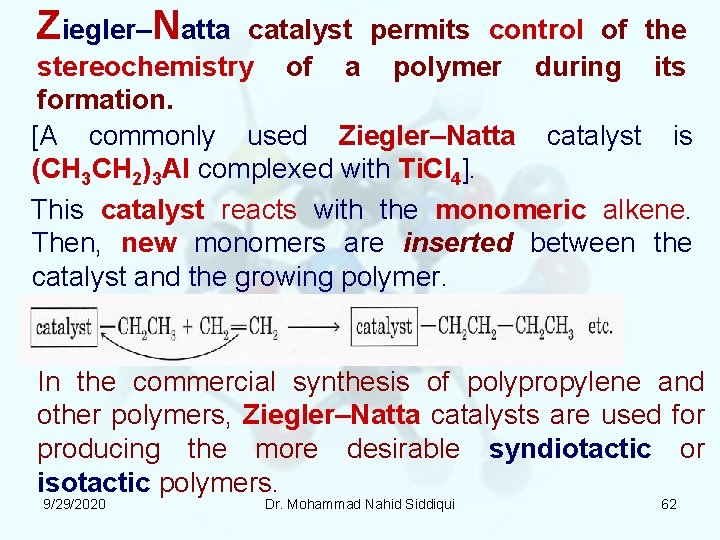
Ziegler–Natta catalyst permits control of the stereochemistry of a polymer during its formation. [A commonly used Ziegler–Natta catalyst is (CH 3 CH 2)3 Al complexed with Ti. Cl 4]. This catalyst reacts with the monomeric alkene. Then, new monomers are inserted between the catalyst and the growing polymer. In the commercial synthesis of polypropylene and other polymers, Ziegler–Natta catalysts are used for producing the more desirable syndiotactic or isotactic polymers. 9/29/2020 Dr. Mohammad Nahid Siddiqui 62
 Nahid siddiqui
Nahid siddiqui Dr nahid siddiqui
Dr nahid siddiqui Nahid farnaz
Nahid farnaz Nahid farnaz
Nahid farnaz Nahid farnaz
Nahid farnaz Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Half redox reaction
Half redox reaction Entire radical to mixed radical
Entire radical to mixed radical Unit 6 test radical functions
Unit 6 test radical functions What is an entire radical
What is an entire radical Areej aftab siddiqui
Areej aftab siddiqui Asim siddiqui azim premji university
Asim siddiqui azim premji university Christison saying
Christison saying Dr ashry
Dr ashry Types of shirkah
Types of shirkah Gazala siddiqui md
Gazala siddiqui md Mahvash siddiqui
Mahvash siddiqui Rehana ladha
Rehana ladha Najma sadeki
Najma sadeki Dr basith ali siddiqui
Dr basith ali siddiqui Ali siddiqui
Ali siddiqui Dr aisha siddiqui
Dr aisha siddiqui Durre siddiqui
Durre siddiqui Sameera siddiqui
Sameera siddiqui Dr rehana siddiqui
Dr rehana siddiqui Free radical substitution
Free radical substitution Antioxidant free radical
Antioxidant free radical Free radical mechanism
Free radical mechanism Free radical halogenation
Free radical halogenation Mohammad ridwan bkn
Mohammad ridwan bkn Dr mohammad aman
Dr mohammad aman Shaik mohammad tajuddin
Shaik mohammad tajuddin Mohammad keshavarz
Mohammad keshavarz Anovulation who classification
Anovulation who classification Alaa mohammad fouad
Alaa mohammad fouad Pearo-nn
Pearo-nn Mohammad irfan bowdoin
Mohammad irfan bowdoin Modified wells score
Modified wells score Mohammad sharifkhani
Mohammad sharifkhani Mohammad vesal
Mohammad vesal Where is the pelvic girdle located
Where is the pelvic girdle located Vlsi
Vlsi Mohammad diab md
Mohammad diab md Dr mohammad khan
Dr mohammad khan Kongres melayu 1939 dan 1940
Kongres melayu 1939 dan 1940 Mohammad sadegh rasooli
Mohammad sadegh rasooli Mohammad sharifkhani
Mohammad sharifkhani Mohammad ali javidian
Mohammad ali javidian Dr elham altaf
Dr elham altaf Mohammad arjomand
Mohammad arjomand Dr mohammad aman
Dr mohammad aman Mohammad alipour
Mohammad alipour Mohammad ghoreishi
Mohammad ghoreishi Mohammad sharifkhani
Mohammad sharifkhani Dr nur mohammad hadi zahalan
Dr nur mohammad hadi zahalan Mohammad ali abtahi
Mohammad ali abtahi Helmholtz free energy and gibbs free energy
Helmholtz free energy and gibbs free energy Negative free energy change
Negative free energy change Free hearts free foreheads
Free hearts free foreheads Ln ksp vs 1/t
Ln ksp vs 1/t