Free Radical Chlorination Experimental Evidence Helps to Determine




- Slides: 15

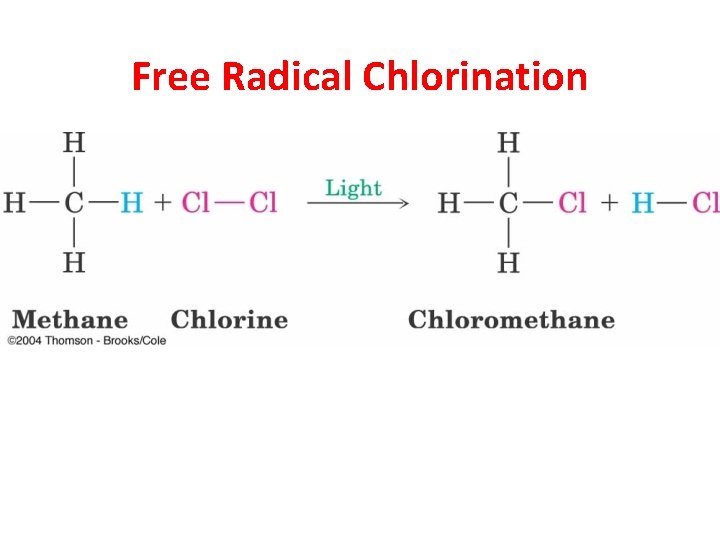
Free Radical Chlorination

Experimental Evidence Helps to Determine Mechanism • Chlorination does not occur at room temperature in the dark. • The most effective wavelength of light is blue that is strongly absorbed by Cl 2 gas. • The light-initiated reaction has a high quantum yield (many molecules of product are formed from each photon of light).

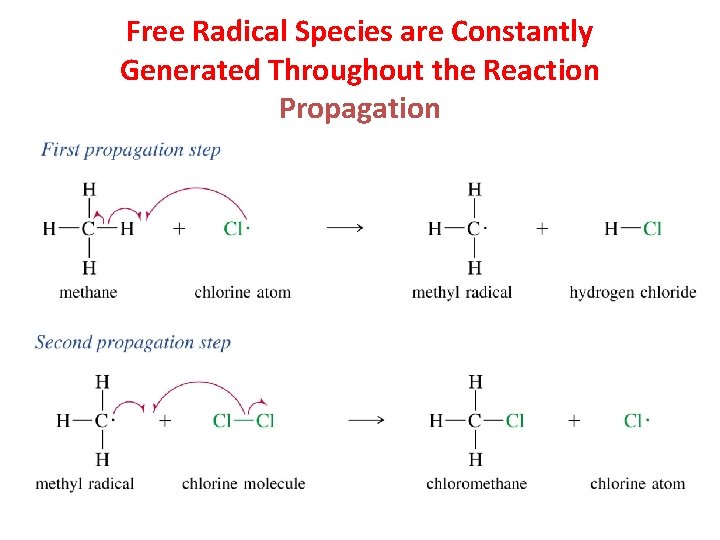
Free Radical Species are Constantly Generated Throughout the Reaction Propagation

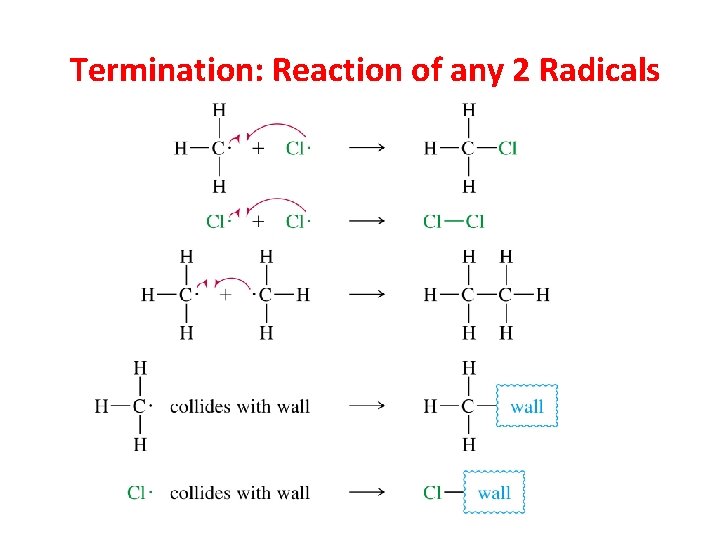
Termination: Reaction of any 2 Radicals

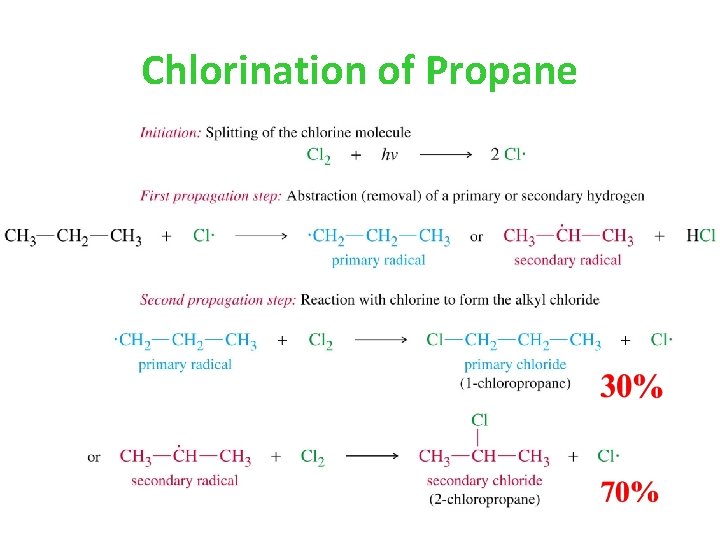
Chlorination of Propane

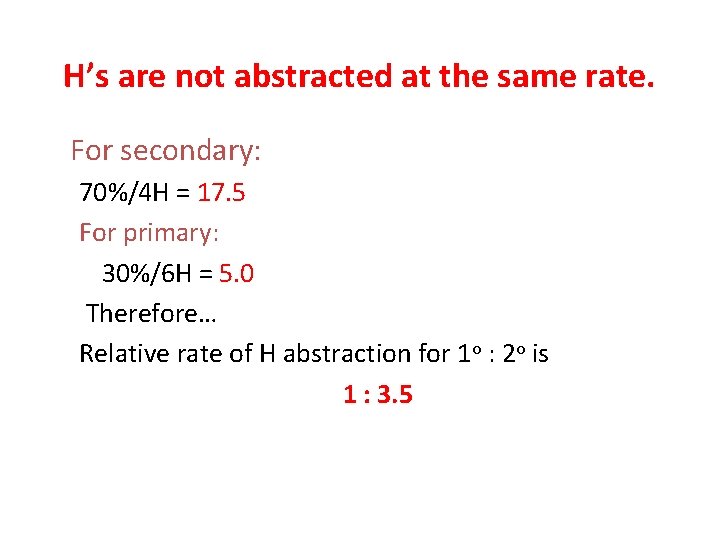
H’s are not abstracted at the same rate. For secondary: 70%/4 H = 17. 5 For primary: 30%/6 H = 5. 0 Therefore… Relative rate of H abstraction for 1 o : 2 o is 1 : 3. 5

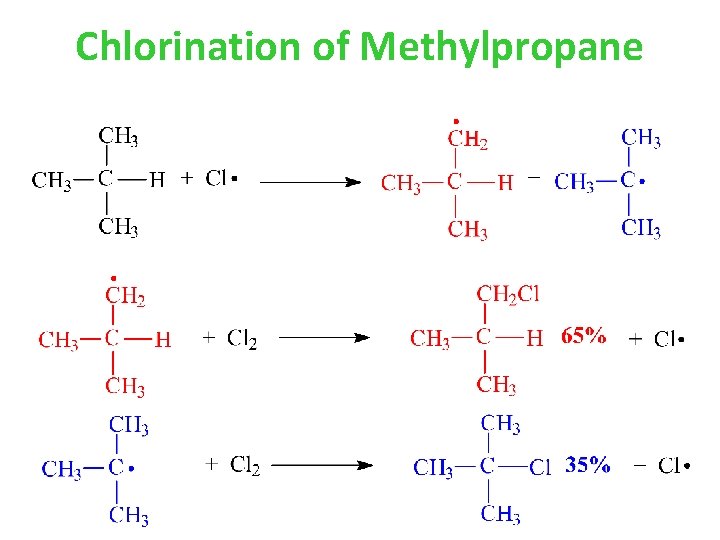
Chlorination of Methylpropane

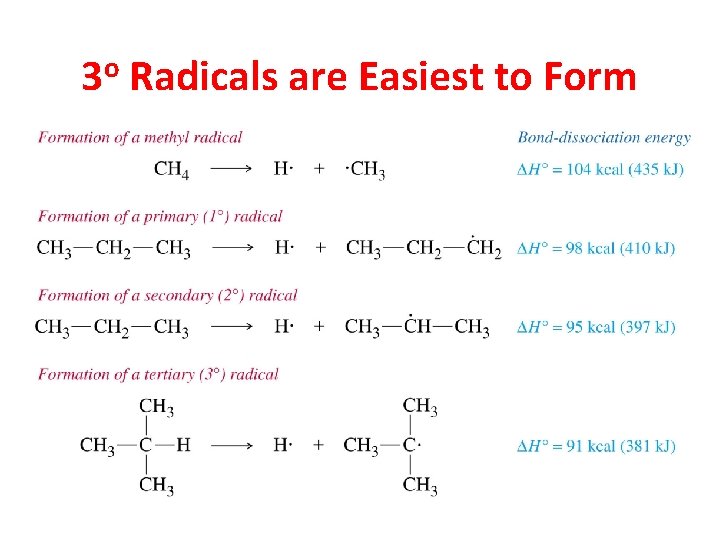
3 o Radicals are Easiest to Form

Tertiary H’s removed five times more readily than primary H’s in chlorination reactions Relative reactivity of 1 o H abstraction: 65% / 9 H = 7. 2 Relative reactivity of 3 o H abstraction: 35% / 1 H = 35 Therefore… Relative rate of H abstraction for 1 o : 3 o 1: 5

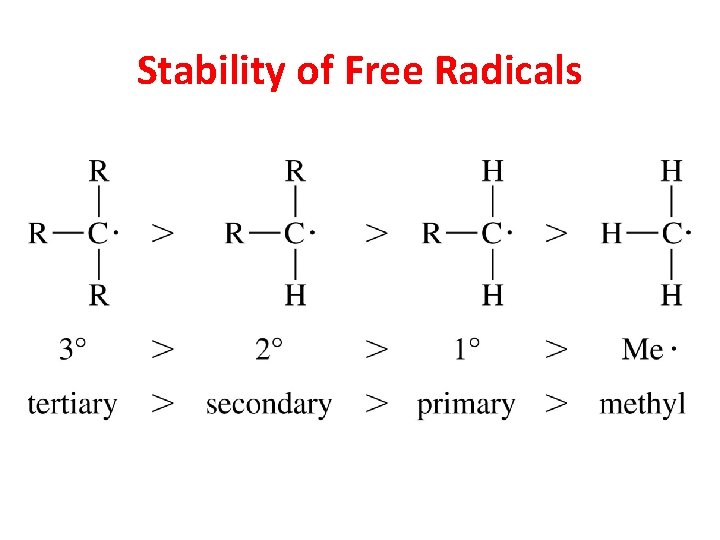
Stability of Free Radicals


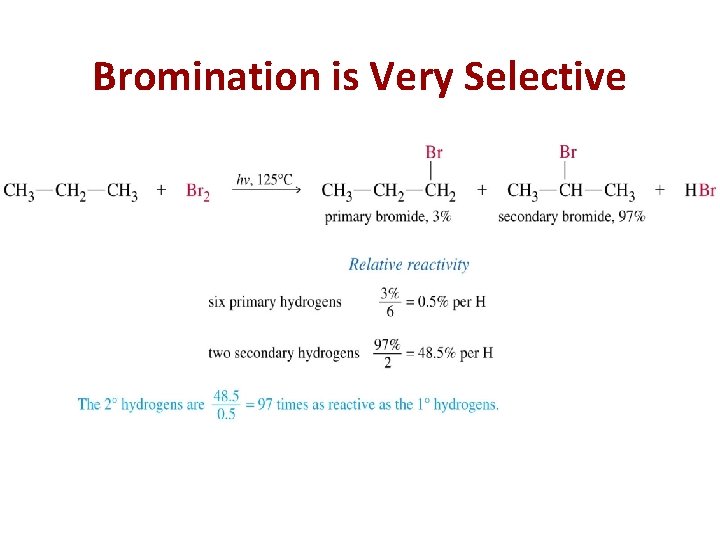
Bromination is Very Selective

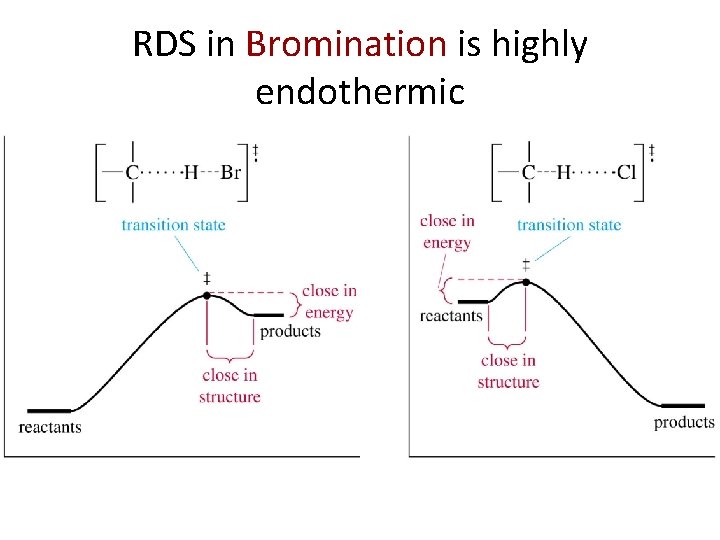
RDS in Bromination is highly endothermic

Consider the free radical monochlorination of 2, 2, 5 -trimethylhexane. Draw all of the unique products (ignore stereoisomers; use zig-zag structures please) and predict the ratio or percent composition of the products. The relative reactivity of H abstraction in a chlorination reaction: 1 o: 2 o: 3 o = 1: 3. 5: 5

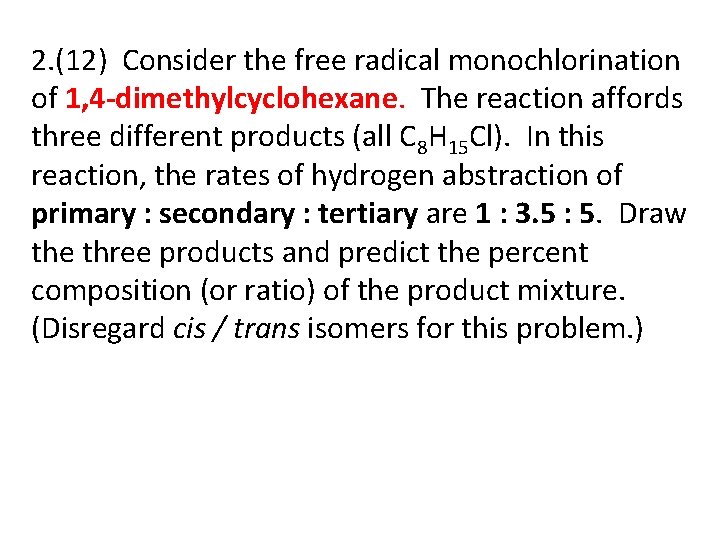
2. (12) Consider the free radical monochlorination of 1, 4 -dimethylcyclohexane. The reaction affords three different products (all C 8 H 15 Cl). In this reaction, the rates of hydrogen abstraction of primary : secondary : tertiary are 1 : 3. 5 : 5. Draw the three products and predict the percent composition (or ratio) of the product mixture. (Disregard cis / trans isomers for this problem. )
 Experimental vs non experimental
Experimental vs non experimental Descriptive vs correlational vs experimental research
Descriptive vs correlational vs experimental research Experimental vs non experimental research
Experimental vs non experimental research Experimental vs nonexperimental research
Experimental vs nonexperimental research Experimental vs non experimental
Experimental vs non experimental Entire radical examples
Entire radical examples Unit 6 radical functions homework 6 radical equations
Unit 6 radical functions homework 6 radical equations What is an entire radical
What is an entire radical Double pot method for disinfection of wells
Double pot method for disinfection of wells Lucas test reagent
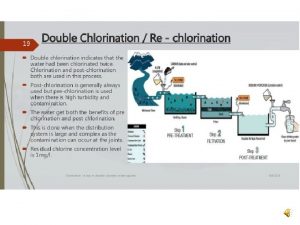
Lucas test reagent Breakpoint chlorination definition
Breakpoint chlorination definition Objective of disinfection
Objective of disinfection Chlorination
Chlorination Side chain oxidation
Side chain oxidation Index
Index Experimental evidence
Experimental evidence